Abstract
The daily requirement of a 70-kg male for creatine is about 2 g; up to half of this may be obtained from a typical omnivorous diet, with the remainder being synthesized in the body Creatine is a carninutrient, which means that it is only available to adults via animal foodstuffs, principally skeletal muscle, or via supplements. Infants receive creatine in mother’s milk or in milk-based formulas. Vegans and infants fed on soy-based formulas receive no dietary creatine. Plasma and muscle creatine levels are usually somewhat lower in vegetarians than in omnivores. Human intake of creatine was probably much higher in Paleolithic times than today; some groups with extreme diets, such as Greenland and Alaskan Inuit, ingest much more than is currently typical. Creatine is synthesized from three amino acids: arginine, glycine and methionine (as S-adenosylmethionine). Humans can synthesize sufficient creatine for normal function unless they have an inborn error in a creatine-synthetic enzyme or a problem with the supply of substrate amino acids. Carnivorous animals, such as lions and wolves, ingest much larger amounts of creatine than humans would. The gastrointestinal tract and the liver are exposed to dietary creatine in higher concentrations before it is assimilated by other tissues. In this regard, our observations that creatine supplementation can prevent hepatic steatosis (Deminice et al. J Nutr 141:1799–1804, 2011) in a rodent model may be a function of the route of dietary assimilation. Creatine supplementation has also been reported to improve the intestinal barrier function of the rodent suffering from inflammatory bowel disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Creatine intake is a hot topic with athletes. It has been shown that there was an ergogenic effect of creatine during resistance training and that subjects with initially low levels of intramuscular creatine obtained better results with dietary supplementation of creatine (Burke et al. 2003). Creatine is only known to be required for a single enzymatic reaction, that of creatine kinase which interconverts creatine and phosphocreatine in tissues with a rapid, high demand for ATP (Wallimann et al. 2011). More than 90 % of the body’s creatine and phosphocreatine is present in muscle (Brosnan and Brosnan 2007), with some of the remainder being found in the brain (Braissant et al. 2011). Creatine turnover occurs by way of a simple, nonenzymatic chemical dehydration to creatinine which is removed in the urine. About 1.5–2.0 % of the body’s pool of creatine and phosphocreatine is thought to be lost each day by such spontaneous removal (Crim et al. 1975). Thus, an equivalent amount of creatine must be replaced each day by synthesis and/or dietary intake.
Creatine synthesis
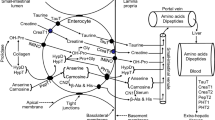
Creatine synthesis is a relatively straightforward process, involving only two enzymes. Arginine:glycine amidinotransferase (AGAT) is present in kidney (Guthmiller et al. 1994), brain (Braissant et al. 2001) and pancreas (Da Silva et al. 2014a). It condenses the amino acids arginine and glycine to give guanidinoacetic acid (GAA). The second enzyme, guanidinoacetic acid methyltransferase (GAMT) is present in liver (Ogawa et al. 1988), brain (Braissant et al. 2001) and pancreas (to a much lesser extent, Da Silva et al. 2014a). It transfers a methyl group from S-adenosylmethionine (SAM) to GAA to give creatine. Most of the body’s supply of creatine is synthesized in liver, but it is believed that creatine for brain can be synthesized within the brain itself (Braissant et al. 2001). Creatine synthesized in liver must be secreted into the bloodstream by an unknown mechanism (Da Silva et al. 2014a) and then actively transported into muscle by the creatine transporter (SLC6A8), a Na+- and Cl− -dependent symporter (Brosnan and Brosnan 2007). The steps of creatine synthesis and transport are shown in Fig. 1. There is a small amount of SLC6A8 at the blood–brain barrier, so creatine can be moved from blood to brain, but only slowly (Braissant 2012).
Inborn errors have been recognized in AGAT, GAMT and SLC6A8 with very serious consequences: deficiency of creatine in brain and muscle, mental retardation, seizures and speech delay (Sykut-Cegielska et al. 2004). In humans, brain is much more severely affected than muscle, either because muscle has another mechanism to transport creatine (Pyne-Geithman et al. 2004) or because dietary creatine has much freer access to muscle and heart than to brain, as shown by creatine uptake studies in rodents (Ipsiroglu et al. 2001). In the case of the brain, the lower access of peripheral creatine to the CNS is the low permeability of the blood:brain barrier for creatine due to a lack of transporters in the astrocytes surrounding the blood:brain barrier (Braissant et al. 2001). Muscle energy metabolism is seriously disturbed in AGAT knockout mice on creatine-free chow because of creatine deficiency and it can be reversed by treatment with dietary creatine (Nabuurs et al. 2013). It has been noted that supplementation with large doses of creatine in AGAT or GAMT deficiency will normalize the situation if started at birth (Schulze and Battini 2007; Braissant et al. 2011). To date, there is no satisfactory treatment for the transporter defect (Braissant et al. 2011). The inheritance of these genetic disorders of creatine synthesis is autosomal recessive and they are thought to be relatively rare, although probably underdiagnosed (Sykut-Cegielska et al. 2004). It has been argued that AGAT and GAMT deficiency disorders should be included in newborn screening since early detection can lead to very positive results for the infant (Longo et al. 2011; Pasquali et al. 2014). Little information is available on the frequency of heterozygosity and characteristics of carriers for these disorders. In a study of two Tunisian siblings with GAMT deficiency, both parents showed levels of GAMT activity of about 20–25 % of control levels (Nasrallah et al. 2012). Another study involving relatives showed increased GAA in one individual when the patient had GAMT deficiency, and low plasma GAA and creatine + creatinine in a relative when the patient had AGAT deficiency (Carducci et al. 2002). There is also one recent report on an individual showing somewhat elevated urinary GAA and low creatine; this probably represents a heterozygous GAMT deficiency (Nasrallah et al. 2015). Thus it is possible that some heterozygotes would need to receive at least part of their creatine from the diet when their own creatine synthesis is compromised.
Creatine synthesis requires arginine and glycine for the first step, and SAM as a methyl donor in the second step. Both arginine and glycine can be synthesized by mammals, but especially young growing animals may need supplementary arginine and glycine in the diet (Wu et al. 2004; Wang et al. 2013). In patients with an inborn error of the urea cycle, other than arginase I deficiency, arginine becomes a dietary essential amino acid (Brosnan and Brosnan 2010). If there are insufficient substrates, creatine synthesis would be curtailed. The best sources of arginine and glycine are also very good sources of creatine, so a deficient diet could put the person or animal in double jeopardy. The availability of SAM depends on the supply of one-carbon groups, which in turn depends on the level of folate, vitamin B12, vitamin B2, and one-carbon precursors such as serine, glycine, histidine, tryptophan and choline (Brosnan et al. 2015). Supplementation of the diet of rats with creatine conserves SAM and decreases plasma homocysteine (Stead et al. 2001), so it might be expected that such supplementation would overcome deficiencies in the one-carbon supply for creatine synthesis and conserve the groups for other essential pathways. In neonatal piglets, plasma homocysteine can be decreased by giving an oral supplement containing betaine, choline, creatine and vitamin B6, whereas none of the individual components was effective alone (Cote-Robitaille et al. 2015). These studies are relatively clear-cut in experimental animals where completely adequate diets are fed. The situation in humans is much less clear, with some reports that creatine supplementation does decrease plasma homocysteine (McCarty 2001) and others that it does not (Steenge et al. 2001). A recent study by Peters et al. (2015) on Bangladeshi adults who were taking 3 g of creatine per day did not show a significant decrease in total homocysteine in plasma. The creatine groups, however, did experience a decline in GAA and in those receiving creatine and folic acid, the decline in GAA was significantly associated with a decline in plasma total homocysteine. It should also be noted that more than 50 % of the subjects in this study had hyperhomocysteinemia so there are probably many factors affecting homocysteine concentrations in addition to methyl supply. In an earlier study, 21 g of creatine, and even more so a combination treatment with 9 g of arginine plus 21 g of creatine, given for 4 days to human subjects resulted in an increase of homocysteine levels (Jahangir et al. 2009). Changes in homocysteine induced by creatine supplementation may depend on the C677T polymorphism of methylenetetrahydrofolate reductase (MTHFR) (Petr et al. 2013). In addition, acute variations especially in the reduced form of homocysteine, as opposed to total homocysteine, are related to changes in serum creatine levels induced by physical exercise (Sotgia et al. 2007).
It is known that creatine synthesis is regulated physiologically in kidney by down-regulation of AGAT, both at the transcriptional and activity levels in animals (McGuire et al. 1984) and in humans (Derave et al. 2004). More recently, AGAT retroregulation by creatine levels has been shown in developing brain cells (Hanna-El-Daher et al. 2015). Thus, taking a creatine supplement has been shown to decrease the rate of endogenous synthesis, shifting the metabolic burden of creatine synthesis away from the recipient animal or person. Such a decrease would be expected to result in conservation of arginine, glycine and SAM.
Creatine intake from the diet
Creatine is a “carninutrient”, which means that it is only available in the diet via animal foodstuffs, primarily from muscle meats (including fish) and, to a lesser extent, from dairy products. It is interesting to note that liver contains only about 10 % as much creatine as does muscle meat (Harris et al. 1997). We have reported that infants receive some creatine in mothers’ milk, or somewhat more in milk-based formulas, but much less or none in milk-free or entirely soy-based formula (Edison et al. 2013). Lacto-ovo vegetarians receive little dietary creatine (Solis et al. 2014), while vegans and infants fed on soy-based formulas receive no dietary creatine (Edison et al. 2013). Thus normal dietary intake of creatine is low in milk-fed infants, forcing them to synthesize about 90 % of their daily requirement (Edison et al. 2013). This observation has important implications for neonatal amino acid metabolism and for infants suffering from creatine deficiency syndromes. The relatively small amounts of creatine coming from milk or formula would not help very much in overcoming the problems of an inborn error of creatine synthesis since these disorders typically require relatively high therapeutic doses of creatine (Sykut-Cegielska et al. 2004) so it is essential to recognize an inborn error of creatine synthesis at birth to enable immediate creatine supplementation. A recent study by Solis et al. (2014) reported that vegetarians had low plasma creatine but that brain levels were the same as those in omnivores, indicating that dietary creatine intake did not determine the brain creatine concentration. On the other hand, muscle of vegetarians did contain less creatine than that of omnivores (Burke et al. 2003). These observations provide further evidence for endogenous synthesis of creatine in the CNS (Braissant et al. 2001).
Dietary creatine is readily absorbed regardless of the source (Harris et al. 2002), although there can be some losses during cooking depending upon the conditions used (Harris et al. 1997). Boiling meat causes an increased conversion of creatine to creatinine, especially at low pH (Harris et al. 1997); boiling either chicken breast or stewing beef for 20 min retained about 90 % of the creatine in the raw meat, whereas more prolonged boiling (up to 60 min) lost up to 30 % of the original creatine. In addition, some creatine is leached into the cooking fluid and would be lost if the broth were discarded (Purchas et al. 2004). Cambero et al. (1992) reported no change in creatine content of beef broth heated at temperatures from 55 to 95 °C for 60 min. Thus, simply considering the creatine content of raw meat is not an accurate guide to dietary creatine intake. This problem becomes particularly important when trying to determine the creatine intake of our ancestor, Paleolithic man, since it is not clear how the meat was handled before it was consumed. Many groups did, and some still do, eat raw fish (sushi) or meat (steak tartare) or cured fish (smoked salmon) or cured meat. For example, 50 years ago Alaskan Arctic Eskimos were reported to eat much of their meat uncooked (Ho et al. 1972). People in a number of countries use air-drying to preserve fish or meat, a method which is reported to preserve the creatine content of the food (Harris et al. 1997). This is also observed in salted and dry-cured ham, even after months of dry-curing (Mora et al. 2010). Whatever the state of the meat or fish, it certainly has more creatine than other foods. Hunter-gatherer societies often ate large amounts of animal foods; for example, diets of Alaskan and Greenland natives consisted of more than 95 % animal foods (Cordain et al. 2002) so their intake of creatine would be much higher than ours is today. The estimated intake of food from animal/fish sources by a variety of hunter-gatherer societies ranged from 0.5 to 1.0 kg per day (Kuipers et al. 2010), providing approximately 2 to 4 g creatine per day in the raw food (Harris et al. 1997).
Wild carnivores, such as wolves and lions, ingest much creatine. For example, a 35 kg wolf would eat about 4 kg of meat per day with a creatine content of approximately 30 mmol/kg, thus providing about 16 g creatine per day (Harris et al. 1997). There is no information available on creatine synthesis or concentrations in these animals, but this amount is likely to be more creatine than the wolf would require in a day.
Newly reported effects of creatine
Some effects of creatine may be related to the route taken in the assimilation of dietary creatine, for instance the gastrointestinal tract and the liver are both exposed to dietary creatine before it is taken up by muscles and other tissues.
Intestine
Intestinal epithelial cells are held together by tight and adherens junctions in the apical junctional complex (Ivanov et al. 2010). These structures are in close contact with the F-actin filaments of the cytoskeleton, and ATP hydrolysis is involved in their maintenance (Ivanov et al. 2010). These structures are illustrated in Fig. 2. In inflammatory states, the barrier function of the apical junction is disordered: intestinal epithelial cells are exposed to a hypoxic environment. Hypoxia-inducible transcription factors (HIFs) are activated in hypoxic situations. In the case of inflammatory bowel disease, HIF upregulates genes for creatine kinase and creatine supplementation in mouse colitis models has been shown to attenuate the inflammatory response (Glover et al. 2013). This may have clinical consequences, since chronic inflammation associated with intestinal colitis is a major risk factor for colitis-associated colon cancer. Thus, these observations provide a compelling argument for creatine supplementation as an adjuvant therapy to promote epithelial restitution and ameliorate mucosal inflammation via enhanced cellular energetics of intestinal epithelial cells (Glover et al. 2013).
Brain
In a recent review, Joncquel-Chevalier Curt and coworkers propose that creatine is a neurotransmitter in the CNS which might modulate GABAergic and/or glutamatergic neurons (Jonquel-Chevalier Curt et al. 2015).
Liver
We have observed that creatine supplements given to rats fed a high-fat diet (35 % fat by weight) prevent the hepatic steatosis which is usually observed in this model (Deminice et al. 2011). Creatine supplementation has a similar effect on Zucker fatty rats on a normal rodent diet (7 % fat by weight) (unpublished observations). Liver would not normally contain much creatine since it synthesizes it for other tissues, but in the supplemented rats the 11.7-fold elevation of creatine concentration in plasma resulted in a sevenfold increase in creatine in liver (unpublished observations). Healthy liver does not express creatine kinase (Brosnan et al. 1990) so there must be some more direct effect of creatine rather than phosphocreatine on lipid synthesis or oxidation or on energy metabolism (da Silva et al. 2014b). It will be interesting to see whether a simple creatine supplementation will also positively affect non-alcoholic fatty liver disease (NAFLD) in humans.
Adipose tissue
A new function for creatine in adipose tissue has been proposed by Kazak et al. (2015). They report that beige adipocytes (white adipocytes turning brown-like) can use a novel substrate cycle for thermogenesis. Mitochondria from these cells can use the mitochondrial creatine kinase isoform to phosphorylate creatine to phosphocreatine; creatine and inorganic phosphate may then be liberated from phosphocreatine with resultant heat production. Pharmacological inhibition of creatine synthesis diminishes this thermogenesis mechanism. These data are in accord with the previously reported phenotype of knockout mice with both cytosolic and mitochondrial brain-type isoforms deleted that present with a defect in thermoregulation and severe hypothermia when exposed to the cold (Streijger et al. 2009). These novel findings may have important implications for patients (and experimental animals) with genetic defects in creatine synthesis.
Conclusions and perspectives
Creatine is synthesized in the human from three amino acids, glycine, arginine and methionine. While sufficient creatine can be made in most people to replace the daily losses as creatinine, it appears that omnivores who ingest creatine in their diets tend to have higher levels of creatine in plasma and muscle than do vegetarians. Creatine synthesis in some individuals is compromised because of mutations in the genes encoding AGAT or GAMT. Patients who are homozygotes for an inborn error in one of these enzymes will generally need supplementary creatine but it is possible that even some heterozygotes have sufficiently impaired creatine synthesis so that they too might benefit from a dietary supplement. Inborn errors of glycine, arginine or SAM synthesis may also interfere with creatine synthesis, requiring an exogenous source of creatine.
Abbreviations
- AGAT:
-
Arginine:glycine amidinotransferase
- GAA:
-
Guanidinoacetic acid
- GAMT:
-
Guanidinoacetate methyltransferase
- HIF:
-
Hypoxia inducible transcription factor
- SAM:
-
S-adenosylmethionine
- SLC6A8:
-
Creatine transporter
References
Braissant O (2012) Creatine and guanidinoacetate transport at blood-brain and blood-cerebrospinal fluid barriers. J Inherit Metab Dis 35:655–664
Braissant O, Henry H, Loup M, Eilers B, Bachmann C (2001) Endogenous synthesis and transport of creatine in the rat brain: an in situ hybridization study. Mol Brain Res 86:193–201
Braissant O, Henry H, Béard E, Uldry J (2011) Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids 40:1315–1324
Brosnan JT, Brosnan ME (2007) Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 27:241–261
Brosnan JT, Brosnan ME (2010) Creatine metabolism and the urea cycle. Mol Genet Metab 100:S49–S52
Brosnan MJ, Chen L, Van Dyke TA, Koretsky AP (1990) Free ADP levels in transgenic mouse liver expressing creatine kinase. Effects of enzyme activity, phosphagen type, and substrate concentration. J Biol Chem 265:20849–20855
Brosnan ME, MacMillan L, Stevens JR, Brosnan JT (2015) Division of labour: how does folate metabolism partition between one-carbon metabolism and amino acid oxidation? Biochem J 472:135–146
Burke DG, Chilibeck PD, Parise G, Candow DG, Mahoney D, Tarnolpolsky M (2003) Effect of creatine and weight training on muscle creatine and performance in vegetarians. Med Sci Sports Exerc 35:1946–1955
Cambero MI, Seuss I, Honikel KO (1992) Flavor compounds of beef broth as affected by cooking temperature. J Food Sci 57:1285–1290
Carducci C, Birarelli M, Leuzzi V, Carducci C, Battini R, Cioni G, Antonozzi I (2002) Guanidinoacetate and creatine plus creatinine assessment in physiologic fluids: and effective diagnostic tool for the biochemical diagnosis of arginine:glycine amidinotransferase and guanidinoacetate methyltransferase deficiencies. Clin Chem 48:1772–1778
Cordain L, Eaton SB, Miller JB, Mann N, Hill K (2002) The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr 56:S42–S52
Cote-Robitaille M-E, Girard CL, Guay F, Matte JJ (2015) Oral supplementation of betaine, choline, creatine and vitamin B6 and their influence on the development of homocysteinaemia in neonatal piglets. J Nutr Sci 4:e31
Crim MC, Calloway DH, Margen S (1975) Creatine metabolism in men: urinary creatine and creatinine excretions with creatine feeding. J Nutr 105:428–438
Da Silva RP, Clow K, Brosnan JT, Brosnan ME (2014a) Synthesis of guanidinoacetate and creatine from amino acids by rat pancreas. Brit J Nutr 111:571–577
Da Silva RP, Kelly KB, Leonard K-A, Jacobs RL (2014b) Creatine reduces hepatic TG accumulation in hepatocytes by stimulating fatty acid oxidation. Biochim Biophys Acta 1841:1639–1646
Deminice R, da Silva RP, Lamarre SG, Brown C, Furey GN, McCarter SA, Jordao AA, Kelly KB, King-Jones K, Jacobs RL, Brosnan ME, Brosnan JT (2011) Creatine supplementation prevents the accumulation of fat in the livers of rats fed a high-fat diet. J Nutr 141:1799–1804
Derave W, Marescau B, vanden Eede E, Eijnde BO, de Deyn P, Hespel P (2004) Plasma guanidino compounds are altered by oral creatine supplementation in healthy humans. J Appl Physiol 97:852–857
Edison EE, Brosnan ME, Aziz K, Brosnan JT (2013) Creatine and guanidinoacetate content of human milk and infant formulas: implications for creatine deficiency syndromes and amino acid metabolism. Brit J Nutr 110:1075–1078
Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, Bayless AJ, Dobrinskikh E, Kendrick AA, Kelly CJ, Burgess A, Miller L, Kominsky DJ, Jedlicka P, Colgan SP (2013) Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci USA 110:19820–19825
Guthmiller P, Van Pilsum JF, Boen JR, McGuire DM (1994) Cloning and sequencing of rat kidney l-arginine:glycine amidinotransferase. Studies on the mechanism of regulation by growth hormone and creatine. J Biol Chem 269:17556–17560
Hanna-El-Daher L, Béard E, Henry H, Tenenbaum L, Braissant O (2015) Mild guanidinoacetate increase under partial guanidinoacetate methyltransferase deficiency strongly affects brain cell development. Neurobiol Dis 79:14–27
Harris RC, Lowe JA, Warnes K, Orme CE (1997) The concentration of creatine in meat, offal and commercial dog food. Res Vet Sci 62:58–62
Harris RC, Nevill M, Harris DB, Fallowfield JL, Bogdanis GC, Wise JA (2002) Absorption of creatine supplied as a drink, in meat or in solid form. J Sports Sci 20:147–151
Ho K-J, Mikkelson B, Lewis LA, Feldman SA, Taylor CB (1972) Alaskan Arctic Eskino: responses to a customary high fat diet. Am J Clin Nutr 25:737–745
Ipsiroglu OS, Stromberger C, Ilas J, Hӧger H, Mühl A, Stӧckler-Ipsiroglu S (2001) Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life Sci 69:1805–1815
Ivanov AI, Parkos CA, Nusrat A (2010) Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 177:512–524
Jahangir E, Vita JA, Handy D, Holbrook M, Palmisano J, Beal R, Loscalzo J, Eberhardt RT (2009) The effect of l-arginine and creatine on vascular function and homocysteine metabolism. Vasc Med 14:239–248
Jonquel-Chevalier Curt M, Voicu P-M, Fontaine M, Dessein A-F, Porchet N, Mention-Mulliez K, Dobbelaere D, Soto-Ares G, Cheillan D, Vamecq J (2015) Creatine biosynthesis and transport in health and disease. Biochimie 119:146–165
Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, Kajimura S, Gygi SP, Spiegelman BM (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163:643–655
Kuipers RS, Luxwolda MF, Dijck-Brouwer DAJ, Eaton SB, Crawford MA, Cordain L, Muskiet FAJ (2010) Estimated macronutrient and fatty acid intake from an East African Paleolithic diet. Br J Nutr 104:1666–1687
Longo N, Ardon O, Vanzo R, Schwartz E, Pasquali M (2011) Disorders of creatine transport and metabolism. Am J Med Genet 157:72–78
McCarty MF (2001) Supplemental creatine may decrease serum homocysteine and abolish the homocysteine “gender gap” by suppressing endogenous creatine synthesis. Med Hypotheses 56:5–7
McGuire DM, Gross MD, Van Pilsum JF, Towle HC (1984) Repression of rat kidney l-arginine:glycine amidinotransferase synthesis by creatine at a pretranslational level. J Biol Chem 259:12034–12038
Mora L, Hernández-Cázares AS, Sentandreu MA, Toldrá F (2010) Creatine and creatinine evolution during the processing of dry-cured ham. Meat Sci 84:384–389
Nabuurs CI, Choe CU, Veltien A, Kan HE, van Loon LJC, Rodenburg RJT, Matschke J, Wieringa B, Kemp GJ, Isbrandt D, Heerschap A (2013) Disturbed energy metabolism and muscular dystrophy caused by pure creatine deficiency are reversible by creatine intake. J Physiol 591:571–592
Nasrallah F, Kraoua I, Curt MJ-C, Bout M-A, Taieb SH, Feki M, Khouja N, Briand G, Kaabachi N (2012) Guanidinoacetate methyltransferase (GAMT) deficiency in two Tunisian siblings: clinical and biochemical features. Clin Lab 58:427–432
Nasrallah F, Benrhouma H, Kraoua I, Briand G, Omar S, Ben Youssef IT, Kaabachi N (2015) Mixed movement disorders revealing an atypical form of creatine deficiency syndrome. Iran J Neurol 14:47–49
Ogawa H, Date T, Gomi T, Konishi K, Pitot HC, Cantoni GL, Fujioka M (1988) Molecular cloning, sequence analysis, and expression in Escherichia coli of the cDNA for guanidinoacetate methyltransferase from rat liver. Proc Natl Acad Sci USA 85:694–698
Pasquali M, Schwarz E, Jensen M, Yuzyuk T, DeBiase I, Randall H, Longo N (2014) Feasibility of newborn screening for guanidinoacetate methyltransferase (GAMT) deficiency. J Inherit Metab Dis 37:231–236
Peters BA, Hall MN, Liu X, Parvez F, Siddique AB, Shahriar H, Uddin MN, Islam T, Ilievski V, Graziano JH, Gamble MV (2015) Low-dose creatine supplementation lowers plasma guanidinoacetate, but not plasma homocysteine, in a double-blind, randomized, placebo-controlled trial. J Nutr 145:2245–2252
Petr M, Ŝteffl M, Kohliková E (2013) Effect of the MTHFR 677C/T polymorphism on homocysteinemia in response to creatine supplementation: a case study. Physiol Rev 62:721–729
Purchas RW, Rutherfurd SM, Pearce PD, Vather R, Wilkinson BHP (2004) Cooking temperature effects on the forms of iron and levels of several other compounds in beef semitendinosus muscle. Meat Sci 68:201–207
Pyne-Geithman GJ, deGrauw TJ, Cecil KM, Chuck G, Lyons MA, Ishida Y, Clark JF (2004) Presence of normal creatine in the muscle of a patient with a mutation in the creatine transporter: a case study. Mol Cell Biochem 262:35–39
Schulze A, Battini R (2007) Pre-symptomatic treatment of creatine biosynthesis defects. In: Salomons GS, Wyss M (eds) Creatine and creatine kinase in health and disease. Springer, New York
Solis MY, de Salles Painelli V, Artioli G, Roschel H, Otaduy MC, Gualano B (2014) Brain creatine depletion in vegetarians? A cross-sectional 1H-magnetic resonance spectroscopy (1H-MRS) study. Br J Nutr 111:1272–1274
Sotgia S, Carru C, Caria MA, Tadolini B, Deiana L, Zinellu A (2007) Acute variations in homocysteine levels are related to creatine changes induced by physical activity. Clin Nutr 26:444–449
Stead LM, Au KP, Jacobs RL, Brosnan ME, Brosnan JT (2001) Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am J Physiol Endocrinol Metab 281:E1095–E1100
Steenge GR, Verhoef P, Greenhaff PL (2001) The effect of creatine and resistance training on plasma homocysteine concentration in healthy volunteers. Arch Intern Med 161:1455–1456
Streijger F, Pluk H, Oerlemans F, Beckers G, Bianco AC, Ribiero MO, Wieringa B, Van der Zee CEEM (2009) Mice lacking brain-type creatine kinase activity show defective thermoregulation. Physiol Behav 97:76–86
Sykut-Cegielska J, Gradowska W, Mercimek-Mahmutoglu S, Stӧckler-Ipsiroglu S (2004) Biochemical and clinical characteristics of creatine deficiency syndromes. Acta Biochim Polonica 51:875–882
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40:1271–1296
Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477
Wu G, Jaeger LA, Bazer FW, Rhoads JM (2004) Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem 15:442–451
Acknowledgments
This work was supported by Grants from the Canadian Institutes of Health Research (RNL 119957) and the Research Development Corporation (5404-1433-101. We thank Dr. Jennifer R. Stevens for assistance with the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article is a review summarizing the results and conclusions of published studies on human or animal subjects. All of the work carried out in our laboratories was approved by our local Ethics Committees.
Additional information
Handling Editor: T. Wallimann and R. Harris.
Rights and permissions
About this article
Cite this article
Brosnan, M.E., Brosnan, J.T. The role of dietary creatine. Amino Acids 48, 1785–1791 (2016). https://doi.org/10.1007/s00726-016-2188-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2188-1