Abstract
Timing of cortisol collection during pregnancy is an important factor within studies reporting on the association between maternal cortisol and depression during pregnancy. Our objective was to further examine the extent to which reported associations differed across studies according to time of maternal cortisol collection during pregnancy. On December 15, 2016, records were identified using PubMed/MEDLINE (National Library of Medicine), EMBASE (Elsevier; 1974–), Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO), PsycINFO (EBSCO), and Web of Science Core Collection (Thomson Reuters). Unique abstracts were screened using the following inclusion criteria: (1) maternal cortisol assessed during pregnancy; (2) antepartum depression assessed during pregnancy using a screening instrument; (3) reports on the association between maternal cortisol and antepartum depression; (4) provides information on timing of cortisol assessment during pregnancy, including time of day and gestation; and (5) not a review article or a case study. One thousand three hundred seventy-five records were identified, resulting in 826 unique abstracts. Twenty-nine articles met all inclusion criteria. On balance, most studies reported no association between maternal cortisol and antepartum depression (N = 17), and saliva and blood were the most common reported matrices. Morning and second and third trimesters were the most common times of collection during pregnancy. Among studies reporting an association (N = 12), second-trimester and third-trimester cortisol assessments more consistently reported an association and elevated cortisol concentrations were observed in expected recovery periods. Our review adds to the existing literature on the topic, highlighting gaps and strategic next steps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Depression during pregnancy, defined hereafter as antepartum depression, is highly prevalent, affecting approximately 20–25% of pregnant women globally (Gavin et al. 2005; Gelaye et al. 2016). Antepartum depression can have devastating affects for both the mother and infant and is linked to a number of adverse health-related behaviors and outcomes. Such behaviors and outcomes include poor maternal nutrition (Barker et al. 2013), increased substance use (Horrigan et al. 2000), preeclampsia (Kurki et al. 2000), spontaneous preterm delivery (Orr et al. 2002), postpartum depression (Dietz et al. 2007), and impaired fetal and infant growth (Rahman et al. 2004; Hoffman and Hatch 2000). Estimates for the increase in risk for certain outcomes are as high as 39% for preterm birth, 45% for intrauterine growth restriction, and 49% for low birth weight delivery (Grote et al. 2010). Based on such findings, the American College of Obstetricians and Gynecologists (ACOG) now recommends that clinicians screen for depression at least once during the perinatal period, and an understanding of the underlying neurobiological pathways continues to be an important research objective.
One such candidate pathway is the hypothalamic pituitary adrenal (HPA) axis (Pariante and Lightman 2008; Penninx et al. 2013; Knorr et al. 2010; Stetler and Miller 2011; Kino 2015). The HPA axis is one of the body’s stress-response systems and plays a crucial role in homeostatic regulation. Pregnancy is a period of profound physiological changes during which increases in maternal and placental corticotrophin-releasing hormone increase circulating maternal cortisol levels (Brunton et al. 2008; Lachelin 2013; Burke and Roulet 1970; Kirschbaum et al. 2009). Such naturally occurring adaptions are thought to occur to aid in fetal lung maturation (Ballard and Ballard 1972) and fetal growth (Bolten et al. 2011) and to prime the placenta for childbirth (Sandman et al. 2006). Saliva, blood, and urine are biological matrices commonly used to assess cortisol levels during pregnancy and reflect cortisol levels in the past 1 to 24 h. Using such matrices, cortisol has been observed to follow a diurnal trend with peak levels at awakening and nadir levels in the evening. Differences in peak and nadir cortisol levels have also been observed to widen as pregnancy progresses (Brunton et al. 2008). In order to assess long-term cortisol secretions reflecting the past 1 to 3 months, hair has emerged as another matrix of choice (Wosu et al. 2013). A commonly cited concern of hair is cortisol degradation due to prolonged environmental exposure (Dettenborn et al. 2012). Given such differences in the time windows these matrices represent, reviews of the literature for the association between maternal cortisol and antepartum depression can be increasingly complicated.
Previous findings for the association between maternal cortisol levels and antepartum depression have been mixed. When evaluating maternal cortisol concentrations (basal or in response to awakening or stressful stimuli), some investigators report statistically significant differences comparing pregnant women with and without depression (Bjelanovic et al. 2015; Diego et al. 2009; Field et al. 2009; Hoffman et al. 2016; Lommatzsch et al. 2006; Murphy et al. 2015; O’Connor et al. 2014; O’Keane et al. 2011; Parcells 2010; Peer et al. 2013; Voegtline et al. 2013). However, a number of investigators have reported no such differences (Braithwaite et al. 2016; Davis et al. 2007; Deligiannidis et al. 2013; Evans et al. 2008; Glynn and Sandman 2014; Goedhart et al. 2010; Hellgren et al. 2013; Iliadis et al. 2015; Kaasen et al. 2012; Katz et al. 2012; Luiza et al. 2015; Monk et al. 2011; Pluess et al. 2010; Rouse and Goodman 2014; Salacz et al. 2012; Shaikh et al. 2011; Wikenius et al. 2016; Deligiannidis et al. 2016). Reasons for such inconsistencies may be due to differences in methodologies across studies, such as differences in the timing and method of maternal cortisol assessment. Recent reviews on the association between maternal cortisol levels and antepartum depression conclude that pregnant women with antepartum depression present with elevated cortisol concentrations and blunted awakening responses when compared to non-depressed pregnant women (Seth et al. 2016; Serati et al. 2016; Iliadis et al. 2015). However, the role of cortisol collection method and timing of assessment during pregnancy requires additional investigation. Therefore, the objective of this systematic review was to further examine the extent to which reported associations between maternal cortisol levels and antepartum depression differed across studies according to timing of cortisol collection, thereby building upon the work of previous reviews on the topic.
Methods
Studies evaluating the relationship between maternal cortisol and antepartum depression were identified by searching PubMed/MEDLINE (National Library of Medicine), EMBASE (Elsevier; 1974–), Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO), PsycINFO (EBSCO), and Web of Science Core Collection (Thomson Reuters) from inception through December 15, 2016. Controlled vocabulary terms (e.g., MeSH or Emtree terms) were included when available and appropriate. The search strategies were designed and executed by a librarian (PAB). No language limits or year restrictions were applied, and bibliographies of relevant articles were reviewed to identify additional studies. The exact search terms used for each of the databases are provided in the supplementary document.
Records were identified through databases, and duplicates were removed. The abstracts of the remaining records were screened for inclusion using the following eligibility criteria: (1) maternal cortisol assessed during pregnancy; (2) antepartum depression assessed during pregnancy using a screening instrument; (3) reports on the association between maternal cortisol and antepartum depression; (4) provides information on timing of cortisol assessment during pregnancy, including time of day and gestation; and (5) not a review article or a case study. We did not restrict inclusion according to depression screener; however, studies that did not report a depression screener or assessed “blues” or stress more generally were excluded. Findings from all studies were reported, and reported p values ≤ 0.05 were deemed statistically significant.
Results
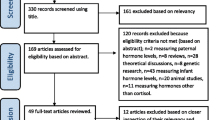
One thousand three hundred seventy-five records were retrieved from the database search, resulting in 826 unique records for screening. Twenty-seven studies met all inclusion criteria, and a scan of the references of these studies resulted in two additional studies that also met all inclusion criteria. In total, 29 studies were included in our systematic review (Fig. 1). The majority of the screened studies failed to meet inclusion because investigators reported on the association between maternal cortisol and infant outcomes (or between maternal depression with infant outcomes), rather than on the association between maternal cortisol and antepartum depression (N = 774 out of 826, 93.7%). Table 1 provides a description of each of the 29 studies; 12 reported a statically significant association between maternal cortisol and antepartum depression (Bjelanovic et al. 2015; Diego et al. 2009; Evans et al. 2008; Field et al. 2009; Hoffman et al. 2016; Lommatzsch et al. 2006; Meliska et al. 2013; Monk et al. 2011; O’Connor et al. 2014; O’Keane et al. 2011; Peer et al. 2013; Voegtline et al. 2013), and 17 studies did not (Braithwaite et al. 2016; Davis et al. 2007; Deligiannidis et al. 2016; Glynn and Sandman 2014; Goedhart et al. 2010; Hellgren et al. 2013; Iliadis et al. 2015; Kaasen et al. 2012; Katz et al. 2012; Luiza et al. 2015; Pedersen et al. 1993; Pluess et al. 2010; Rouse and Goodman 2014; Salacz et al. 2012; Shea et al. 2007; Susman et al. 1999; Wikenius et al. 2016).
Flow diagram of selection process. Superscript letter a denotes reasons for exclusions: does not report on the association between cortisol during pregnancy and depression during pregnancy (n = 774), a review article on the topic (n = 6), reports on the association between cortisol during pregnancy and depression during pregnancy but depression instrument either not mentioned or not compatible with criteria (n = 10), missing important information on time of depression and cortisol collection during pregnancy (n = 4), and met inclusion criteria but not a full article (n = 5)
Study design characteristics of eligible studies
Among the 29 studies, publication dates ranged from 1993 to 2016 and study populations were based exclusively in European or North American countries. The majority of studies sampled study participants from clinical settings (N = 18). Total sample sizes ranged from 29 (Meliska et al. 2013) to 2810 (Goedhart et al. 2010), and reported mean ages ranged from 17 to 37 years. The most common depression screeners reported were the Edinburgh Postnatal Depression Scale followed by the Center for Epidemiologic Studies Depression Scale. The most common matrix reported for the determination of maternal cortisol levels was saliva (N = 14), followed by blood (plasma (N = 4) and serum (N = 3)), urine (N = 3), and hair (N = 2). Two studies used both urine and plasma (Luiza et al. 2015; Pedersen et al. 1993), and one study used both saliva and serum (Kaasen et al. 2012). Among the studies using saliva, blood, and urine, morning collections were most common (8:00 a.m.–11:30 a.m.). Some studies reported multiple collections of maternal cortisol throughout the pregnancy period (N = 18 studies). However, such studies were predominately restricted to the second and third trimesters, and two studies reported no evidence of group by time interactions (Deligiannidis et al. 2016; Hellgren et al. 2013). No clear differences in association were observed across choice of laboratory assay or depression screener.
Study findings according to gestational age of cortisol assessment
Five studies assessed cortisol prior to or at 16-week gestational age (approximately first trimester) (Hoffman et al. 2016; Glynn and Sandman 2014; Goedhart et al. 2010; Luiza et al. 2015; Pluess et al. 2010). Among these studies, one reported a statistically significant association between first-trimester hair cortisol concentrations and antepartum depression scores in week 40 of pregnancy (r = 0.27, p = 0.02) (Hoffman et al. 2016). Eight studies assessed cortisol between 16 to 27-week gestational age (approximately second trimester) (Braithwaite et al. 2016; Davis et al. 2007; Diego et al. 2009; Field et al. 2009; Glynn and Sandman 2014; O’Connor et al. 2014; O’Keane et al. 2011; Hoffman et al. 2016). Among these studies, four reported a statistically significant association. Diego et al. observed elevated mid-morning urinary cortisol concentrations in the second trimester comparing women with and without depression and found that CES-D scores correlated with urinary cortisol concentrations (r = 0.37, p < 0.01) (Diego et al. 2009). O’Connor et al. reported lower initial wakening salivary cortisol in the second trimester among those with depression (r = − 0.22, p < 0.05) but found no evidence of an association between depression and cortisol awakening response (CAR) (O’Connor et al. 2014). Despite no observed difference in second-trimester morning salivary cortisol levels comparing women with and without antepartum depression (16.6 vs. 17.2 nmol/l, p = 0.78), O’Keane et al. observed 67% higher evening salivary cortisol concentrations comparing the two groups (7.1 vs. 4.8 nmol/l, p < 0.02) (O’Keane et al. 2011). Lastly, Hoffman et al. observed an association between second-trimester hair cortisol concentrations and antepartum depression symptom scores at 16 weeks (r = 0.32, p = 0.002) (Hoffman et al. 2016). Sixteen studies assessed cortisol at or after 28-week gestational age (approximately third trimester) (Bjelanovic et al. 2015; Braithwaite et al. 2016; Davis et al. 2007; Deligiannidis et al. 2016; Evans et al. 2008; Field et al. 2009; Glynn and Sandman 2014; Hellgren et al. 2013; Hoffman et al. 2016; Iliadis et al. 2015; Lommatzsch et al. 2006; Monk et al. 2011; O’Connor et al. 2014; Pedersen et al. 1993; Pluess et al. 2010; Salacz et al. 2012). Among these studies, seven reported a statistically significant association, two of which also reported associations in previous trimesters (O’Connor et al. 2014; Hoffman et al. 2016). One study reported elevated third-trimester salivary cortisol among women with antepartum depression at both 8 a.m. (r = 0.239, p = 0.001) and 5 p.m. (r = 0.206, p = 0.005) (Bjelanovic et al. 2015). In another study, first morning urinary cortisol levels were found to be nearly sevenfold higher among the depressed (71.7 vs. 10.5 units, p value = 0.01) (Field et al. 2009). Nearly twofold increases in 3–7 p.m. serum cortisol levels were observed comparing women with antepartum depression to women without antepartum depression (medians 620 vs. 392 nmol/l; p < 0.01) (Lommatzsch et al. 2006). However, correlations between depression scores and cortisol concentrations did not reach statistical significance (data not shown) (Lommatzsch et al. 2006). Two studies reported elevated third-trimester morning salivary cortisol concentrations among small subsets of women with comorbid depression and anxiety, and no difference comparing “depressed only” to healthy controls (Monk et al. 2011; Evans et al. 2008).
Discussion
Reported associations between maternal cortisol and antepartum depression varied across studies. On balance, we observed that (1) the majority of eligible studies reported no statistically significant association between maternal cortisol and antepartum depression (N = 17 out of 29), (2) saliva and blood were the most common biological matrices for cortisol detection, (3) morning was the most common time of day for cortisol collection, (4) second and third trimesters were the most common time of pregnancy for cortisol collections, and (5) among studies reporting an association (N = 12), second-trimester and third-trimester cortisol assessments more consistently reported an association, and elevated cortisol concentrations were observed in expected recovery periods.
Our review confirms and expands upon previous review articles (Brummelte and Galea 2010; Gelman et al. 2015; Glover and Kammerer 2004; Pariante 2014; Serati et al. 2016; Seth et al. 2016; Workman et al. 2012; Iliadis et al. 2015). For example, a recent review of 47 studies, exceeding our number primarily due to the inclusion of studies assessing depression in the postpartum period as well as during pregnancy (Seth et al. 2016), reported that cortisol awakening responses were blunted in the cases of major maternal depression (including antepartum and postpartum depression). The authors further concluded that hypercortisolemia during pregnancy was associated with transient depressive states, while hypocortisolemia during pregnancy was associated with chronic postpartum depression. Another recent review evaluated a wide range of biomarkers and concluded that hypercortisolemia was associated with depression in the weeks immediately before and after delivery (Serati et al. 2016). Observations of hypercortisolemia among individuals with major depression have been observed in both non-pregnant (Vreeburg et al. 2009) and pregnant populations (Pariante and Lightman 2008; Serati et al. 2016; Seth et al. 2016; Burke et al. 2005). Specifically, individuals (males and females) with major depression have been observed to have higher cortisol concentrations in expected recovery periods where cortisol concentrations are expected to be lower (Burke et al. 2005). Stress-related factors have also been associated with other atypical patterns in cortisol, including blunted and steeper diurnal declines (Stawski et al. 2013; Agbedia et al. 2011). Our review adds to this literature by noting that higher cortisol levels have been observed among pregnant women with antepartum depression using matrices reflecting evening levels and that this may be particularly noticeable in the second and third trimesters when circulating cortisol levels are at their highest.
Study design methodologies varied widely, impacting comparisons across eligible studies. First, few of the eligible studies reported on the association between maternal cortisol and antepartum depression in the first trimester of pregnancy. Therefore, we cannot distinguish whether the association between maternal cortisol and antepartum depression is more common in the second and third trimesters due to physiologic changes later on in pregnancy, or whether it appears more common due to limited data in the first trimester of pregnancy. Second, some studies may have been underpowered to detect an association due to small sample sizes. Third, studies likely differed in their determination of gestational age (example: self-reported last menstrual period vs. ultrasound). Therefore, comparison across studies within our trimester categories should be interpreted with some caution. Fourth, the majority of studies utilized biological matrices that reflected acute cortisol levels in the 1 to 24 hours prior to collection (saliva, blood, and urine). Such matrices allow investigators to observe deviations in expected diurnal patterns, however are limited in their ability to assess differences in long-term cortisol secretion. Two studies utilized hair that reflected long-term cortisol levels in the months prior to collection. A commonly cited limitation of this biologic matrix is the potential for cortisol degradation and leaching (Russell et al. 2012). Despite the aforementioned caveats, an understanding of both acute and long-term maternal cortisol levels is necessary in order to further our understanding of the underlying neurobiological pathways at play in antepartum depression.
Our findings highlight specific gaps in the literature for the association between maternal cortisol and antepartum depression. These gaps include the need for findings that integrate multiple maternal cortisol collections spanning all trimesters of pregnancy. Maternal cortisol collections ranging from the first trimester to delivery would allow for analyses of cortisol trajectories across the pregnancy period. Such analyses would help to determine whether women with antepartum depression present with different long-term cortisol trajectories. An additional gap noted in our review of the literature is the need for data from low-income and middle-income countries, where the burden of antepartum depression is often two to three times higher (Lara et al. 2009; Schatz et al. 2012; Barrios et al. 2015; Shidhaye and Giri 2014). Therefore, additional research across a diverse range of study populations and study participants is warranted.
Conclusion
Antepartum depression is an important research priority, and reviews of the existing literature help shed light on strategic next steps, thereby accelerating the pace at which research findings are translated into clinical practice. These strategic next steps potentially include the evaluation of maternal cortisol secretion profiles across pregnancy using longitudinal study design approaches and an expansion of the research question to diverse study populations and participants.
References
Agbedia OO, Varma VR, Seplaki CL, Seeman TE, Fried LP, Li L, Harris GC, Rebok GW, Xue QL, Tan EJ, Tanner E, Parisi JM, Mcgill S, Carlson MC (2011) Blunted diurnal decline of cortisol among older adults with low socioeconomic status. Ann N Y Acad Sci 1231:56–64
Ballard PL, Ballard RA (1972) Glucocorticoid receptors and the role of glucocorticoids in fetal lung development. Proc Natl Acad Sci U S A 69:2668–2672
Barker ED, Kirkham N, Ng J, Jensen SK (2013) Prenatal maternal depression symptoms and nutrition, and child cognitive function. Br J Psychiatry 203:417–421
Barrios YV, Gelaye B, Zhong Q, Nicolaidis C, Rondon MB, Garcia PJ, Sanchez PA, Sanchez SE, Williams MA (2015) Association of childhood physical and sexual abuse with intimate partner violence, poor general health and depressive symptoms among pregnant women. PLoS One 10:e0116609
Bjelanovic V, Babic D, Hodzic D, Bjelanovic A, Kresic T, Dugandzic-Simic A, Oreskovic S (2015) Correlation of psychological symptoms with cortisol and CRP levels in pregnant women with metabolic syndrome. Psychiatr Danub 27(Suppl 2):578–585
Bolten MI, Wurmser H, Buske-Kirschbaum A, Papousek M, Pirke KM, Hellhammer D (2011) Cortisol levels in pregnancy as a psychobiological predictor for birth weight. Arch Womens Ment Health 14:33–41
Braithwaite, E. C., Murphy, S. E. & Ramchandani, P. G. (2016) Effects of prenatal depressive symptoms on maternal and infant cortisol reactivity. Arch Womens Ment Health
Brummelte S, Galea LA (2010) Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuro-Psychopharmacol Biol Psychiatry 34:766–776
Brunton PJ, Russell JA, Douglas AJ (2008) Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol 20:764–776
Burke CW, Roulet F (1970) Increased exposure of tissues to cortisol in late pregnancy. Br Med J 1:657–659
Burke HM, Davis MC, Otte C, Mohr DC (2005) Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology 30:846–856
Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA (2007) Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry 46:737–746
Deligiannidis KM, Kroll-Desrosiers AR, Barton BA, Rothschild AJ (2013) Salivary cortisol response to trier social stress test in healthy third trimester pregnant women and third trimester pregnant women at elevated risk of developing postpartum depression. Neuropsychopharmacology 38:S408–S409
Deligiannidis, K. M., Kroll-Desrosiers, A. R., Svenson, A., Jaitly, N., Barton, B. A., Hall, J. E. & Rothschild, A. J. 2016. Cortisol response to the trier social stress test in pregnant women at risk for postpartum depression. Arch Womens Ment Health
Dettenborn L, Tietze A, Kirschbaum C, Stalder T (2012) The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress 15:578–588
Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH (2009) Prenatal depression restricts fetal growth. Early Hum Dev 85:65–70
Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC (2007) Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry 164:1515–1520
Evans LM, Myers MM, Monk C (2008) Pregnant women’s cortisol is elevated with anxiety and depression—but only when comorbid. Arch Womens Ment Health 11:239–248
Field T, Diego M, Hernandez-Reif M, Deeds O, Holder V, Schanberg S, Kuhn C (2009) Depressed pregnant black women have a greater incidence of prematurity and low birthweight outcomes. Infant Behav Dev 32:10–16
Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T (2005) Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 106:1071–1083
Gelaye B, Rondon MB, Araya R, Williams MA (2016) Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry 3:973–982
Gelman PL, Flores-Ramos M, Lopez-Martinez M, Fuentes CC, Grajeda JP (2015) Hypothalamic-pituitary-adrenal axis function during perinatal depression. Neurosci Bull 31:338–350
Glover V, Kammerer M (2004) The biology and pathophysiology of peripartum psychiatric disorders. Primary Psychiatry 11:37–41
Glynn LM, Sandman CA (2014) Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med 76:355–362
Goedhart G, Vrijkotte TGM, Roseboom TJ, van der Wal MF, Cuijpers P, Bonsel GJ (2010) Maternal cortisol and offspring birthweight: results from a large prospective cohort study. Psychoneuroendocrinology 35:644–652
Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ (2010) A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 67:1012–1024
Hellgren C, Akerud H, Skalkidou A, Sundstrom-Poromaa I (2013) Cortisol awakening response in late pregnancy in women with previous or ongoing depression. Psychoneuroendocrinology 38:3150–3154
Hoffman MC, Mazzoni SE, Wagner BD, Laudenslager ML, Ross RG (2016) Measures of maternal stress and mood in relation to preterm birth. Obstet Gynecol 127:545–552
Hoffman S, Hatch MC (2000) Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol 19:535–543
Horrigan TJ, Schroeder AV, SchaffeR RM (2000) The triad of substance abuse, violence, and depression are interrelated in pregnancy. J Subst Abus Treat 18:55–58
Iliadis SI, Comasco E, Sylven S, Hellgren C, Sundstrom Poromaa I, Skalkidou A (2015) Prenatal and postpartum evening salivary cortisol levels in association with peripartum depressive symptoms. PLoS One 10:e0135471
Kaasen A, Helbig A, Malt UF, Godang K, Bollerslev J, Naes T, Haugen G (2012) The relation of psychological distress to salivary and serum cortisol levels in pregnant women shortly after the diagnosis of a structural fetal anomaly. Acta Obstet Gynecol Scand 91:68–78
Katz ER, Stowe ZN, Newport DJ, Kelley ME, Pace TW, Cubells JF, Binder EB (2012) Regulation of mRNA expression encoding chaperone and co-chaperone proteins of the glucocorticoid receptor in peripheral blood: association with depressive symptoms during pregnancy. Psychol Med 42:943–956
Kino T (2015) Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders. Front Physiol 6:230
Kirschbaum C, Tietze A, Skoluda N, Dettenborn L (2009) Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34:32–37
Knorr U, Vinberg M, Kessing LV, Wetterslev J (2010) Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology 35:1275–1286
Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O (2000) Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol 95:487–490
Lachelin GC 2013. Introduction to clinical reproduction endocrinology
Lara MA, Le HN, Letechipia G, Hochhausen L (2009) Prenatal depression in Latinas in the U.S. and Mexico. Matern Child Health J 13:567–576
Lommatzsch M, Hornych K, Zingler C, Schuff-Werner P, Hoppner J, Virchow JC (2006) Maternal serum concentrations of BDNF and depression in the perinatal period. Psychoneuroendocrinology 31:388–394
Luiza JW, Gallaher MJ, Powers RW (2015) Urinary cortisol and depression in early pregnancy: role of adiposity and race. BMC Pregnancy Childbirth 15:30
Meliska CJ, Martinez LF, Lopez AM, Sorenson DL, Nowakowski S, Kripke DF, Elliott J, Parry BL (2013) Antepartum depression severity is increased during seasonally longer nights: relationship to melatonin and cortisol timing and quantity. Chronobiol Int 30:1160–1173
Monk C, Fifer WP, Myers MM, Bagiella E, Duong JK, Chen IS, Leotti L, Altincatal A (2011) Effects of maternal breathing rate, psychiatric status, and cortisol on fetal heart rate. Dev Psychobiol 53:221–233
Murphy SE, Braithwaite EC, Hubbard I, Williams KV, Tindall E, Holmes EA, Ramchandani PG (2015) Salivary cortisol response to infant distress in pregnant women with depressive symptoms. Arch Womens Ment Health 18:247–253
O’connor TG, Tang W, Gilchrist MA, Moynihan JA, Pressman EK, Blackmore ER (2014) Diurnal cortisol patterns and psychiatric symptoms in pregnancy: short-term longitudinal study. Biol Psychol 96:35–41
O’keane V, Lightman S, Marsh M, Pawlby S, Papadopoulos AS, Taylor A, Moore R, Patrick K (2011) Increased pituitary-adrenal activation and shortened gestation in a sample of depressed pregnant women: a pilot study. J Affect Disord 130:300–305
Orr ST, James SA, Blackmore Prince C (2002) Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol 156:797–802
Parcells DA (2010) Women’s mental health nursing: depression, anxiety and stress during pregnancy. J Psychiatr Ment Health Nurs 17:813–820
Pariante CM (2014) Depression during pregnancy: molecular regulations of mothers’ and children’s behaviour. Biochem Soc Trans 42:582–586
Pariante CM, Lightman SL (2008) The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31:464–468
Pedersen CA, Stern RA, Pate J, Senger MA, Bowes WA, Mason GA (1993) Thyroid and adrenal measures during late pregnancy and the puerperium in women who have been major depressed or who become dysphoric postpartum. J Affect Disord 29:201–211
Peer M, Soares CN, Levitan RD, Streiner DL, Steiner M (2013) Antenatal depression in a multi-ethnic, community sample of Canadian immigrants: psychosocial correlates and hypothalamic-pituitary-adrenal axis function. Can J Psychiatr 58:579–587
Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N (2013) Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 11:129
Pluess M, Bolten M, Pirke KM, Hellhammer D (2010) Maternal trait anxiety, emotional distress, and salivary cortisol in pregnancy. Biol Psychol 83:169–175
Rahman A, Iqbal Z, Bunn J, Lovel H, Harrington R (2004) Impact of maternal depression on infant nutritional status and illness: a cohort study. Arch Gen Psychiatry 61:946–952
Rouse MH, Goodman SH (2014) Perinatal depression influences on infant negative affectivity: timing, severity, and co-morbid anxiety. Infant Behav Dev 37:739–751
Russell E, Koren G, Rieder M, Van Uum S (2012) Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37:589–601
Salacz P, Csukly G, Haller J, Valent S (2012) Association between subjective feelings of distress, plasma cortisol, anxiety, and depression in pregnant women. Eur J Obstet Gynecol Reprod Biol 165:225–230
Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-Demet A, Hobel C (2006) Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides 27:1457–1463
Schatz DB, Hsiao MC, Liu CY (2012) Antenatal depression in East Asia: a review of the literature. Psychiatry Investig 9:111–118
Serati M, Redaelli M, Buoli M, Altamura AC (2016) Perinatal major depression biomarkers: a systematic review. J Affect Disord 193:391–404
Seth S, Lewis AJ, Galbally M (2016) Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: a systematic literature review. BMC Pregnancy Childbirth 16:124
Shaikh K, Premji SS, Rose MS, Kazi A, Khowaja S, Tough S (2011) The association between parity, infant gender, higher level of paternal education and preterm birth in Pakistan: a cohort study. BMC Pregnancy Childbirth 11:88
Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M (2007) The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology 32:1013–1020
Shidhaye P, Giri P (2014) Maternal depression: a hidden burden in developing countries. Ann Med Health Sci Res 4:463–465
Stawski RS, Cichy KE, Piazza JR, Almeida DM (2013) Associations among daily stressors and salivary cortisol: findings from the National Study of Daily Experiences. Psychoneuroendocrinology 38:2654–2665
Stetler C, Miller GE (2011) Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73:114–126
Susman EJ, Schmeelk KH, Worrall BK, Granger DA, Ponirakis A, Chrousos GP (1999) Corticotropin-releasing hormone and cortisol: longitudinal associations with depression and antisocial behavior in pregnant adolescents. J Am Acad Child Adolesc Psychiatry 38:460–467
Voegtline KM, Costigan KA, Kivlighan KT, Laudenslager ML, Henderson JL, Dipietro JA (2013) Concurrent levels of maternal salivary cortisol are unrelated to self-reported psychological measures in low-risk pregnant women. Arch Womens Ment Health 16:101–108
Vreeburg SA, Hoogendijk WJ, Van Pelt J, Derijk RH, Verhagen JC, Van Dyck R, Smit JH, Zitman FG, Penninx BW (2009) Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 66:617–626
Wikenius E, Moe V, Kjellevold M, Smith L, Lyle R, Waagbo R, Page CM, Myhre AM (2016) The association between hair cortisol and self-reported symptoms of depression in pregnant women. PloS One 11:e0161804
Workman JL, Barha CK, Galea LAM (2012) Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci 126:54–72
Wosu AC, ValdimarsdÓttir U, Shields AE, Williams DR, Williams MA (2013) Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Ann Epidemiol 23:797–811
Funding
The National Institute of Health Training Grant in Psychiatric Epidemiology (T32-MH-017119) and the Eunice Kennedy Shriver Institute of Child Health and Human Development (R01-HD-059835) supported this research. Neither funding source had any further role in the study design, data collection and analysis, data interpretation, manuscript writing, or decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Orta, O.R., Gelaye, B., Bain, P.A. et al. The association between maternal cortisol and depression during pregnancy, a systematic review. Arch Womens Ment Health 21, 43–53 (2018). https://doi.org/10.1007/s00737-017-0777-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00737-017-0777-y