Abstract
We recently reported that dietary supplementation with l-proline (proline) during gestation improved embryonic survival in C57BL/6J mice. The objective of the present study was to test the hypothesis that the effect of maternal proline supplementation on embryonic survival can be carried forward to the first generation female offspring. In the F0 generation, pregnant dams were fed a purified diet supplemented with 0 (control) or 5 g proline/kg diet. The F1 female adult offsprings were bred to fertile males. Fetal survival at embryonic day (E)12.5 and reproductive outcomes at term birth were recorded. The concentrations of amino acids, ammonia, and urea in plasma and amniotic fluid, as well as concentrations of polyamines in placental tissues and amniotic fluid at E12.5 were determined. Results showed that the F1 generation female offspring from proline-supplemented dams had higher (P < 0.05) concentrations of glutamate and taurine in plasma; of putrescine and spermidine in placental tissues; and of glycine, taurine, and spermidine in amniotic fluid at E12.5, as compared with F1 generation female offsprings from dams without proline supplementation. Concentration of proline in the plasma of offspring mice from proline-supplemented dams were lower (P < 0.05), as compared with the control group. No differences in fetal survival, reproductive outcomes, or concentrations of ammonia and urea in plasma and amniotic fluid were observed between the two groups of F1 female offspring. Collectively, our results indicate that the benefits of maternal proline supplementation during gestation on improving embryonic survival and fetal growth in F0 females are not transmitted to their F1 generation females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growing evidence indicates that developmental exposure to environmental factors (e.g., nutrients, stress, and toxic chemicals) is associated with metabolic and physiological modifications in humans and animals (Abu-Saad and Fraser 2010; Armitage et al. 2004; Chan et al. 2018; Connor et al. 2012). The phenotypic effects have been proposed to be mediated through epigenetic mechanisms (Ji et al. 2016). In contrast to the classic genetics, epigenetics is defined as stable and heritable alterations of gene expression through covalent modifications of DNA and core histones without changes in the DNA sequence (Wang et al. 2012). Maternal protein nutrition during gestation and lactation is a key factor that affects fetal growth, neonatal development, offspring health, and diseases later in life (Abu-Saad and Fraser 2010; Guzman et al. 2006; Herring et al. 2018; Hogenkamp et al. 2015; Zambrano et al. 2005). It has been reported that amino acid limitation or imbalance in maternal diet affects reproductive performance in the rodent (Chan et al. 2018; Faria Tda et al. 2008; Winship et al. 2018; Zambrano et al. 2005), a widely used animal model for studying human reproduction. Interestingly, epidemiological studies have indicated that malnutrition during the gestation period can exert a transgenerational effect in humans and animals (Kim 2005; Padmanabhan et al. 2013; Youngson and Whitelaw 2008).
l-Proline (proline) is one of the most abundant amino acids in the milk of human (Davis et al. 1994), mice (Meier et al. 1965), and other animals (Wu and Knabe 1994). The abundance of proline in milk indicates a nutritional role of proline for neonatal growth and development (Wu 2010). Proline has been reported to have various biological functions, such as protein synthesis and tissue growth (Ball et al. 1986; Wu et al. 2014), metabolism (Wu et al. 2008), maintaining cellular structure (Mussini et al. 1967), regulating intracellular redox state (Phang et al. 2010; Wu 2018; Wu et al. 2019), wound healing (Barbul 2008; Mussini et al. 1967), and immune response (Wu 2009). Furthermore, proline serves not only as a building block of proteins, but also as a nitrogenous substrate for endogenous synthesis of arginine, glutamate, and polyamines in mammals (Wu et al. 2008). Polyamines are key regulators of DNA and protein synthesis, cell proliferation, and differentiation in both the small intestine and placenta of mammals, including pigs (Kong et al. 2014; Li and Wu 2018; Wang et al. 2014; Wu 2018; Wu et al. 2005, 2017) and sheep (Wu et al. 2008). Moreover, compelling evidence from animal studies shows that reduced placental and fetal growth is associated with reductions in placental proline transport in gestating dams with either naturally occurring or malnutrition-induced growth retardation (Wu et al. 2008). Although proline is synthesized in mammals via the interorgan metabolism of amino acids (Wu 2010), it may function as a conditionally essential amino acid during gestation and lactation in response to rapid growth and development of conceptus and neonates (Brunton et al. 2012; Wu et al. 2008).
We have recently reported that maternal proline supplementation during gestation enhances fetal survival, reproductive performance, and amino acid transport from dam to fetus in mice (Liu et al. 2018). It is unknown whether this effect can be carried to female offspring after the cessation of proline administration. In the present study, female offspring from control or proline-supplemented dams in our previous study (Liu et al. 2018) were weaned at 3 weeks of age and fed an AIN-93G purified diet. The offspring female mice were mated for the following measurements: fetal survival at embryonic day (E) 12.5, and reproductive performance at term birth, as well as the concentrations of amino acids and polyamines in plasma and amniotic fluid. To our knowledge, this is the first study to investigate a transgenerational effect of maternal proline supplementation on reproductive performance, amino acid and polyamine metabolism in female offspring.
Materials and methods
Chemicals
Proline and alanine were purchased from Sangon Biotech (Shanghai, China). Amino acid standards for high-performance liquid chromatography (HPLC) analysis were obtained from Sigma-Aldrich (St. Louis, MO, USA). Unless indicated, all other chemicals were obtained from Sigma-Aldrich.
Mice and diets
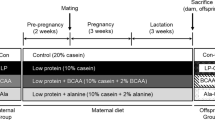
This study was conducted in accordance with the guidelines for animal protocols approved by Institutional Animal Care and Use Committee of China Agricultural University. Twenty-four adult virgin female C57BL/6J mice (F0 generation) received dietary supplementation with either 5 g proline/kg diet or 3.9 g alanine/kg diet (isonitrogenous control) during gestation, as we previously described (Liu et al. 2018). All offspring were weaned at 3 weeks of age. Female offspring (F1 generation) from proline- or alanine-supplemented dams (n = 24 per group) were fed an AIN-93G purified diet (Reeves et al. 1993). The basal diet contained the following amino acids (g/kg diet): alanine, 5.2; arginine, 5.3; aspartic acid + asparagine, 12.9; cystine, 3.6; glutamic acid + glutamine, 37.4; glycine, 3.0; histidine, 4.5; Isoleucine, 8.8; leucine, 17.0; lysine, 13.3; methionine, 4.9; phenylalanine, 8.0; proline, 18.0; serine, 9.2; threonine, 7.5; tryptophan, 2.2; tyrosine, 7.9; and valine, 11.4. Mice were mated with fertile male mice of the same genetic background. Fetal survival at E12.5, reproductive performance at term birth, the concentrations of amino acids, ammonia, and urea in plasma and amniotic fluid, concentrations of polyamines in placenta and amniotic fluid at E12.5, were determined as described (Liu et al. 2018). The presence of a vaginal plug was considered as E0.5. During the whole experimental period, mice were maintained in a controlled environment with the temperature of 23 °C and a 12-h light/dark cycle. The mice had free access to feed and water during the whole experimental period. Twelve mice per group were euthanized at E12.5 for the determination of fetal survival. Reproductive performance of F1 generation female mice at term birth and their feed intake during the whole experimental period was recorded.
Blood, placenta, and amniotic fluid collection
At E12.5, twelve mice from each group were randomly selected and intraperitoneally anesthetized with sodium pentobarbital (40 mg/kg body weight). Blood samples from the retroorbital were collected and plasma was obtained after centrifugation at 3000g for 15 min. Plasma samples were stored at − 80 °C for later analysis. After blood collection, uterine horns were quickly exposed, and the number and weight of fetuses were recorded. Placenta and amniotic fluid were immediately obtained and stored at − 80 °C until analysis.
Analysis of amino acids, ammonia, and urea in plasma and amniotic fluid
Amino acids in plasma and amniotic fluid were analyzed by HPLC methods involving pre-column derivatization with ο-phthaldialdehyde (Li et al. 2014; Yin et al. 2017). Briefly, 200 μl of plasma or amniotic fluid were deproteinized with 200 μl of 1.5 mmol/L HClO4, followed by the addition of 100 μl of 2 mmol/L K2CO3. The amino acids in the neutralized supernatant fluid were analyzed by HPLC (Waters Inc.). The chromatogram for amino acid standards is shown in Supplementary Fig. 1. Ammonia and urea were determined using assay kits from Nanjing Jiancheng Biochemistry (Nanjing, China), according to the protocols provided by the manufacturer.
Analysis of putrescine, spermidine, and spermine in placenta and amniotic fluid
Putrescine, spermidine, and spermine in placenta and amniotic fluid were analyzed by HPLC methods involving pre-column derivatization with ο-phthaldialdehyde and N-acetyl-l-cysteine (Dai et al. 2014). Briefly, 25 mg of grounded placenta powder or 100 μL amniotic fluid in a 1.5-mL eppendorf tube was mixed with 100 μL ice-cold 1.5 mmol/L HCLO4, followed by neutralization with 50 μL of 2 mmol/L K2CO3. After centrifugation at 15,000g at 4 °C for 10 min, 50 μL of the supernatant fluid was used for polyamines analysis by HPLC.
Statistical analysis
Values are expressed as means ± SEMs. Data were analyzed by the unpaired Student’s t test. All analyses were performed by the SAS statistical software (SAS Inst., Inc., Cary, NC). Probability values ≤ 0.05 were taken to indicate statistical significance.
Results
Fetal survival and reproductive performance
The F1 generation female mice from dams fed an AIN-93G diet supplemented with 5 g proline/kg diet or 3.9 g alanine/kg diet (isonitrogenous control) during gestation appeared normal and healthy. The basal diet contained 18 g proline/kg diet during the whole experimental period. All the female offsprings had normal estrus cycles and delivered healthy pups. As shown in Table 1, there were no significant differences (P > 0.05) in the number of total or live-born fetuses, maternal proline intake, fetal weight, or placental weight between F1 female offspring from proline-supplemented dams and alanine-supplemented dams at E12.5. In line with data at E12.5, maternal proline administration did not affect (P > 0.05) reproductive performance, including litter size, birth weight of F1 generation female mice, or their proline intake throughout the experimental period (Table 2).
Concentrations of amino acids, ammonia, and urea in plasma and amniotic fluid
As shown in Table 3, lower concentrations of free proline were observed in the plasma of F1 female offspring from proline-supplemented dams, compared with those from alanine-supplemented dams at E12.5 (P < 0.05). However, there was no difference (P > 0.05) in the concentration of proline in amniotic fluid between F1 female offspring from proline-supplemented dams and those from alanine-supplemented dams (Table 3). The concentrations of glutamate and taurine in the plasma of F1 female offspring from proline-supplemented dams were greater (P < 0.05) than those from alanine-supplemented dams (Table 3). Additionally, concentrations of glycine and taurine in the amniotic fluid of F1 female offspring from proline-supplemented dams were greater (P < 0.05) than those from alanine-supplemented dams (Table 3). Concentrations of other amino acids in plasma and amniotic fluid did not differ (P > 0.05) between the two groups of F1 female offspring (Table 3). Furthermore, concentrations of ammonia and urea in maternal plasma and amniotic fluid were not different (P > 0.05) between these two groups of female mice (Table 4).
Concentrations of polyamines in placenta and amniotic fluid
Placental concentrations of putrescine and spermidine in F1 female offspring from proline-supplemented dams were greater (P < 0.05) than those from alanine-supplemented dams (Table 5). Concentrations of spermine in the placenta of F1 female offspring from proline-supplemented dams were similar (P > 0.05) to those of spermidine in F1 female offspring from alanine-supplemented dams (Table 5). Additionally, the concentration of spermidine in the amniotic fluid of F1 female offspring from proline-supplemented dams was greater (P < 0.05) than that from alanine-supplemented dams at E12.5 (Table 5). The concentrations of putrescine and spermine in amniotic fluid did not differ between the two groups of mice (Table 5).
Discussion
Maternal proline supplementation during gestation improves polyamine synthesis in the conceptus, as well as embryonic/fetal survival and growth (Wu et al. 2008, 2014). In the present study, we found no significant differences in the number of fetuses at E12.5 or reproductive performance at term birth for F1 female offspring from proline-supplemented dams, as compared with F1 generation female mice from alanine-supplemented dams. In contrast, we found that maternal proline supplementation during gestation alters amino acid and polyamine metabolism in the first generation female offspring of C57BL/6J mice.
Both paternal and maternal factors have been reported to be associated with metabolic and physiological modifications in offspring, but maternal factors seem to be more influential (Seckl and Holmes 2007). Epidemiologic evidence suggests that maternal protein availability during gestation affects offspring health and development of metabolic diseases later in life (Abu-Saad and Fraser 2010; Wu et al. 2017). Restricting amino acid availability leads to impaired reproductive development in female and male offspring (Chan et al. 2018; Faria Tda et al. 2008; Winship et al. 2018; Zambrano et al. 2005). Importantly, the adverse effects induced by the limitation of amino acids can be carried forward to subsequent generations through covalent modifications of DNA and core histones (Ji et al. 2016). In addition, an imbalance of amino acids in the diet has been reported to affect fetal growth, neonatal development, as well as the reproductive performance of offspring (Abu-Saad and Fraser 2010; Armitage et al. 2004; Chan et al. 2018; Faria Tda et al. 2008; Guzman et al. 2006; Winship et al. 2018). Based on this scenario, a sufficient and balanced provision of amino acid is required for optimal growth and development of neonates (Brunton et al. 2012; Wu 2009).
Recent studies indicate that proline functions as a signaling molecule in cellular processes, such as energy sensing, intracellular redox status, and cell differentiation (Barbul 2008; Phang 2019; Wu et al. 2011, 2017, 2019). Additionally, proline serves as a major substrate for polyamine synthesis in placenta and intestinal epithelial cells (Wu et al. 2008). Polyamines (putrescine, spermidine, and spermine) are essential for placental growth and angiogenesis (Wu et al. 2005). All these lines of evidence indicate a potential role of proline in fetal-neonatal growth and development (Ball et al. 1986; Brunton et al. 2012; Wu et al. 2011). In our recent study, we have demonstrated that maternal proline supplementation enhances fetal survival at E12.5 and reproductive performance at term birth (Liu et al. 2018). The present work was conducted to determine whether the effect of proline supplementation could be carried over to F1 female offspring after cessation of proline administration. We did not observe an effect of proline supplementation to F0 female mice during gestation on fetal survival at E12.5 or reproductive performance at term birth for their F1 female offspring, thus excluding a transgenerational effect of proline on these indicators of reproductive function (Tables 1 and 2). Thus, not all amino acids have a transgenerational effect on embryonic/fetal survival and growth. However, birth weight may not be the only indicator of the health of progeny. Beneficial changes in metabolic profiles of amino acids and polyamines during gestation may have long-term effects on postnatal growth and metabolism. Future studies are warranted to examine this possibility.
The reason why maternal proline intake does not have a transgenerational effect on fetal survival or growth in F1 female offspring as maternal protein nutrition does is currently unknown. However, several factors may provide insights into this finding. First, we supplemented proline to the AIN-93G basal diet, whereas a low protein diet was used in previous studies (Guzman et al. 2006; Zambrano et al. 2005). The synthesis of proteins in the fetus depends on sufficient proline and a balanced provision of all proteinogenic amino acids (Brunton et al. 2012; Wu et al. 2008). A low protein diet reduced both the availability and possibly the balance of all amino acids, thus exerting a deleterious effect on fetal survival and development as well as male reproductive development in offspring, which can affect the reproductive function of the subsequent generations (Guzman et al. 2006; Zambrano et al. 2005). Second, DNA or histone methylation has been proposed as the epigenetic mechanisms that confer a transgenerational effect on reproduction in animals. In our study, proline supplementation during gestation might not be sufficient to alter epigenetic modifications in the dams or transmit them to the next generation, thus exerting no effect on fetal survival or reproductive performance.
Amino acids in the amniotic fluid are critical for fetal and placental development due to their functions as substrates for the synthesis of proteins and low molecular weight substances which possess enormous biological functions in metabolism and physiology (Bazer et al. 2015; Kwon et al. 2003; Li and Wu 2018; Wu et al. 2014). In the previous study, enhanced fetal survival was associated with elevated concentrations of arginine, aspartic acid, glutamine, proline, and tryptophan in the amniotic fluid (Liu et al. 2018). However, changes in both fetal survival and the concentrations of some amino acids in amniotic fluid for proline-supplemented F0 female mice were not observed in their F1 female offspring that did not receive proline supplementation. This result further supports the notion that different amino acids have different effects on conceptus metabolism, survival, growth, and development (Bazer et al. 2015; Wu et al. 2008, 2014).
The requirement for proline for whole-body protein synthesis is the highest among all amino acids base on a per-gram basis (Li and Wu 2018). In our previous study, we observed a positive correlation between placental development and the concentrations of proline and polyamines (putrescine, spermidine, and spermine) in the amniotic fluid (Liu et al. 2018). Proline is a major nitrogenous substrate for the synthesis of polyamines (Wu et al. 2008) that are key regulators of placental angiogenesis, trophoblast growth, and embryogenesis (Kong et al. 2014; Wang et al. 2014; Wu et al. 2005). However, we found that the concentration of proline was decreased in the plasma of F1 female offspring from proline-supplemented dams. A modest decrease in the concentration of proline in maternal plasma may not have an adverse effect on embryonic/fetal survival and growth, as shown in the present study with mice (Table 3). It is possible that maternal proline supplementation may program the offspring to express its genome for reducing endogenous synthesis of proline in favor of the channeling of substrates for the synthesis of other molecules such as arginine, polyamines, glycine, and glutamate (Wu 2013). Besides polyamines, amino acids are vital for animal metabolism, growth and health (Hou and Wu 2018; Li and Wu 2018; Wu 2018). Thus, whether F1 offspring from proline-supplemented dams are less sensitive to the development of diet-induced obesity or vascular dysfunction during gestation warrants further investigation. Nonetheless, given insufficient maternal intake of proline by mammals (e.g., pigs) during gestation (Ji et al. 2017), future studies should be undertaken to define transgenerational impacts of maternal amino acid nutrition on postnatal growth, health, and reproductive performance through epigenetic and other mechanisms.
In summary, results of our study indicated that maternal dietary proline supplementation during gestation affected the concentrations of glutamate, proline, and taurine in plasma, of glycine and taurine in amniotic fluid, and of polyamines in the placental tissues and amniotic fluid of F1 female offspring, without affecting fetal survival or reproductive performance. This effect was accompanied by decreased proline availability in maternal plasma possibly as an adaptational mechanism for regulating amino acid and polyamine metabolism in the conceptus and offspring. To our knowledge, this is the first study determining a transgenerational effect of proline on amino acid and polyamine metabolism in animals.
References
Abu-Saad K, Fraser D (2010) Maternal nutrition and birth outcomes. Epidemiol Rev 32:5–25. https://doi.org/10.1093/epirev/mxq001
Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L (2004) Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol London 561:355–377. https://doi.org/10.1113/jphysiol.2004.072009
Ball RO, Atkinson JL, Bayley HS (1986) Proline as an essential amino-acid for the Young-Pig. Br J Nutr 55:659–668. https://doi.org/10.1079/Bjn19860072
Barbul A (2008) Proline precursors to sustain mammalian collagen synthesis. J Nutr 138:2021S–2024S. https://doi.org/10.1093/jn/138.10.2021S
Bazer FW, Johnson GA, Wu G (2015) Amino acids and conceptus development during the peri-implantation period of pregnancy. Adv Exp Med Biol 843:23–52. https://doi.org/10.1007/978-1-4939-2480-6_2
Brunton JA, Baldwin MP, Hanna RA, Bertolo RF (2012) Proline supplementation to parenteral nutrition results in greater rates of protein synthesis in the muscle, skin, and small intestine in neonatal Yucatan miniature piglets. J Nutr 142:1004–1008. https://doi.org/10.3945/jn.111.154534
Chan KA, Jazwiec PA, Gohir W, Petrik JJ, Sloboda DM (2018) Maternal nutrient restriction impairs young adult offspring ovarian signaling resulting in reproductive dysfunction and follicle loss. Biol Reprod 98:664–682. https://doi.org/10.1093/biolre/ioy008
Connor KL, Vickers MH, Beltrand J, Meaney MJ, Sloboda DM (2012) Nature, nurture or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. J Physiol London 590:2167–2180. https://doi.org/10.1113/jphysiol.2011.223305
Dai Z, Wu Z, Wang J, Wang X, Jia S, Bazer FW, Wu G (2014) Analysis of polyamines in biological samples by HPLC involving pre-column derivatization with o-phthalaldehyde and N-acetyl-l-cysteine. Amino Acids 46:1557–1564. https://doi.org/10.1007/s00726-014-1717-z
Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Lewis DS, Lee DR, Reeds PJ (1994) Amino acid composition of human milk is not unique. J Nutr 124:1126–1132. https://doi.org/10.1093/jn/124.7.1126
Faria Tda S, Brasil Fde B, Sampaio FJ, Ramos Cda F (2008) Maternal malnutrition during lactation alters the folliculogenesis and gonadotropins and estrogen isoforms ovarian receptors in the offspring at puberty. J Endocrinol 198:625–634. https://doi.org/10.1677/JOE-08-0121
Guzman C, Cabrera R, Cardenas M, Larrea F, Nathanielsz PW, Zambrano E (2006) Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol London 572:97–108. https://doi.org/10.1113/jphysiol.2005.103903
Herring CM, Bazer FW, Johnson GA, Wu G (2018) Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp Biol Med 243:525–533. https://doi.org/10.1177/1535370218758275
Hogenkamp A, Knippels LM, Garssen J, van Esch BC (2015) Supplementation of mice with specific nondigestible oligosaccharides during pregnancy or lactation leads to diminished sensitization and allergy in the female offspring. J Nutr 145:996–1002. https://doi.org/10.3945/jn.115.210401
Hou Y, Wu G (2018) l-Glutamate nutrition and metabolism in swine. Amino Acids 50:1497–1510. https://doi.org/10.1007/s00726-018-2634-3
Ji Y, Wu Z, Dai Z, Sun K, Wang J, Wu G (2016) Nutritional epigenetics with a focus on amino acids: implications for the development and treatment of metabolic syndrome. J Nutr Biochem 27:1–8. https://doi.org/10.1016/j.jnutbio.2015.08.003
Ji Y, Wu Z, Dai Z, Wang X, Li J, Wang B, Wu G (2017) Fetal and neonatal programming of postnatal growth and feed efficiency in swine. J Anim Sci Biotechnol 8:42. https://doi.org/10.1186/s40104-017-0173-5
Kim YI (2005) Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr 135:2703–2709. https://doi.org/10.1093/jn/135.11.2703
Kong X, Wang X, Yin Y, Li X, Gao H, Bazer FW, Wu G (2014) Putrescine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. Biol Reprod 91:106. https://doi.org/10.1095/biolreprod.113.113977
Kwon H, Spencer TE, Bazer FW, Wu G (2003) Developmental changes of amino acids in ovine fetal fluids. Biol Reprod 68:1813–1820. https://doi.org/10.1095/biolreprod.102.012971
Li P, Wu G (2018) Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50:29–38. https://doi.org/10.1007/s00726-017-2490-6
Li X, Bazer FW, Johnson GA, Burghardt RC, Frank JW, Dai Z, Wang J, Wu Z, Shinzato I, Wu G (2014) Dietary supplementation with l-arginine between days 14 and 25 of gestation enhances embryonic development and survival in gilts. Amino Acids 46:375–384. https://doi.org/10.1007/s00726-013-1626-6
Liu N, Dai Z, Zhang Y, Chen J, Yang Y, Wu G, Tso P, Wu Z (2018) Maternal l-proline supplementation enhances fetal survival, placental development and nutrient transport in mice. Biol Reprod. https://doi.org/10.1093/biolre/ioy240
Meier H, Hoag WG, McBurney JJ (1965) Chemical characterization of inbred-strain mouse milk. I. Gross composition and amino acid analysis. J Nutr 85:305–308. https://doi.org/10.1093/jn/85.3.305
Mussini E, Hutton JJ Jr, Udenfriend S (1967) Collagen proline hydroxylase in wound healing, granuloma formation, scurvy, and growth. Science 157:927–929
Padmanabhan N, Jia D, Geary-Joo C, Wu X, Ferguson-Smith AC, Fung E, Bieda MC, Snyder FF, Gravel RA, Cross JC, Watson ED (2013) Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell 155:81–93. https://doi.org/10.1016/j.cell.2013.09.002
Phang JM (2019) Proline metabolism in cell regulation and cancer biology: recent advances and hypotheses. Antioxid Redox Signal 30:635–649. https://doi.org/10.1089/ars.2017.7350
Phang JM, Liu W, Zabirnyk O (2010) Proline metabolism and microenvironmental stress. Annu Rev Nutr 30:441–463. https://doi.org/10.1146/annurev.nutr.012809.104638
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951. https://doi.org/10.1093/jn/123.11.1939
Seckl JR, Holmes MC (2007) Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endoc 3:479–488. https://doi.org/10.1038/ncpendmet0515
Wang J, Wu Z, Li D, Li N, Dindot SV, Satterfield MC, Bazer FW, Wu G (2012) Nutrition, epigenetics, and metabolic syndrome. Antioxid Redox Signal 17:282–301. https://doi.org/10.1089/ars.2011.4381
Wang X, Ying W, Dunlap KA, Lin G, Satterfield MC, Burghardt RC, Wu G, Bazer FW (2014) Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod 90:84. https://doi.org/10.1095/biolreprod.113.114637
Winship AL, Gazzard SE, Cullen McEwen LA, Bertram JF, Hutt KJ (2018) Maternal low protein diet programmes low ovarian reserve in offspring. Reproduction 156:299–311. https://doi.org/10.1530/rep-18-0247
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17. https://doi.org/10.1007/s00726-009-0269-0
Wu G (2010) Functional amino acids in growth, reproduction, and health. Adv Nutr 1:31–37. https://doi.org/10.3945/an.110.1008
Wu G (2013) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton
Wu G (2018) Principles of animal nutrition. CRC Press, Boca Raton
Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr 124:415–424. https://doi.org/10.1093/jn/124.3.415
Wu G, Bazer FW, Hu J, Johnson GA, Spencer TE (2005) Polyamine synthesis from proline in the developing porcine placenta. Biol Reprod 72:842–850. https://doi.org/10.1095/biolreprod.104.036293
Wu G, Bazer FW, Datta S, Johnson GA, Li P, Satterfield MC, Spencer TE (2008) Proline metabolism in the conceptus: implications for fetal growth and development. Amino Acids 35:691–702. https://doi.org/10.1007/s00726-008-0052-7
Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li X, McKnight JR, Satterfield MC, Spencer TE (2011) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063. https://doi.org/10.1007/s00726-010-0715-z
Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417. https://doi.org/10.1146/annurev-animal-022513-114113
Wu G, Bazer FW, Johnson GA, Herring C, Seo H, Dai Z, Wang J, Wu Z, Wang X (2017) Functional amino acids in the development of the pig placenta. Mol Reprod Dev 84:870–882. https://doi.org/10.1002/mrd.22809
Wu Z, Hou Y, Dai Z, Hu CA, Wu G (2019) Metabolism, nutrition, and redox signaling of hydroxyproline. Antioxid Redox Signal 30:674–682. https://doi.org/10.1089/ars.2017.7338
Yin J, Li Y, Zhu X, Han H, Ren W, Chen S, Bin P, Liu G, Huang X, Fang R, Wang B, Wang K, Sun L, Li T, Yin Y (2017) Effects of long-term protein restriction on meat quality, muscle amino acids, and amino acid transporters in pigs. J Agric Food Chem 65:9297–9304. https://doi.org/10.1021/acs.jafc.7b02746
Youngson NA, Whitelaw E (2008) Transgenerational epigenetic effects. Annu Rev Genom Hum G 9:233–257. https://doi.org/10.1146/annurev.genom.9.081307.164445
Zambrano E, Rodriguez-Gonzalez GL, Guzman C, Garcia-Becerra R, Boeck L, Diaz L, Menjivar M, Larrea F, Nathanielsz PW (2005) A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol London 563:275–284. https://doi.org/10.1113/jphysiol.2004.078543
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31572412, 31572410, 31625025, and 31272450), and Texas A&M AgriLife Research (H-8200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethical approval
This animal study was approved by the Animal Use and Care Committee of China Agricultural University.
Additional information
Handling Editor: J. M. Phang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, N., Dai, Z., Zhang, Y. et al. Maternal l-proline supplementation during gestation alters amino acid and polyamine metabolism in the first generation female offspring of C57BL/6J mice. Amino Acids 51, 805–811 (2019). https://doi.org/10.1007/s00726-019-02717-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-019-02717-2