Abstract
This study investigated the effect of agmatine (Agm) in proliferation of ovine trophecdoderm cells (oTr1) as well as the importance of the arginine decarboxylase (ADC) and agmatinase (AGMAT) alternative pathway for synthesis of polyamines in ovine conceptuses during the peri-implantation period of pregnancy. Morpholino antisense oligonucleotides (MAOs) were used to inhibit translation of mRNAs for ODC1 alone, AGMAT alone, and their combination. Rambouillet ewes (N = 50) were assigned randomly to the following treatments on Day 8 of pregnancy: MAO control (n = 10); MAO-ODC1 (n = 8); MAO-ADC (n = 6); MAO-ODC1:MAO-ADC (n = 9); or MAO-ODC1:MAO-AGMAT (n = 9). Ewes were ovario-hysterectomized on Day 16 of pregnancy to obtain uterine flushings, uterine endometrium, and conceptus tissues. Inhibition of translation of both ODC1 and AGMAT resulted in 22% of ewes having morphologically and functionally normal (elongated and healthy) conceptuses designated MAO-ODC1:MAO-AGMAT (A). But, 78% of the MAO-ODC1:MAO-AGMAT ewes had morphologically and functionally abnormal (not elongated and fragmented) conceptuses designated MAO-ODC1:MAO-AGMAT (B). The pregnancy rate was less (22%; P < 0.05) for MAO-ODC1:MAO-AGMAT ewes than for MAO-control (80%), MAO-ODC1 (75%), MAO-ADC (84%), and MAO-ODC1:MAO-ADC (44%) ewes. Moreover, inhibition of translational of both ODC1 and AGMAT mRNAs increased expression of ADC, SLC22A1, SLC22A2, and SLC22A3 mRNAs, as well as abundances of agmatine, putrescine, spermindine, and spermine in conceptus tissue. However, MAO-ODC1:AGMAT(B) ewes had greater abundances of agmatine, putrescine, and spermidine and reduced amounts of spermine in uterine flushes. Thus, in vivo knockdown of translation of ODC1 and AGMAT mRNAs increased expression of genes for the synthesis and transport of polyamines in ovine conceptuses during the peri-implantation period of pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ovine conceptus undergoes dramatic morphological changes (spherical to tubular to filamentous forms) during the peri-implantation period of pregnancy (Gray et al. 2006). These changes in morphology maximize surface area of contact between trophectoderm and uterine luminal (LE) epithelium for implantation and for the uptake of histotroph that includes molecules either secreted or transported into the uterine lumen (Bazer et al. 2010a, 2012; Spencer et al. 2017). The morphological transition in ovine conceptuses coincides with the onset of secretion of interferon tau (IFNT), the antiluteolytic pregnancy recognition signal in ruminants. IFNT abrogates the mechanism for pulsatile secretion of luteolytic prostaglandin F2α (PGF) that is required for maintenance of a functional corpus luteum to produce progesterone, the essential hormone for pregnancy (Bazer et al. 1997, 2011, 2012; Spencer et al. 2004).
Secretions both from uterine LE and glandular (GE) epithelia, as well as select transport of nutrients into the uterine lumen are regulated by progesterone and IFNT (Gray et al. 2002). Histotroph includes amino acids, glucose, growth factors, hormones, cytokines, enzymes, adhesion proteins, and polyamines, which are essential for growth, development, and survival of the conceptus (Gray et al. 2002, 2006; Bazer et al. 2014; Lenis et al. 2016).
Polyamines (putrescine, spermidine, and spermine) are polycationic molecules that contain two or more amino groups (–NH3 +) and are present in all eukaryotic and prokaryotic cells (Lefèvre et al. 2001). The polyamines promote DNA and protein synthesis and enhance proliferation of trophectoderm cells, as well as survival, growth, and development of the ovine conceptus. Adequate levels of polyamines in the uterine lumen during the peri-implantation period of pregnancy are required for survival of conceptuses (Wu et al. 2004, 2013; Kong et al. 2014; Lenis et al. 2016).
There are two pathways for the synthesis of polyamines in ovine conceptuses. In the classical pathway, Arg is converted to ornithine by arginase and ornithine is decarboxylated by ornithine decarboxylase (ODC1) to produce putrescine which is the substrate for spermidine synthase to generate spermidine, and spermidine is catabolized to spermine by spermine synthase (Wu and Morris 1998; Wu and Meininger 2000; Kwon et al. 2003; Wang et al. 2014). In the alternative pathway for production of polyamines, the ovine conceptus, arginine decarboxylase (ADC) decarboxylates Arg to form agmatine, and agmatine is converted to putrescine by agmatinase (AGMAT) (Wang et al. 2015; Lenis et al. 2016). AGMAT is present in the rat brain with the highest activity in the hypothalamus, a region rich in imidazoline receptors, and lowest in the striatum and cortex. In both areas, cellular activity for AGMT is greatest in the mitochondrial matrix (Sastre et al. 1996). For many years, it was believed that only the “classical pathway” for synthesis of polyamines existed in mammals (Sastre et al. 1996; Sekowska et al. 1998). However, our laboratory discovered the presence of the alternative pathway in ovine conceptuses whereby Arg is decarboxylated by ADC to produce agmatine, which is then converted to putrescine by agmatinase (Wang et al. 2014).
Agm is an amine with a wide variety of physiological and pharmacological effects (Piletz et al. 2013). Agm has been found to have anti-tumor and cyto-protective effects on nerve, colon, renal, and other different cells lines (Wolf et al. 2007; Molderings and Haenisch, 2012). On the other hand, we have demonstrated that l-Arg has important biological effects during the pre-implantation period of pregnancy, stimulating the proliferation, migration, and production of IFNT in ovine trophoectoderm cells (oTr1) (Lenis et al. 2016).
Synthesis of polyamines and Agm by ovine conceptuses requires Arg in histotroph (Wu et al. 2009, 2013; Wang et al. 2014; Lenis et al. 2016). However, polyamines and Agm can be transported into trophectoderm cells from uterine histotroph by solute carrier family 22 (SLC22) (organic cation transporter). SLC22 members 1 (SLC22A1), 2 (SLC22A2), and 3 (SLC22A3) are transporters for polyamines and Agm. SLC22A1 preferentially transports spermidine and spermine, while SLC22A2 and SLC22A3 preferentially transport putrescine. Transport of Arg is carried out by SLC7A1 (solute carrier family 7 member 1) in ovine trophectoderm cells (Gao et al. 2009a). SLC7A1 is a member of solute carrier family (Sala-Rabanal et al. 2013).
Insulin growth factors (IGFs) act as autocrine, endocrine, and paracrine hormones to regulate essential processes for conceptus development during the peri-implantation period of pregnancy (Rechler and Nissley 1985). The molecular structure for insulin growth factor type I (IGF1) and insulin growth factor type II (IGF2) are similar to pro-insulin and they stimulate proliferation and differentiation of a variety of cells (Kim et al. 2008). IGF2 acts via its receptor (IFGR2) to promote growth, development, and differentiation of the conceptus, as well as migration and invasion of trophectoderm cells in domestic animals (Leroith et al. 1995).
We previously reported that the amounts of Agm produced by uterine epithelia and taken up by the conceptus may be sufficient to allow some, but not all, conceptuses to survive when morpholino antisense oligonucleotides (MAOs) were used to inhibit translation of mRNAs for ODC1 and ADC by trophectoderm cells. Considering that l-Arg stimulates the proliferation, migration, and production of IFNT in oTr1, this study was designed to: (1) evaluate the effect of Agm on the proliferation of in vitro cultured oTr1; and (2) understand the importance of the alternative pathway for synthesis of polyamines in ovine conceptus development during the peri-implantation period of pregnancy. This required use of MAOs to inhibit translation of ODC1 mRNA alone, AGMAT mRNA alone, the combination of MAOs for ODC1 and ADC, and particularly the combination of ODC1 and AGMAT mRNA. This is because the uterus produces agmatine and MAOs do not block translation of ADC mRNA in uterine epithelia.
Materials and methods
Cell culture
Our established oTr1 cells (Wang et al. 2015) were seeded and propagated for 48 h in in vitro in complete medium (CM), DMEM/F-12 (Dulbecco modified Eagle medium/Nutrient Mixture F-12, Gilbco BRL, Grand Island, NYU, USA), 10% fetal bovine serum (BFS) (Gibco BRL), 50 U/ml Penicillin, 50 μg/ml Streptomycin, 0.1 mM NEAA (nutritionally non-essential amino acids), 1 mM sodium pyruvate, 2 mM of glutamine, and 4 μg/ml of insulin, until reaching 90% confluence. After 48 h we split the cells using trypsin in order to obtain the number of cells needed for each treatment, while the remaining cells were cryo-preserved using DMSO in a ratio of 1:3.
For cell proliferation assays, oTr1 cells (passages 6–12) were subcultured for 12 h (10,000 cells/well) in 24-well plates (Costar # 3524; Corning, NY, USA) in CM until reaching 50% confluence. For the initiation of the experiments, the cells were cultured in FBS-free CM-I and insulin for 24 h and then cultured in CM-II, a medium lacking Agm, for 6 h. Subsequently, cells were cultured in basal medium (BM) which contained different concentrations of Agm: BM I, 0 μM; BM II, 50 μM; BM III, 100 μM; BM IV, 150 μM; BM V, 200 μM; and BM VI, 500 μM. The doses of Agm used in were based on concentrations of Agm in uterine flushings from ewes during the peri-implantation period of pregnancy (Wang et al. 2014). This experiment was carried out with two different controls. The first was a negative control lacking Agm (BM I), and the second was a negative control in which oTr1 cells were cultured in commercial medium (DMEM/F-12. Each experiment was carried ouit in triplicate and the medium was changed at 48 h of the 96-h period of culture.
Cell numbers were determined and described previously (Raspotnig et al. 1999). The oTr1 cells were removed from the medium by vacuum aspiration, and each well was washed twice with PBS (1×). Then, the cells were fixed and incubated in 50% ethanol for 30 min, and then the ethanol was removed by aspiration. Fixed cells were stained for 4 min with Janus Green B which was dissolved in phosphate-buffered saline (PBS; 0.2 w/v, pH 7.2) at room temperature. Staining solution was removed by aspiration and the dish was washed twice with PBS (1×). Immediately thereafter the cells were lysed with 0.5 N HCl and the absorbance of the supernatant read at 595 nm using a microplate reader. The number of cells was calculated from the absorbance reading using the following formula: cell number = (absorbance − 0.00462)/0.00006926.
Animal model
Multiparous Rambouillet-type ewes (n = 50) were synchronized to estrus using a commercially available Eazi-Breed CIDR (Pfizer, New York, New York) device for 12 days followed by an intramuscular injection of 20 mg Lutalyse (Pfizer New York, New York) at the time of CIDR removal. Estrus (Day 0) was detected in ewes by vasectomized rams and the ewes were subsequently mated to intact rams of known fertility. All experimental and surgical procedures were approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Morpholino design
Morpholino antisense oligonucleotides (MAOs) were designed and synthesized to inhibit initiation of translation of mRNAs for ODC1, ADC, and AGMAT which encode for enzymes involved in the synthesis of polyamines via the classical arginase-ODC1 pathway and the ADC-AMAT non-classical pathway in trophectoderm cells of ovine conceptuses. The MAO for ODC1 had the sequence 5′-ACTCT TCATT ACCAA AGTTG TTCAT-3′ and targeted the starting codon of ODC1 mRNA (GenBank accession no. NM_002539). The MAO for ADC had the sequence 5′ TTTCTCTCA GGTAGCCAGCCATGCC′3 (GenBank accession no. NM_001293722.1) and targeted the starting codon of ADC. For AGMAT the MAO sequence was 5′ TGGACGCCAGCAGCCGCAGCATC′3 and targeted the starting codon of AGMT mRNA (GenBank accession no. NM_ XM_004013796.3). For the control, MAO, the sequence was 5′-CCTCT TACCT CAGTTACAAT TTATA-3′ and targeted a splice site mutant of Homo sapiens hemoglobin β-chain (HBB) gene (GenBank accession no. GU324922). All morpholinos were synthesized with a 3′-lissamine tag modification (red color) for convenient confirmation of the cellular uptake of each MAO by conceptus trophectoderm.
Experimental design and tissue collection
For morpholino delivery into the uterine lumen, we performed surgery on Day 8 post-mating as previously described by Wang et al. (2014). The ewes were assigned randomly to the following treatments: MAO control (n = 10); MAO-ODC1 (n = 8); MAO-ADC (n = 6); MAO-ODC1:MAO-ADC (n = 9); or MAO-ODC1:MAO-AGMT (n = 9). The development and normal implantation of ovine conceptuses is unaffected by surgery or morpholino delivery (Wang et al. 2014). MAO control, MAO-ODC1, MAO-ADC, and MAO-AGMAT were complexed to lissamine as described previously (Wang et al. 2014). The MAO combination treatments (100 nmol MAO-ODC1 plus 100 nmol MAO-ADC and 100 nmol MAO-ODC1 plus 100 nmol MAO-AGMAT) were prepared with Gene Tools Endo-Porter delivery reagent (200 µl) and diluted to a final volume of 1.2 ml with OPTI-MEM (Invitrogen; Carlsbad, CA, USA). All MAOs were injected into the lumen of the uterine horn ipsilateral to the ovary with a CL. On Day 16, ewes were ovario-hysterectomized to obtain uterine flushings, uterine endometrium, and conceptus tissues. The ligated uterine horn ipsilateral to the CL was flushed with 10 ml sterile phosphate-buffered saline (PBS), pH 7.2. The presence or absence of a functional CL and the presence of a conceptus in the uterine flushing was recorded and pregnancy rate (percent of ewes pregnant that were mated) determined for each treatment group. For pregnant ewes with a conceptus, the morphology of the conceptus was recorded as small, thin, fragile, fragmented, elongated, and/or normal. After photographing each conceptus using a digital camera, the conceptus was immediately removed from the uterine flush with a transfer pipette, and the recovered volume of uterine flushing recorded. Portions of each conceptus and Sections (0.5 cm) from the mid-portion of the uterine horn ipsilateral to the CL were placed in optimal-cutting temperature compound (VWR, Houston, TX, USA), frozen in liquid nitrogen, and stored at − 80 °C or fixed in freshly prepared 4% (wt/vol) paraformaldehyde in PBS, pH 7.2, for 48 h, and then in 70% ethanol for 24 h. The uterine flushings were centrifuged (5000×g for 15 min), aliquoted, and stored at − 80 °C until analyzed.
Slot blot assay of IFNT
We performed slot blot protein analyses to quantify the abundance of IFNT in uterine flushings as described by Lenis et al. (2016). For this assay, 10 µg of protein from each uterine flush was used to optimize analyses by slot blotting. The signal for each sample was expressed relative to the immunoreactivity for proteins in uterine flushings from MAO-control ewes. The abundance of IFNT was quantified by measuring the intensity of light emitted from correctly sized bands under ultraviolet light using a ChemiDoc EQ system and Quantity One software (Bio-Rad, Hercules, CA). The data are expressed as IFNT intensity in uterine flushes per 10 µg total protein.
RNA isolation, cDNA synthesis, and qPCR
Approximately 20 mg of conceptus tissue was lysed and homogenated in lysis buffer. The RNA extraction was conducted using the QIAGEN RNeasy® Mini Kit according to the manufacturer’s instructions. Quantification of RNA was performed using the NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific Inc.). cDNA was synthesized from 1000 ng RNA using SuperScript™ First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA). Synthesized cDNA was used as a template for real-time PCR amplification using SYBR® Green PCR Mastermix (Applied Biosystems, Warrington, UK).
Quantitative real-time PCR
All primers were designed using Primer-BLAST software (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) and evaluated for the optimal concentration with conceptus cDNA. Quantitative analysis of gene expression was performed using quantitative real-time PCR (qPCR). All qRT-PCR was carried out using 5 ng cDNA in 1 µl, 5 pm of primers in 1 and 5 µl of SYBR Green PCR MasterMix (Applied Biosystems). Primer sequences are described in Table 1. The final reaction volume was adjusted to 10 µl with RNase/DNase-free water. The qPCR was carried out using the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
All reaction programs had four stages of cycling conditions: (1) 50 °C for 2 min, (2) 95 °C for 10 min, (3) 40 cycles at 95 °C for 15 s, 60 °C for 1 min, and (4) 95 °C for 15 s and 60 °C for 15 s. A dissociation curve was generated to ensure specificity of amplification. Relative mRNA expression was normalized to the previously validated housekeeping gene (18S ribosomal RNA) and calculated using the comparative Ct method (2− ΔΔCT method). Significant differences were determined using the least squares means and orthogonal contrasts for multiple comparisons using the general lineal model (GLM) statistical discovery software JMP (SAS Institute, Cary, NC).
Quantitative immunofluorescence microscopy
Lissamine-labeled MAOs were analyzed by fluorescence microscopy to confirm their effective delivery into the trophectoderm. Cryosections of the OCT containing conceptuses (10 µm) were prepared and placed directly into mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. Also, the translational knockdown efficiency of MAO-ODC1, MAO-AGMAT, MAO-ADC and their combinations was evaluated in frozen sections of conceptuses or uteri by immunofluorescence microscopy as previously described (Wang et al. 2014). For each primary antibody, images were captured with identical microscope and detector settings to facilitate comparisons of spatial distribution and fluorescence intensities among samples from ewes on the various treatments. Signals were quantified using Image J software (Version 1.47, National Institutes of Health) and standardized procedures described previously (Arqués et al. 2012).
RNA quantification
Total RNA in conceptus tissue was determined using the fluorometric method and a microplate reader as described by Rezaei et al. (2013). The RNA fluorescence complex was measured with an excitation wavelength of 365 nm and an emission wavelength of 590 nm. Results are expressed as μg of RNA per 20 mg of conceptus tissue.
Analyses for putrescine, spermidine, spermine, and agmatine
Concentrations of polyamines, and Agm were determined in uterine flushings and conceptuses as described previously (Wang et al. 2014; Dai et al. 2014). Briefly, uterine flushings (100 µl), and conceptuses (15 mg) were acidified with 100 µl of 1.5 M HClO4 and neutralized with 50 µl of 2 M K2CO3. The neutralized extracts were analyzed for polyamines and Agm after making 1:2.5 dilutions for conceptuses and no dilution for uterine flushes. Polyamines were subjected to high-performance liquid chromatography analyses involving precolumn derivatization with o-phthaldialdehyde (OPA) reagent I. Reagent I was prepared as described previously (Wang et al. 2014b).
Statistics
Normality of quantitative parameters was assessed using normal probability plots and the Kolmogorov–Smirnov test generated with the univariate procedure of SAS. To determine the effect of treatment on oTr1 cell proliferation, we used a mixed model one-way analysis of variance (ANOVA) followed by Duncan’s adjustment of error rate. Protein amount and volumes of uterine flushes were expressed as mean ± standard deviation (SD) except that pregnancy rate is expressed as a binominal (Yes: pregnant vs No: not pregnant). To determine the effect of treatment on protein and volume of collected uterine flushes and to compare gene expression levels among treatment groups, we used a mixed model one-way analysis of variance (ANOVA) followed by Duncan’s adjustment of error rate. The effect of treatment on pregnancy rates was analyzed using Fisher exact Test. Statistical analyses were done using software SAS® (version 9.2, SAS Institute, Cary, NC, USA). For all analyses, P ≤ 0.05 was defined as significant.
Results
Agmatine and proliferation of oTr1 cells
There was no dose-dependent effect of Agm on proliferation of oTr1 cells (P > 0.05) during the first 48 h of culture. Furthermore, proliferation of oTr1 cells in the basal medium, with or without Agm, had a lower (P > 0.05) rate of cell proliferation than for oTr1 cells cultured in CM (Fig. 1a). At 96 h of culture, Agm at concentrations of 100 and 150 μM reduced proliferation of oTr1 at 96 h of culture by 11.5 and 15.9%, respectively, compared to the basal medium without Agm (0 μM) and by 16 and 7%, respectively, when compared with Agm at doses of 200 and 500 μM (Fig. 1b). When comparing proliferation oTr1 cells in basal medium there was a decrease (P > 0.05) in proliferation at all concentrations of Agm compared to that for oTr1 cells in CM.
Effect of agmatine on the proliferation of ovine trophectoderm cells at 48 h (a) and 96 h (b) of culture. Agm did not affect the proliferation of ovine trophectoderm cells at 48 h of culture; however, at 96 h of culture agmatine at doses of 100 and 150 μM reduced the proliferation of ovine trophectoderm cells when compared to the control (0 μM agmatine)
MAO delivery and effect of knockdown of translation of ODC1, ADC, AGMAT mRNAs in ovine conceptuses
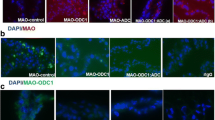
We confirmed efficiencies for MAO delivery and translational knockdown of ODC1 and ADC mRNAs independently and the combination of ODC1 with ADC and AGMAT using immunofluorescence microscopy (Fig. 2a). For each treatment group, MAO uptake by conceptuses was confirmed by the presence of the pink-colored 3′-lissamine in conceptus trophectoderm (Fig. 2a). The presence of ODC1, AGMAT, and ADC proteins is evident in the first panel of Fig. 2b–d, whereas little or none of those respective proteins were detectable by immunofluorescence analyses following inhibition of translation of mRNAs for ODC1, ADC, and AGMAT (Fig. 2b–d). These results indicate that MAOs were delivered efficiently into trophectoderm cells of the conceptus and that knockdown of translation of ODC1, ADC, and AGMAT mRNAs to ODC1, ADC, and AGMAT proteins in trophectoderm cells was successful.
MAO delivery and knockdown ODC1, ADC, and AGMAT in ovine conceptus trophectoderm on Day 16 of pregnancy. a The images confirm delivery of lissamine-tagged MAOs into tropohectoderm of conceptuses in all treatment groups: MAO control, MAO-ODC1, MAO-ADC, MAO-ODC1:ADC, MAO-ODC1: AGMAT (a), and MAO-ODC1: AGMAT (b). b Immunofluorescence images in conceptus trophectoderm confirmed inhibition of translation of mRNAs for ODC1, ADC, and AGMAT compared with conceptus trophectoderm of conceptuses from MAO control ewes. c The knockdown of translation of AGMAT mRNA was confirmed in the second panel by comparing the immunofluorescence signal with MAO-control. d The knockdown translation of ADC mRNA was confirmed by comparing the immunofluorescence signal in panel 2 with the MAO control panel. Purified nonrelevant rabbit IgG was substituted for the primary antibody as a negative control
Effects of in vivo knockdown of translation of mRNAs for ODC1, AGMAT, and ADC or their combination on conceptus development
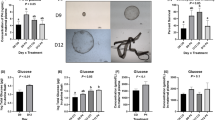
We investigated in vivo translational knockdown of ADC and ODC1 alone, as well as in combination with AGMAT and ADC on conceptus development between Days 8 and 16 of pregnancy. The morphologies of MAO control, MAO-ODC1, MAO-ADC, and 60% of the MAO-ODC1:MAO-ADC conceptuses were normal. However, the MAO-ODC1:MAO-AGMAT combination knockdown resulted in two phenotypes of conceptuses. The first phenotype was represented by 22% (n = 2) of conceptuses that appeared to be morphologically and functionally normal (elongated and healthy) and designated MAO-ODC1:MAO-AGMAT (A). The second phenotype represented by 78% (n = 4) of the conceptuses was morphologically and functionally abnormal (not elongated and fragmented) and designated MAO-ODC1:MAO-AGMAT (B) (Fig. 3a). Only two MAO-ODC1:MAO-AGMAT (B) conceptuses could be analyzed in detail because they were completely fragmented.
Gross morphology of ovine conceptus on Day 16 of pregnancy following inhibition of translation of ODC1, ADC, ODC1 plus ADC and ODC1 plus AGMAT mRNAs. In vivo knockdown of ODC1, ADC, and ODC1:ADC resulted in normal and healthy phenotypes of ovine conceptus, while the combination knockdown (ODC1:AGMAT) resulted in two phenotypes based on their morphological and functional development. The MAO-ODC1:AGMAT (a) conceptuses were normal, healthy and elongated while MAO-ODC1:AGMAT (b) conceptuses were abnormal, fragmented and not elongated. a The morphology of MAO control (n = 6), MAO-ODC1 (n = 5), MAO-ADC (n = 7), MAO-ODC1:ADC (n = 4), and MAO-OC1:AGMAT (a) conceptuses was normal and conceptuses elongated. However, MAO-ODC1:AGMAT (b) (n = 2) conceptuses were morphologically abnormal (small, fragmented and not elongated). b Differences in conceptuses’ morphologies, protein and IFNT in uterine flushes, as well as total RNA and gene expression in conceptus tissue of MAO-ODC1:MAO-AGMAT (a) and MAO-ODC1:MAO-AGMAT (b) conceptuses
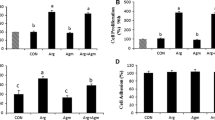
The overall pregnancy rate for all ewes in this study was 60%. However, the pregnancy rate was lower (P < 0.05) for MAO-ODC1:MAO-AGMAT ewes (22%) compared with MAO control (80%), MAO-ODC1 (75%), MAO-ADC (84%), and MAO-ODC1:MAO-ADC (44%) ewes (Fig. 4b). There was no significant effect of treatment on volume of uterine flushing recovered (Fig. 4a). Total protein was greater (P < 0.05) in uterine flushings from MAO-ODC1:MAO-ADC, MAO-ODC1:MAO-AGMAT (A), and MAO-ODC1:MAO-AGMAT (B) ewes compared with MAO control, MAO-ODC1, and MAO-ADC ewes (Fig. 2c). The relative abundance of IFNT in 10 µg of protein in uterine flushings was not different (P > 0.05) between MAO-ODC1, MAO-ADC, MAO-ODC1:MAO-ADC, and MAO-ODC1:MAO-AGMAT (A) ewes compared with MAO control ewes. However, the MAO-ODC1:MAO-AGMAT (B) had less (P < 0.05) IFNT in uterine flushings (Fig. 5b, c).
Effects of knockdown of ODC1, ADC, ODC1:ADC, and ODC1:AGMAT on recovery volume of uterine flushes, pregnancy rate and total protein in uterine flushes. a Recovery volume of uterine flushes was not different (P > 0.05) among treatment groups. b The pregnancy rates were affected (P < 0.05) by treatment with MAO-ODC1:AGMAT ewes having the lowest pregnancy rate. c The total recoverable protein in uterine flushes from MAO-ODC1: MAO-ADC, MAO-ODC1:MAO-AGMAT (a), and MAO-ODC1:MAO-AGMAT (b) was different (P < 0.05) than that for MAO control ewes. Significant effects are indicated by different superscript letters (P < 0.05). Data are presented as mean and SEM
Interferon tau (IFNT) in uterine flushings from Day 16 of pregnancy and total RNA (µg) in conceptus tissue. In vivo knockdown of ODC1 (n = 6), ADC (n = 5), the combination ODC1:ADC (n = 4), and the combination ODC1:AGMAT phenotype (a) did not affect the abundance of IFNT in uterine flushings; however, IFNT in uterine flushes from MAO-ODC1:AGMAT (b) ewes (n = 2) was less (P < 0.05) that for MAO control ewes. Total RNA in conceptuses from ODC1, ADC, ODC1:ADC, and ODC1:AGMAT (a) and (b) ewes was different (P < 0.05) as indicated by different superscript letters (P < 0.05). Data are presented as mean and SEM
In vivo translational knockdown of MAO-ODC1, MAO-ADC, MAO-ODC1:MAO-ADC, MAO-ODC1:MAO-AGMAT (A), and MAO-ODC1:MAO-AGMAT (B) affects total RNA in conceptus tissue
The polyamines (putrescine, spermidine, and spermine) are essential for embryonic development by stimulating RNA translation, DNA and protein synthesis, cell proliferation and migration, and growth and differentiation of tissues. There was an effect of MAO treatment on total RNA in conceptus tissue. Conceptuses from MAO-ODC1, MAO-ADC, MAO-ODC:MAO-ADC, MAO-ODC:MAO-AGMAT (A), and MAO-ODC:MAO-AGMAT (B) ewes had less (P < 0.05) RNA than conceptuses from MAO-control ewes (Fig. 5a).
Inhibition of translation of the combination of ODC1 and AGMAT mRNAs increased expression of ADC, SLC22A1, SLC22A2, and SLC22A3 mRNAs. SLC22A1, SLC22A2, and SLC22A3 transport polyamines and agmatine into cells. ADC is an essential enzyme that converts arginine to agmatine for synthesis of polyamines in conceptus tissue. Double knockdown of ODC1 and AGMAT affected the expression of ODC1 and IFNT mRNAs
The effects of MAOs on expression of genes related to transport of polyamines, Agm and Arg in conceptus tissue for synthesis of polyamines were evaluated using quantitative real time PCR. For AGMAT mRNA, there was no effect of MAO treatment on expression (Fig. 6a). For ADC mRNA, conceptuses from MAO-ODC1:MAO-AGMAT (B) ewes had a 126-fold greater (P < 0.01) expression of ADC than conceptuses from MAO-Control ewes (Fig. 6b). The expression of ODC1 mRNA was less (P < 0.05) for conceptuses from MAO-ODC1:MAO-AGMAT (A) ewes (Fig. 6c).
Effects of knockdown of ODC1, ADC, ODC1:ADC, and ODC1:AGMAT on expression of mRNAs for AGMAT (agmatinase; a), ADC (arginine decarboxylase; b), and ODC1 (ornithine decarboxylase 1; c) in conceptus tissue. Conceptuses from MAO-ODC1:AGMAT (b) ewes had greater relative expression (P < 0.05) of ADC mRNAs compared with other treatments while conceptuses from MAO-ODC1:AGMAT (a) ewes expressed less (P < 0.05) mRNAs for ODC1. All quantitative data are presented as means and SEM
Expression of IFNT mRNA was less (P < 0.05) for conceptuses from MAO-ODC1:MAO-AGMAT (B) ewes compared with MAO-control (Fig. 7a). Conceptuses from MAO-ODC1, MAO-ODC1:MAO-ADC, and MAO-ODC1:MAO-AGMAT (B) ewes expressed less (P < 0.05) IGF2 mRNA only when compared with conceptuses from MAO-ADC ewes. There was no effect (P > 0.05) of MAO treatment on expression of IGF2 mRNA (Fig. 7b). Conceptuses from MAO-ODC1:MAO-AGMAT (A) ewes had greater (P < 0.05) expression of SLC22A1 (5.9-fold), SLC22A2 (9.8-fold), and SLC22A3 (8.1-fold) mRNAs than conceptuses from MAO-control ewes (Fig. 8a–c), while conceptuses from MAO-ODC1:AGMAT (B) ewes had even greater (P < 0.05) expression of SLC22A1 (41-fold), SLC22A2 (50-fold), and SLC22A3 (56-fold) compared with conceptuses from MAO-control ewes (Fig. 8a–c). There was not a significant effect of MAO treatment on expression of SLC7A1 by conceptuses (Fig. 8d).
Effects of knockdown of ODC1, ADC, ODC1:ADC, and ODC1:AGMAT on expression of mRNAs for IFNT (interferon tau; a) and IGF2 (Insulin growth factor II; b) in conceptus tissue. a Conceptuses from MAO-ODC1: MAO-AGMAT (B) ewes expressed less (P < 0.05) IFNT mRNA compared with conceptuses from MAO-control, MAO:ODC1, MAO-ADC, and MAO-ODC1:MAO-ADC ewes. b Conceptuses from MAO-ODC1, MAO-ODC1:ADC, and MAO-ODC1:MAO-AGMAT (b) ewes expressed less (P < 0.05) mRNA for IGF2 only when compared with conceptuses from MAO:ADC ewes. All quantitative data are presented as means and SEM
Effects of knockdown of ODC1, ADC, ODC1:ADC, and ODC1:AGMAT on expression of mRNAs for: a SLC22A1 (organic cation transporter type 1; b SLC22A2 (organic cation transporter type 2; c SLC22A3 (organic cation transporter type 3; and d) SLC7A1 (solute carrier family 7, organic cation transporter, y + system, member 1, d) in conceptus tissue. Conceptuses from MAO-ODC1:MAO-AGMAT (a, b) ewes had greater (P < 0.05) relative expression of mRNAs for SLC22A1, SLC22A2, and SLC22A3 than conceptuses from MAO-control ewes. There was no effect of MAO on expression of SLC7A1 by conceptuses. All quantitative data are presented as means and SEM
In vivo knockdown of translation of ODC1, ADC, and AGMAT mRNAs alone and in combination reduced the abundance of agmatine, putrescine, spermindine, and spermine in conceptus tissue, while MAO-ODC1:AGMAT (B) ewes had increased abundances of agmatine, putrescine, and spermidine and reduced the amounts of spermine in uterine flushes
High-performance liquid chromatography was used to determine abundances of polyamines and agmatine in conceptus tissue. Agmatine, putrescine, spermidine, and spermine were less abundant (P < 0.05) in conceptus tissue from MAO-ODC1:MAO-AGMAT (B) ewes compared with MAO control ewes (Fig. 9a). The amounts of agmatine, putrescine, and spermidine were greater (P < 0.05) in conceptus tissues from MAO-ODC1 ewes, while the abundance of spermine was not different compared with MAO control ewes (Fig. 9a–d).
The abundances of agmatine, putrescine, spermidine, and spermine in ovine conceptuses following inhibition of translation of mRNAs for ODC1, ADC, and AGAMT alone or in combination. In vivo knockdown of both ODC1 and AGMAT in conceptuses decreased the concentrations (nmol/g of tissue) of agmatine (a), putrescine (b), spermidine (c), and spermine (d), while conceptuses from MAO-ODC1 ewes had greater (P < 0.05) amounts of agmatine, putrescine, and spermidine compared with MAO-control ewes. Means with different superscript letters are different (P < 0.05). All quantitative data are presented as means and SEM
Uterine flushes from MAO-ODC1:MAO-AGAMT (B) ewes had greater amounts (P < 0.05) of agmatine, putrescine, and spermidine and less (P < 0.05) of spermine compared with MAO-control ewes (Fig. 10a–c). Uterine flushings from MAO-ODC1:MAO-AGAMT (A) ewes had greater amounts (P < 0.05) of putrescine (Fig. 10b) and less (P < 0.05) of spermine compared with MAO-control ewes (Fig. 10d).
The abundances of agmatine, putrescine, and spermidine in uterine flushings of ewes in which translation of mRNAs for ODC1, ADC, and AGAMAT alone or in combination was inhibited. In vivo knockdown of translation of ODC1 in combination with AGMAT in ovine conceptuses increased the abundances (P < 0.05) of agmatine (a), putrescine (b), and spermidine (c) (nmol) in uterine flushes, while conceptuses from MAO-ODC1:MAO-AGMAT (a, b) ewes had less spermine (P < 0.05). Means with different superscript letters were different (P < 0.05). All quantitative data are presented as means and SEM
Discussion
Polyamines are organic cations in all cells as they are essential for cell–cell interactions, cell signaling, and proliferation, differentiation and migration of cells (Sala-Rabanal et al. 2013). During the peri-implantation period of pregnancy, polyamines play a crucial role in regulating gene expression, RNA translation, DNA and protein synthesis, and production of IFNT, as well as regulation of angiogenesis and stimulation of placental growth (Wu et al. 2004; Wang et al. 2014).
This is the first report of the effect of Agm on proliferation of a trophectoderm cell line and of the in vivo knockdown of translation of mRNAs for both ODC1 and AGMAT in ovine conceptuses. We further evaluated the effect of different concentrations of Agm on the proliferation of oTr1 cells and found no stimulatory effects of Agm. Those results confirmed those in our previous report (Lenis et al. 2016). However, in the present study, 100 and 150 μM doses of Agm significantly decreased oTr1 cell proliferation when compared to proliferation of oTr1 cells in culture medium alone. It has also been reported that Agm inhibits proliferation of tumor cell lines (Wolf et al. 2007). On the other hand, when the proliferation of oTr1 cells cultured in BM was compared with that for cells grown in CM, a decrease in cell proliferation was found. This supports the idea that commercial cell culture media are designed to improve cell viability, proliferation, and metabolic activity when compared to the custom media manufactured in the laboratory (Jayme et al. 1997).
In the present study, knockdown of ODC1 or ADC did not significantly disrupt development of the conceptus or its functionality assessed as production of IFNT by trophectoderm cells. However, 20% of conceptuses from MAO-ODC1:MAO-ADC ewes were fragmented. However, for MAO-ODC1:MAO-AGAMT (B) the percentage of fragmentation was 100% and they had greater amount of total protein in uterine flushes compared with MAO-control ewes. We previously reported greater amounts of the total protein in uterine flushes containing fragmented conceptuses. Further, MAO-ODC1:MAO-AGMAT (A) and MAO-ODC1:MAO-AGMAT (B) ewes had lower (P < 0.05) pregnancy rates compared to other MAOs treatments. These results indicate that production of polyamines by the conceptus is an essential process for growth and development, as well as secretion of IFNT for establishment and maintenance of pregnancy in sheep (Wang et al. 2014).
Our previous studies evaluated the single effect of in vivo translational knockdown of ODC1 mRNA in ovine conceptuses during the peri-implantation period of pregnancy (Wang et al. 2014). That study led to the discovery of an alternative pathway to produce polyamines (ADC/AGMAT) which was sufficient to support growth and development of one-half of the ovine conceptuses deficient in ODC1 mRNA translation, while the other 50% of the conceptuses were retarded in their development (Wang et al. 2014).
In a subsequent study, the inhibition of translation of both ODC1 and ADC mRNAs also resulted in two phenotypes of conceptuses. Thirty-three percent of the conceptuses were morphologically normal and 67% of the conceptuses were morphologically and functionality abnormal. Interestingly the abundances of Agm, putrescine, and spermidine were greater for conceptuses from ewes in which there was double knockdown of ODC1 and ADC proteins compared with conceptuses from control ewes. Additionally, for abnormal conceptuses from double knockdown (ODC1:ADC) only the amount of Agm was greater in tissues. Those results indicated that Agm is produced by uterine epithelia and that the amount of Agm in histotroph was sufficient to allow some ovine conceptuses to survive the knockdown of translation of both ODC1 and ADC mRNAs. Thus, the arginase:ODC1 metabolic pathway can compensate for the ADC:AGMAT pathway for synthesis of polyamines and vice versa in an attempt to maintain the pool of polyamines in the conceptus for homeostasis (Wang et al. 2014). In the present study, the conceptuses were not able to use the Agm from histotroph due to knockdown of translation of AGMAT.
IFNT is the antiluteolytic pregnancy recognition signaling hormone required for maintenance of a functional corpus luteum which produces progesterone in ruminants, the essential hormone for pregnancy, (Bazer et al. 1997, 2010b, 2012; Spencer et al. 2006). During maternal recognition of pregnancy, the integrity of the mononuclear cells of the conceptus trophectoderm determinate the adequate production and secretion of IFNT between Days 10 and 21 to 25 of pregnancy (Spencer and Bazer 2004). We determined the effect of double knockdown ODC1 and AGMAT mRNAs to prevent utilization of Agm by the conceptus. Interestingly, only 22% of the conceptuses exhibited a normal morphological phenotype, while 78% of the conceptuses failed to elongate and showed an abnormal morphological phenotype and functionality based in the significant decrease in secretion of IFNT. These results confirmed that the integrity and functionality of ovine conceptus is reflected in its production of IFNT during the peri-implantation period of pregnancy (Spencer et al. 1999; Bazer et al. 1997; Wang et al. 2014).
IGF1 and IGF2 possess mitogenic and differentiative properties related with development of the conceptus and placental development in humans and domestic animals (Satterfield et al. 2008). The biological action of the IGFs depends on interactions between IGF1 and IGF2 and their receptors, insulin growth factor 1 (IGF1R) and insulin growth factor 2 (IGF2R), as well as IGF binding proteins (IGFBP 1-7).
Both IGF1 and IGF2 are produced by conceptuses and endometrial tissue and they are components of uterine luminal histotroph in cattle and sheep (Irwin et al. 1999). Our previous results indicated that IGF1 and IGF2 are essential for blastocyst growth and development (Satterfield et al. 2008). IGF2 activates cell signaling in conceptus tissue such as phosphatidylinositol 3-kinase (PI3 K)/protooncogenic protein kinase 1 (AKT1), mammalian target of rapamycin (mTOR), ribosomal protein S6 kinase (RPS6 K), ERK1/2, and P38 MAPK (Kim et al. 2008). However, in the present study, there were no significant differences in IGF2 gene expression between conceptuses from MAO:ODC1-MAO-AGMAT (B) and MAO-control ewes due to extreme differences among ewes in conceptus development. However, MAO-ODC1, MAO-ODC1:MAO-ADC, and MAO:ODC1-MAO-AGMAT (B) phenotype conceptuses expressed less IGF2 mRNA than MAO-ADC conceptuses that were morphologically and functionally normal.
Polyamines, Arg and Agm are transported from histotroph to conceptus tissue (Bazer et al. 2011; Wang et al. 2015; Lenis et al. 2016) as they are essential for conceptus development during the peri-implantation and placentation periods of pregnancy (Zhao et al. 2008; Wang et al. 2014). The solute carrier transporter family is involved in transport of polyamines and it is the second largest group of membrane proteins in the human genome with 384 members (Abdulhussein and Wallace 2014). Transport of polyamines into conceptus tissue is also essential for its development during pregnancy (Wang et al. 2014). The abundance of Arg in the uterine lumen and the expression of SLC7A1 and SLC7A2 mRNAs in uterine epithelia and conceptuses, respectively, increased during the peri-implantation period of pregnancy which coincides with period of rapid elongation of ovine conceptuses (Gao et al. 2009a, b). However, in the present study, there was no effect of MAO treatment on expression of the Arg transporter (SLC7A1) in ovine conceptuses.
The homeostatic mechanism to maintain stable protein levels in cells is a coordinated process between protein synthesis, post-translational modifications, transport, and degradation. Ribosomes are involved in those biological processes for ensuring accurate translation of mRNAs for the proteins (Hilal and Spahn 2015). The regulation of gene transcription and translation of mRNAs for proteins depends on both the intracellular context and requirements of the cell. For certain proteins, mRNA translation is regulated by different biological process such as stabilization and destabilization of the mRNAs (DiFederico et al. 1999).
In the present study, MAO:ODC1-MAO-AGMAT (A) conceptuses were morphologically and functionally normal, and expression of mRNAs for SLC22A1 (5.9-fold), SLC22A2 (9.8-fold), and SLC22A3 (8.1-fold) was greater than for conceptuses from MAO-control ewes. Interestingly, for MAO:ODC1-MAO-AGMAT (B) conceptuses, there was even a greater increase in expression of mRNAs for SLC22A1 (41-fold), SLC22A2 (50-fold), and SLC22A3 (56-fold), and ADC (126-fold) when compared with conceptuses from MAO-control ewes. Inhibition of both ODC1 and AGMAT mRNAs reduces the capacity of the trophectoderm to synthesize polyamines and this has been linked to increases in expression of polyamine transporters to increase their intracellular levels (Moinard et al. 2005). The overexpression of SLC22 family members, as well as ADC in conceptuses from MAO:ODC1-MAO-AGMAT (A) and MAO:ODC1-MAO-AGMAT (B) ewes, suggests that the conceptuses are modifying gene expression to assure access to available polyamines required for their survival. However, conceptuses from MAO:ODC1-MAO-AGMAT (B) ewes mostly failed to develop due to the absence of both ODC1 and AGMAT for synthesis of putrescine and, presumably, an inability to take up sufficient polyamines from the uterine luminal fluid (Fig. 10).
Deficiencies in different mechanisms may explain the disruption of phenotype in conceptuses with double knockdown ODC1 and AGMAT mRNAs. The morphological and functional abnormalities are likely due to insufficient concentrations of polyamines which would adversely affect ribosome assembly and translation of mRNAs critical for development of the conceptus. Recent studies using inhibitors of polyamine transporters in combination with difluoromethylornithine (irreversible inhibitor of ornithine decarboxylase) demonstrated that cellular depletion of polyamines resulted in the accumulation of mRNAs which activated apoptosis in a cancer model (Hilal and Spahn 2015). The substantial increase in expression of mRNAs for the SLC22A family members and ADC may increase the abundance of specific micro-RNAs (miRNAs) that inhibit translation of mRNAs and increase degradation of proteins. Mir132 is an example of one miRNA that decreases the abundance of proteins without modification of mRNAs, while Mir125b leads to degradation of both proteins and mRNAs (Fig. 11) (Baek et al. 2008).
In summary, polyamines are critical for the integrity and functionality in the ovine conceptuses and for a successful outcome of pregnancy in sheep. In vivo knockdown of translation of both ODC1 and AGMAT RNAs resulted in over-expression of genes related to synthesis and transport of polyamines in ovine conceptuses during the peri-implantation period of pregnancy. This suggests that the depletion of polyamines in ovine conceptuses is highly detrimental to their survival and development.
Abbreviations
- ADC :
-
Arginine decarboxylase
- AGMAT:
-
Agmatinase
- Arg:
-
l-arginine
- Agm:
-
Agmatine
- OAZ:
-
Antienzyme
- MAO:
-
Morpholino
- IFNT:
-
Interferon tau
- IGF2:
-
Insulin growth factor type 2
- NO:
-
Nitric oxide
- ODC1 :
-
Ornithine decarboxylase
- SLC22A1:
-
Organic cationic transporter type 1
- SLC22A2:
-
Organic cationic transporter type 2
- SLC22A3:
-
Organic cationic transporter type 3
- SLC7A1:
-
Solute carrier family 7 member 1
References
Abdulhussein AA, Wallace HM (2014) Polyamines and membrane transporters. Amino acids 46(3):655–660
Arqués O, Chicote I, Tenbaum et al (2012) Standardized relative quantification of immunofluorescence tissue staining. Protoc Exch 10. https://doi.org/10.1038/protex.2012.008
Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP (2008) The impact of microRNAs on protein output. Nature 455:64–71
Bazer FW, Spencer TE, Ott TL (1997) Interferon tau: a novel pregnancy recognition signal. Am J Reprod Immunol 37:412–420
Bazer FW, Spencer TE, Johnson GA et al (2010a) Uterine receptivity to implantation of blastocysts in mammals. Front Biosci (Schol Ed) 3:745–767
Bazer FW, Wu G, Spencer TE et al (2010b) Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod 16:135–152
Bazer FW, Wu G, Johnson GA et al (2011) Uterine histotroph and conceptus development: select nutrients and secreted phosphoprotein 1 affect mechanistic target of rapamycin cell signaling in ewes. Biol Reprod 85:1094–1107
Bazer FW, Kim J, Song G et al (2012) Select nutrients, progesterone, and interferon tau affect conceptus metabolism and development. Ann N Y Acad Sci 1271:88–96
Bazer FW, Wu G, Johnson GA et al (2014) Environmental factors affecting pregnancy: endocrine disrupters, nutrients and metabolic pathways. Mol Cell Endocrinol 398:53–68
Dai Z, Wu Z, Wang Jia S, Bazer FW, Wu G (2014) Analysis of polyamines in biological samples by HPLC involving pre-column derivatization with O-phthalaldehyde and N-acetyl-L-cysteine. Amino Acids 46:1557–1564
DiFederico E, Genbacev O, Fisher SJ (1999) Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol 155:293–301
Gao H, Wu G, Spencer TE et al (2009a) Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod 80:86–93
Gao H, Wu G, Spencer TE et al (2009b) Select nutrients in the ovine uterine lumen. III. Cationic amino acid transporters in the ovine uterus and peri-implantation conceptuses. Biol Reprod 80:602–609
Gray CA, Burghardt RC, Johnson GA et al (2002) Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 124:289–300
Gray CA, Abbey CA, Beremand PD et al (2006) Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biol Reprod 74:383–394
Hilal T, Spahn CM (2015) Ribosome rescue and protein quality control in concert. Mol Cell 57:389–390
Irwin JC, Suen LF, Martina NA et al (1999) Role of the IGF system in trophoblast invasion and pre-eclampsia. Hum reprod 14(2):90–98
Jayme D, Watanabe T, Shimada T (1997) Basal medium development for serum-free culture: a historical perspective. Cytotechnology 23:95–101
Kim J, Wende AR, Sena S et al (2008) Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol 22:2531–2543
Kong X, Wang X, Yin Y et al (2014) Putrescine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. Biol Reprod 91(5):106–111
Kwon H, Wu G, Bazer FW et al (2003) Developmental changes in polyamine levels and synthesis in the ovine conceptus. Biol Reprod 69:1626–1634
Lefèvre PL, Palin MF, Murphy BD (2011) Polyamines on the reproductive landscape. Endocr Rev 32(5):694–712
Lenis YY, Wang X, Tang W et al (2016) Effects of agmatine on secretion of interferon tau and catecholamines and expression of genes related to production of polyamines by ovine trophectoderm cells. Amino Acids 48(10):2389–2399
LeRoith D, Werner H, Beitner-Johnson D et al (1995) Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev 16(2):143–163
Moinard C, Cynober L, de Bandt JP (2005) Polyamines: metabolism and implications in human diseases. Clin Nutr 24(2):184–197
Molderings GJ, Haenisch B (2012) Agmatine (decarboxylated l-arginine): physiological role and therapeutic potential. Pharmacol Ther 133:351–365
Piletz JE, Aricioglu F, Cheng JT et al (2013) Agmatine: clinical applications after 100 years in translation. Drug Discov Today 18(17):880–893
Raspotnig G, Fauler G, Jantscher A (1999) Colorimetric determination of cell numbers by janus green staining. Anal Biochem 275(1):74–83
Rechler MM, Nissley SP (1985) The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol 47(1):425–442
Rezaei R, Knabe A, Tekwe D et al (2013) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44(3):911–923
Sala-Rabanal M, Li DC, Dake GR et al (2013) Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol Pharm 10(4):1450–1458
Sastre M, Regunathan S, Galea E et al (1996) Agmatinase activity in rat brain: a metabolic pathway for the degradation of agmatine. J Neurochem 67(4):1761–1765
Satterfield MC, Hayashi K, Song G et al (2008) Progesterone regulates FGF10, MET, IGFBP1, and IGFBP3 in the endometrium of the ovine uterus. Biol Reprod 79(6):1226–1236
Sekowska A, Bertin P, Danchin A (1998) Characterization of polyamine synthesis pathway in Bacillus subtilis 168. Mol Microbiol 29(3):851–858
Spencer TE, Bazer FW (2004) Uterine and placental factors regulating conceptus growth in domestic animals. J Anim Sci 82:4–13
Spencer TE, Gray A, Johnson GA et al (1999) Effects of recombinant ovine interferon tau, placental lactogen, and growth hormone on the ovine uterus. Biol Reprod 61:1409–1418
Spencer TE, Johnson GA, Bazer FW et al (2004) Implantation mechanisms: insights from the sheep. Reproduction 128:657–668
Spencer TE, Johnson GA, Bazer FW et al (2006) Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses. Reprod Fertil Dev 19:65–78
Spencer TE, Forde N, Lonergan P (2017) Insights into conceptus elongation and establishment of pregnancy in ruminants. Reprod Fertil Dev 29:84–100
Wang X, Wei Y, Dunlap KA et al (2014) Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod 90:84
Wang X, Burghardt RC, Romero JJ et al (2015) Functional roles of arginine during the peri-implantation period of pregnancy. III. Arginine stimulates proliferation and interferon tau production by ovine trophectoderm cells via nitric oxide and polyamine-TSC2-MTOR signaling pathways. Biol Reprod 92:75
Wolf C, Brüss M, Hänisch B et al (2007) Molecular basis for the antiproliferative effect of agmatine in tumor cells of colonic, hepatic, and neuronal origin. Mol Pharmacol 71(1):276–283
Wu G, Meininger CJ (2000) Arginine nutrition and cardiovascular function. J Nutr 130:2626–2629
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Bazer FW, Cudd TA et al (2004) Maternal nutrition and fetal development. J Nutr 134:2169–2172
Wu G, Bazer FW, Davis TA et al (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wu G, Bazer FW, Satterfield MC et al (2013) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Zhao YC, Chi YJ, Yu YS et al (2008) Polyamines are essential in embryo implantation: expression and function of polyamine-related genes in mouse uterus during peri-implantation period. Endocrinology 149(5):2325–2332
Acknowledgements
Funding from the Sustainability Strategy 2013–2014, from CODI University of Antioquia (UdeA), Medellín, Colombia Scholarship “Becas Doctorado UdeA 2014” was used to support YYL, a PhD student in Veterinary Science, Faculty of Agrarian Science, Antioquia University). Funding for the research and related activities was from the Agriculture and Food Research Initiative Competitive Grants (2016-67015-24958 to FWB and 2015-67015-23276 to GW) from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All experimental and surgical procedures were approved by the Institutional Animal Care and Use Committee at Texas A&M University.
Additional information
Handling Editor: F. Erdmann.
Rights and permissions
About this article
Cite this article
Lenis, Y.Y., Elmetwally, M.A., Tang, W. et al. Functional roles of agmatinase during the peri-implantation period of pregnancy in sheep. Amino Acids 50, 293–308 (2018). https://doi.org/10.1007/s00726-017-2515-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2515-1