Abstract
Over the last few years, consistent data have demonstrated that creatine (Cr) supplementation prevents the accumulation of fat in rat liver as well as the progression of fatty liver disease in different situations. Studies have demonstrated that Cr is effective and prevents fatty liver in high-fat and choline-deficient diets and in hepatoma cells in vitro. Because Cr synthesis is responsible for a considerable consumption of hepatic methyl groups, studies have tested the idea that Cr supplementation could modulate phospholipid formation and VLDL secretion. Studies have also demonstrated Cr is able to modulate the expression of key genes related to fatty acid oxidation in hepatocyte cell culture and in rat liver. However, to date, the mechanism by which Cr exerts protective effects against fatty liver is poorly understood. Therefore, the present review aims to summarize the studies involving the therapeutic use of Cr supplementation on fatty liver disease and to explore the mechanisms involved in one-carbon and fatty acid metabolism for the preventive effects of Cr supplementation on fat liver accumulation. Although a small number of studies have been conducted to date, we consider Cr as a new and promising therapeutic strategy to control fat accumulation in the liver as well as the progression of fatty liver disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Creatine (Cr) is one of the most popular supplements proposed as an ergogenic aid since Harris et al. (1992) demonstrated Cr supplementation increases muscle Cr and phosphocreatine (PCr) content. Over the last few years, interest in Cr supplementation has increased markedly in the medical field because of the beneficial effects found in a number of muscular and neurological diseases including McArdle disease, Duchenne dystrophy, myasthenia gravis, myotrophic lateral sclerosis and Parkinson’s disease (Gualano et al. 2010, 2011; Wallimann et al. 2011). Considering the therapeutic effects demonstrated in some human metabolic disorders as dyslipidemia (Earnest et al. 1996) and type 2 diabetes (Gualano et al. 2011; Alves et al. 2012), we have tested Cr supplementation in animal models of fatty liver.
Fatty liver is the early stage of nonalcoholic fatty liver disease (NAFLD), a multi causal disease that includes a pathologic spectrum ranging from simple accumulation of lipids in the liver to nonalcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and hepatocellular carcinoma (Tilg and Moschen 2010). NAFLD is frequently accompanying by obesity, insulin resistance and dyslipidemia; however, the arising and progression of NAFLD is still poorly understood (Schwenger and Allard 2014). Recent studies have proposed that inflammation and/or fibrosis determine the long-term prognosis of the disease (Tilg and Moschen 2010). So far, there is no specific treatment for NAFLD or a single drug to address its related comorbidities (Hardy et al. 2015). Most NAFLD therapies include administration of antiobesity drugs, insulin-sensitizing medications and lipid-lowering agents as sibutramine, PPARγ agonists (thiazolidinediones), metformin and statins (Gawrieh and Chalasani 2015; Hardy et al. 2015). Many of these drugs are expensive and have several known side effects, e.g., rhabdomyolysis with statins. Last few years, findings from our laboratory and others have demonstrated preventive effects of Cr treatment on liver fat accumulation. Thus, the present review aims to summarize the studies dealing with the therapeutic use of Cr supplementation on fatty liver disease, to explore the mechanisms involved and to provide perspectives on this promising new therapeutic approach for fatty liver disease.
Cr function and physiology
Cr kinase catalyzes the reaction of ATP and Cr which results in phosphocreatine and ADP, maintaining ATP levels in situations of high energy consumption (Bessman and Carpenter 1985). In addition, this process is also responsible for the translocation of ATP from mitochondrial production sites to the cytosol, where the bulk of ATP will be consumed; Cr will then diffuse back to mitochondria (Wallimann et al. 2011). The low molecular weight of Cr and the difference in cytosolic concentrations of the compounds involved allow the flow of ATP to cellular sites of energy consumption (Brosnan and Brosnan 2007).
Cr can be obtained from the diet and also be endogenously synthesized from amino acids. Dietary Cr comes mainly from meat and fish and can be found in small amounts in milk and dairy products. In human omnivores about half of the daily Cr requirement (~1 g/day) comes from the diet, while the rest is endogenously synthesized in the liver (Brosnan and Brosnan 2007). Vegetarians have a very low Cr intake and consequently de novo Cr synthesis provides most of their needs. The endogenous synthesis of Cr is dependent on three amino acids, glycine, arginine and methionine (Brosnan et al. 2011) and three enzymes, arginine:glycine amidinotransferase (AGAT), methionine adenosyltransferase (MAT) and guanidinoacetate methyltransferase (GAMT). In the kidneys, AGAT facilitates the conversion of glycine and arginine into ornithine and guanidinoacetate (GAA). AGAT activity seems to be the limiting step in Cr synthesis. This enzyme is up-regulated by growth hormone and down-regulated by dietary Cr. GAA synthesis may be regulated by arginine levels (Brosnan et al. 2011). An entire glycine molecule is incorporated into Cr, which also contains an amidino group from arginine and a methyl group from methionine. MAT catalyses the production of S-adenosylmethionine (SAM). GAMT employs SAM to methylate GAA to Cr. High activities of MAT and GAMT are found in the liver which is considered the main site of Cr synthesis (Brosnan et al. 2011). However, Cr supplementation decreases AGAT activity and GAA plasma concentration, but not hepatic GAMT activity, which suggests that de novo Cr synthesis is primarily regulated in the kidney (da Silva et al. 2009). Cr and PCr are spontaneously converted to creatinine, which is excreted in the urine. The average daily creatinine excretion is estimated to be 1.7 % of the total body Cr pool and these losses have to be supplied by diet or de novo Cr synthesis (da Silva et al. 2009; Wyss and Kaddurah-Daouk 2000).
Fatty liver disease pathogenesis
As obesity is the most important risk factor for NAFLD, dietary habits are strongly correlated with NAFLD pathogenesis including an excess of energy intake and the quality of diet (Schwenger and Allard 2014). High consumption of saturated fat, cholesterol, simple carbohydrates and a low intake of fiber, antioxidants, vitamins and polyunsaturated fatty acids are correlated with NASH (Musso et al. 2003; Vos and Lavine 2013). In addition, some single-nucleotide polymorphisms, as in patatin-like phospholipase domain—containing three genes and in chromosome 10 are associated with NAFLD and NASH (Rotman et al. 2010).
The pathogenesis of NAFLD is still not completely understood. A higher flow of non-esterified fatty acids (NEFA) from adipose tissue, dietary fatty acids, decreased lipid oxidation and lipid export from liver, and an increased hepatic de novo lipogenesis are indicated as the possible causes of hepatic triacylglycerol (TAG) accumulation. More than a decade ago, Day and James (1998) proposed the two-hit model of NAFLD progression, suggesting that hepatic lipid accumulation is the first “hit” and the increase in oxidative stress and lipid peroxidation as the second “hit”. More recently, Tilg and Moschen (2010) proposed a ‘multiple parallel hits hypothesis’ suggesting that inflammation mediators derived from a number of parallel processes and different tissues, especially adipose tissue and gut, play a key role in the development of liver inflammation and NAFLD progression evidencing the complexity of the disease and its treatment.
Hepatocytes can take up NEFA from the bloodstream and these, together with fatty acids derived from endogenous lipogenesis, may be esterified to TAG. Subjects with insulin resistance have higher levels of circulating NEFA due to a lower suppressive effect of insulin in adipocytes (Lewis et al. 2002). NEFA from adipose tissue are thought to be the main source for hepatic TAG synthesis in NAFLD subjects followed by fatty acids produced by de novo lipogenesis (DNL) (Donnelly et al. 2005; Kumashiro et al. 2011).
DNL is a metabolic pathway in which excess carbohydrates are converted to fatty acids. Insulin activates sterol regulatory element binding protein 1c (SREBP-1c), a transcription factor that promotes the expression of lipogenic genes, such as acetyl-CoA carboxylase and fatty acid synthase, key enzymes in fatty acid synthesis. Hepatic insulin resistance is characterized by an impairment of insulin signaling to downregulate hepatic gluconeogenesis. Therefore, excess glucose becomes available for conversion into fatty acids through DNL (Kawano and Cohen 2013). Moreover, NEFA can activate cytochrome P450 activity, oxidative stress and lipid peroxidation (Postic and Girard 2008; Lewis et al. 2002). The progression of NAFLD is related to an increase in lipid peroxidation, activation of hepatic stellate cells, collagen production and fibrogenesis. Mitochondrial dysfunction may also increase oxidative stress in steatotic livers; NAFLD and insulin resistance are associated with decreased mitochondrial respiratory capacity in animal models (Garcia-Ruiz et al. 2013).
Peroxisome proliferator-activated receptor (PPAR) alpha (PPARα) is a transcription factor highly expressed in liver, heart and brown adipose tissue that increases mitochondrial and peroxisomal β-oxidation and down-regulates inflammatory genes (Jay and Ren 2007). PPARα is less expressed in animal models of NAFLD (Tailleux et al. 2012 and PPARα knockout mice have increased susceptibility to develop NASH (Abdelmegeed et al. 2011). PPARγ has a central role in adipose tissue metabolism, enhancing adipogenesis, fatty acid uptake and esterification. White and brown adipose tissues are the sites where PPARγ is largely expressed (Ahmadian et al. 2013). Although the hepatic effects of PPARγ are not clearly defined, hepatic PPARγ inactivation reduced liver steatosis in mice fed high fat diet and in a lipoatrophic mouse model (Gavrilova et al. 2003).
The treatment of NAFLD should address lifestyle changes first. Weight loss and exercise are capable of ameliorating insulin resistance and reducing fatty liver (Larson-Meyer et al. 2006; Johnson and George 2010). Several drugs, especially insulin-sensitizing agents, have been tested in NAFLD subjects. Metformin and thiazolidinedione may decrease fatty liver; however, more research is necessary to clarify their benefits and possible side effects (Schwenger and Allard 2014; Musso et al. 2010; Lavine et al. 2011). Omega-3 polyunsaturated fatty acids, vitamin E, ursodeoxycholic acid and probiotics have been used in human trials (Schwenger and Allard 2014; Musso et al. 2010; Scorletti et al. 2014). In addition, supplementation of betaine has been shown to decrease hepatic fat and oxidative stress (Kharbanda et al. 2007; Deminice et al. 2015a). Recently, Cr supplementation has been shown to prevent fatty liver in animal models of NAFLD together with the modulation of the expression of genes in one-carbon and fatty-acids metabolism (Deminice et al. 2011, 2015b).
Cr supplementation prevents fatty liver
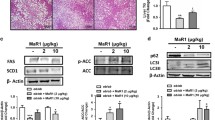
Over the last few years, consistent data have demonstrated that Cr treatment/supplementation may decrease the accumulation of fat in the liver as well as the progression of fatty liver disease in different situations (Table 1). The first evidence for a protective effect of Cr supplementation against fatty liver disease was reported by Demince et al. (2011), who demonstrated that 1 % Cr added to the diet prevented hepatic fat accumulation, TAG and lipid peroxidation induced by 3 weeks of high-fat diet in rats. The effects of Cr supplementation on liver fat were clearly evident in the Oil-Red-O—stained histological sections as presented in Fig. 1. These results were novel and suggested that Cr may exercise therapeutic properties on hepatic lipid metabolism.
Cr supplementation prevents liver fat accumulation and lipid peroxidation in rats fed a high-fat diet. Total hepatic fat (a), liver triglycerides (b), liver TBARS (c) and liver sections stained with Oil-red-O (d). Rats were fed control (c), high-fat (HF), or HF diet supplemented with Cr (HFC) for 3 weeks. From Deminice et al. (2011) with permission
The pioneering data of Deminice et al. (2011) were confirmed later by da Silva et al. (2014) and Deminice et al. (2015b) using two different approaches. da Silva et al. (2014) employed oleate-treated McArdle RH-7777 rat hepatoma cells (McA cell) to investigate the role of Cr in regulating hepatic lipid metabolism. Cr reduced cellular TAG accumulation by 40–60 % in cells incubated with oleate concentrations ranging from 0.1 to 0.4 mM. This study presented important new information about the protective effects of Cr on the accumulation of fat in the liver: (1) the protective effect on lipid metabolism is specific to Cr and cannot be duplicated by similar structural compounds; (2) the triglyceride reduction observed in McA cells after Cr treatment was dose-dependent; and (3) incubating cells with the pan-lipase inhibitor diethyl p-nitrophenylphosphate (E600) did not diminish the effect of Cr, demonstrating that the TAG reduction brought about by Cr does not depend on lipolysis. da Silva et al. (2014) proposed that the protective role of Cr is associated with increased fatty acid oxidation and TAG secretion.
One year later, Deminice et al. (2015b) fed a choline-deficient diet to rats for 4 weeks. They found that Cr prevented the accumulation of fat in the liver, hepatic oxidative stress and increased levels of plasma TNF-α and homocysteine. Changes in key genes of β-oxidation may explain the protective effect of Cr against fatty liver induced by a choline-deficient diet in rats. Taken together, these results provide a body of evidence that Cr is effective in protecting against hepatic fat accumulation in vitro and in studies in rodents.
Interestingly, studies from Choe et al. (2013) and Stockebrand et al. (2013) have demonstrated that Cr deficiency protected mice from hepatic and adipose tissue fat accumulation when exposed to high-fat diet for 8–10 weeks. AGAT-deficient mice had severe phosphocreatine reduction and paradoxically were protected against hepatic fat accumulation induced by a high-fat diet. These mice showed chronic AMP-activated protein kinase (AMPK) activation, which may protect against increased adiposity induced by a high-fat diet. However, the AGAT-deficient mice presented with markedly reduced body weight mass, lower body fat content, increased diet intake, lower locomotor activity, reduced body tension and severe scoliosis (Choe et al. 2013). Furthermore, the AGAT-deficient mice did not gain weight when fed a high fat diet. These remarkable abnormalities highlight the importance of Cr and (P)Cr in body homeostasis and suggest that AGAT-deficient mice are not an ideal model to study such comorbidities, related to energy metabolism, as NAFLD (Choe et al. 2013; Stockebrand et al. 2013).
Does altered one-carbon or fatty acid metabolism account for the preventative effects of Cr supplementation on liver fat accumulation?
Although the benefits presented above, the mechanism by which Cr supplementation prevents hepatic fat accumulation is not totally known. Studies have based their hypothesis in two metabolic pathways: the modulatory capacity of Cr supplementation on methyl balance and fatty acid metabolism. Effects of Cr in these pathways are detailed below.
Methyl balance modulation has a limited role in the prevention of liver fat accumulation by Cr supplementation
Hepatic SAM is a universal donor of methyl groups for the methylation of many compounds, including DNA, RNA, hormones, neurotransmitters, phospholipids (PL), proteins and others (Selhub 2002). Decreased hepatic SAM levels play a key role in liver fat accumulation and in the progression of fatty liver disease (Noureddin et al. 2015). Methionine and choline deficient diets have long been employed to study the mechanisms of fatty liver disease and its progression in rodents (Ghoshal et al. 1983). Methionine- and/or choline- deficient diets cause decreased SAM and increased accumulation of triglycerides in the liver (Caballero et al. 2010) as well as increased plasma homocysteine concentration and hepatic oxidative stress (Deminice et al. 2015b). In contrast, supplementation with methyl donors (e.g., S-adenosylmethionine, choline, betaine) prevents these perturbations (Kwon do et al. 2009; Deminice et al. 2015b; Al Rajabi et al. 2014). This is attributed to the fact that SAM is required for the synthesis of PL via phosphatidylethanolamine N-methyltransferase (PEMT); PC is an essential component of VLDL (Li et al. 2006). Studies using diets deficient in methyldonors have shown impaired SAM availability and PL synthesis which reduces the hepatic secretion of VLDL, resulting in hepatic TAG accumulation (Jacobs et al. 2008; Vance 2013). In addition, PEMT knockout mice have severe steatosis (Jacobs et al. 2010) and humans with PEMT gene (V175M) polymorphism exhibited increased susceptibility to NASH (Song et al. 2005). Indeed, impaired hepatic PL synthesis via PEMT plays a role in hepatic fat accumulation and NASH progression (Li et al. 2006; Jacobs et al. 2008).
Cr is also a methylated compound and Cr synthesis requires a SAM molecule (Brosnan et al. 2011). Because Cr synthesis is responsible for a considerable consumption of hepatic SAM, it has been shown that Cr supplementation modulates methyl balance and decreases Hcy formation (Stead et al. 2001, 2006; Edison et al. 2007; Deminice et al. 2009). Cr supplementation increases intramuscular and liver Cr concentration, promotes a remarkable (~90 %) down-regulation of renal AGAT activity in the rat and decrease in GAA availability (Deminice et al. 2011; Edison et al. 2007); moreover, Cr supplementation reduces endogenous Cr and homocysteine synthesis, (Stead et al. 2001; Edison et al. 2007; Deminice et al. 2009, 2011). Stead et al. (2001) showed that methylation demand and homocysteine formation are down-regulated in rats fed 0.4 % Cr, and up-regulated when fed 0.36 % GAA diet. Homocysteine levels were reduced by 25 % by Cr, and increased by 50 % by GAA, while plasma Met levels were unchanged (Stead et al. 2001). Stead et al. (2006) after a reevaluation of the methyl balance concluded that Cr and PL synthesis are both major consumers of SAM in the human liver.
As Cr and PL consume SAM from the same hepatic pool, Deminice et al. (2011) first hypothesized that suppression of Cr synthesis by Cr supplementation could cause a sparing effect on hepatic SAM; this would be expected to increase PL formation via PEMT, enhance VLDL secretion and prevent hepatic fat accumulation in rats exposed to a high-fat diet. This hypothesis was based on earlier studies with betaine supplementation, which increases SAM availability, regulates PL synthesis and normalizes VLDL assembly (Kharbanda et al. 2007), thereby preventing both non-alcoholic and alcoholic fatty liver. However, these protective effects of Cr supplementation could not be totally explained by modulation of methyl balance and consequent increase in SAM availability. Cr supplementation prevented the decrease in hepatic SAM caused by high-fat diet; however, PL levels and PEMT mRNA were unaltered (Deminice et al. 2011). Data from da Silva et al. (2014) corroborate with these findings while McA RH-7777 cells neither expresses PEMT nor enzymes responsible for the GAA synthesis, however, had TAG synthesis and accumulation decreased when treated with Cr. Deminice et al. (2015b) demonstrated Cr supplementation prevented hepatic fat accumulation of rats fed a choline-deficient diet, without changes in PEMT gene expression, liver PL levels or triglyceride transfer protein (MTP). These results also argue against the hypothesis that the modulation of one-carbon metabolism by Cr supplementation could explain the protective effects against fatty liver.
Cr supplementation prevents fatty liver by modulation of fatty acid metabolism
Because of the inconclusive results on the mechanism by which Cr prevented liver fat accumulation in the different situations presented above, studies have examined the specific effects of Cr supplementation on lipid metabolism, particularly on fatty acid oxidation. These studies evaluated the abundance of genes involved in fatty acid and phospholipid metabolism (Deminice et al. 2011, 2015b; da Silva et al. 2014). Their results demonstrated that rats fed high-fat diet had reduced PPARα mRNA levels as well as those of its targets CPT1α, LCAD and CD36 compared to controls, and that these changes were reversed by Cr supplementation. A high-fat and choline-deficient diet down-regulated PPARα gene expression as well as some of their downstream targets involved in fatty acid oxidation including UCP2, PGC1a, LCAD and CPT1; these were all normalized to control levels in rats fed a Cr supplemented diet (Deminice et al. 2011, 2015b). PPARα and PPARγ are both important regulators of lipid metabolism and play a crucial role in the progression and pathophysiology of fatty liver disease (Takasawa et al. 2008; Matsusue et al. 2003; Gavrilova et al. 2003; Zhang et al. 2015). PPARα-null mice exhibited both hepatic steatosis and decreased expression of transcripts involved in fatty acid oxidation (Gao et al. 2015). da Silva et al. (2014) demonstrated that Cr treatment directly regulate lipid metabolism; promoting increased PPARα, CPT1a in oleate-treated McA RH-7777 rat hepatoma cells. This, however, was not found when Cr analogs were used (creatinine and GAA). Recently, Kazak et al. (2015) provided evidence that Cr metabolism regulates energy expenditure in both beige and brown adipose tissue. Using a proteomic approach, they demonstrated an up-regulation of GAMT and mitochondrial CK (CKMT2) gene expression and CK activity in beige-fat mitochondria of mice exposed to 4 °C. UCP1 deficient mice (UCP1−/−) gradually acclimated to 4 °C showed increased expression of genes related to thermogenesis, as Dio2 and Pgc-1α. Furthermore, genes related to Cr metabolism had increased expression showing an up-regulation of Cr metabolism as a compensatory mechanism to maintain thermogenesis in the UCP1−/− mice. In addition, Cr was able to increase respiration of beige-fat mitochondria when ADP was limiting (Kazak et al. 2015). These prominent results suggest that manipulation of energy expenditure using Cr metabolism target drugs or Cr supplementation may be a new approach to treat subjects with metabolic diseases (Kazak et al. 2015).
Activation of AMPK has also been speculated as a possible anti-fatty liver mechanism of Cr supplementation. Ceddia and Sweeney (2004) demonstrated a significant ~2-fold increase in the phosphorylation of both α-1 and α-2 AMPK isoforms after Cr supplementation. Additionally, it has been demonstrated that changes in PCr:Cr ratio modulates AMPK activity (Pontikos et al. 1998). AMPK is known as a fatty acid metabolism modulator, including hepatic lipid metabolism (Viollet et al. 2009). Metformin and the thiazolidinediones (antidiabetic and obesogenic drugs), alleviate fatty liver in humans and rodents by downregulating lipid metabolism through AMPK activation (Zhou et al. 2001). However, da Silva et al. (2014) demonstrated that Cr treatment did not alter AMPK nor lipase activity in oleate-treated McA RH-7777 rat hepatoma cells. Therefore, the protective effects of Cr mediated AMPK activation must be better investigated.
Conclusions
In conclusion, recent studies in vivo and in vitro have demonstrated that Cr treatment prevents liver fat accumulation, hepatic inflammation and, i.e., oxidative stress, perturbations involved in the progression of fatty liver disease. The effects of Cr do not appear to be explained by modulation of methyl balance. Novel results, however, demonstrate that Cr substantially modulates the expression of transcription factors (PPARα and PPARγ), and of key genes related to fatty acid metabolism. These data suggests a possible mechanism whereby Cr supplementation prevents liver fat accumulation through the modulation of fatty acid metabolism.
Currently, there is no approved drug therapy for fatty liver disease and the optimal treatment remains uncertain. Most treatments include administration of antiobesity drugs, insulin-sensitizing medications and lipid-lowering agents such as sibutramine, PPARγ agonists (thiazolidinediones), metformin, vitamin E and statins. Many of these drugs are expensive and have several known side effects, e.g., some thiazolidinediones have demonstrated weight gain, bone loss/fracture risk, increased risk of myocardial and bladder cancer (Hardy et al. 2015). In addition, other drug treatments are still on debate e.g. statins (Pastori et al. 2015). Cr supplementation has already shown to elicit beneficial effects on glucose metabolism in diabetic subjects (Alves et al. 2012; Gualano et al. 2011) and in blood lipids in hyperlipidemic subjects, (Earnest et al. 1996). Recent findings indicate that Cr supplementation may prevent liver fat accumulation, classifying Cr as a promising therapeutic approach for fatty liver disease and related metabolic disturbances. Future studies must address the dose, safety and efficacy of Cr supplementation in NAFLD subjects, both as a single treatment and combined with lifestyle changes.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- BHMT:
-
Betaine-homocysteine S-methyltransferase
- CK:
-
Creatine kinase
- DNL:
-
De novo lipogenesis
- McA:
-
McArdle
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- NEFA:
-
Non-esterified fatty acids
- PE, PEMT:
-
Phosphatidylethanolamine N-methyltranferase
- PL:
-
Phosphatidylcholine
- SAH:
-
S-adenosylhomocysteine
- SAM:
-
S-adenosylmethionine
- TAG:
-
Triacylglycerols
References
Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ (2011) PPARα expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr 141(4):603–610. doi:10.3945/jn.110.135210
Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM (2013) PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 19(5):557–566. doi:10.1038/nm.3159
Al Rajabi A, Castro GS, da Silva RP, Nelson RC, Thiesen A, Vannucchi H, Vine DF, Proctor SD, Field CJ, Curtis JM, Jacobs RL (2014) Choline supplementation protects against liver damage by normalizing cholesterol metabolism in Pemt/Ldlr knockout mice fed a high-fat diet. J Nutr 144(3):252–257. doi:10.3945/jn.113.185389
Alves CR, Ferreira JC, de Siqueira-Filho MA, Carvalho CR, Lancha AH Jr, Gualano B (2012) Creatine-induced glucose uptake in type 2 diabetes: a role for AMPK-alpha? Amino Acids 43(4):1803–1807. doi:10.1007/s00726-012-1246-6
Bessman SP, Carpenter CL (1985) The creatine-creatine phosphate energy shuttle. Annu Rev Biochem 54:831–862. doi:10.1146/annurev.bi.54.070185.004151
Brosnan JT, Brosnan ME (2007) Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 27:241–261. doi:10.1146/annurev.nutr.27.061406.093621
Brosnan JT, da Silva RP, Brosnan ME (2011) The metabolic burden of creatine synthesis. Amino Acids 40(5):1325–1331. doi:10.1007/s00726-011-0853-y
Caballero F, Fernandez A, Matias N, Martinez L, Fucho R, Elena M, Caballeria J, Morales A, Fernandez-Checa JC, Garcia-Ruiz C (2010) Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-l-methionine and glutathione. J Biol Chem 285(24):18528–18536. doi:10.1074/jbc.M109.099333
Ceddia RB, Sweeney G (2004) Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J Physiol 555:409–421. doi:10.1113/jphysiol.2003.056291
Choe CU, Nabuurs C, Stockebrand MC, Neu A, Nunes P, Morellini F, Sauter K, Schillemeit S, Hermans-Borgmeyer I, Marescau B, Heerschap A, Isbrandt D (2013) L-arginine:glycine amidinotransferase deficiency protects from metabolic syndrome. Hum Mol Genet 22(1):110–123. doi:10.1093/hmg/dds407
da Silva RP, Nissim I, Brosnan ME, Brosnan JT (2009) Creatine synthesis: hepatic metabolism of guanidinoacetate and creatine in the rat in vitro and in vivo. Am J Physiol Endocrinol Metab 296(2):E256–E261. doi:10.1152/ajpendo.90547.2008
da Silva RP, Kelly KB, Leonard KA, Jacobs RL (2014) Creatine reduces hepatic TG accumulation in hepatocytes by stimulating fatty acid oxidation. Biochim Biophys Acta 1841 11:1639–1646. doi:10.1016/j.bbalip.2014.09.001
Day CP, James OF (1998) Steatohepatitis: a tale of two “hits”? Gastroenterology 114(4):842–845
Deminice R, Portari GV, Vannucchi H, Jordao AA (2009) Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. Br J Nutr 102(1):110–116. doi:10.1017/S0007114508162985
Deminice R, da Silva RP, Lamarre SG, Brown C, Furey GN, McCarter SA, Jordao AA, Kelly KB, King-Jones K, Jacobs RL, Brosnan ME, Brosnan JT (2011) Creatine supplementation prevents the accumulation of fat in the livers of rats fed a high-fat diet. J Nutr 141(10):1799–1804. doi:10.3945/jn.111.144857
Deminice R, da Silva RP, Lamarre SG, Kelly KB, Jacobs RL, Brosnan ME, Brosnan JT (2015a) Betaine supplementation prevents fatty liver induced by a high-fat diet: effects on one-carbon metabolism. Amino Acids 47(4):839–846. doi:10.1007/s00726-014-1913-x
Deminice R, de Castro GS, Francisco LV, da Silva LE, Cardoso JF, Frajacomo FT, Teodoro BG, Dos Reis Silveira L, Jordao AA (2015b) Creatine supplementation prevents fatty liver in rats fed choline-deficient diet: a burden of one-carbon and fatty acid metabolism. J Nutr Biochem 26(4):391–397. doi:10.1016/j.jnutbio.2014.11.014
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Investig 115(5):1343–1351. doi:10.1172/JCI23621
Earnest CP, Almada AL, Mitchell TL (1996) High-performance capillary electrophoresis-pure creatine monohydrate reduces blood lipids in men and women. Clin Sci 91(1):113–118
Edison EE, Brosnan ME, Meyer C, Brosnan JT (2007) Creatine synthesis: production of guanidinoacetate by the rat and human kidney in vivo. Am J Physiol Renal Physiol 293(6):F1799–F1804. doi:10.1152/ajprenal.00356.2007
Gao Q, Jia Y, Yang G, Zhang X, Boddu PC, Petersen B, Narsingam S, Zhu YJ, Thimmapaya B, Kanwar YS, Reddy JK (2015) PPARα-deficient ob/ob obese mice become more obese and manifest severe hepatic steatosis due to decreased fatty acid oxidation. Am J Pathol 185(5):1396–1408. doi:10.1016/j.ajpath.2015.01.018
Garcia-Ruiz C, Baulies A, Mari M, Garcia-Roves PM, Fernandez-Checa JC (2013) Mitochondrial dysfunction in non-alcoholic fatty liver disease and insulin resistance: cause or consequence? Free Radical Res 47(11):854–868. doi:10.3109/10715762.2013.830717
Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML (2003) Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 278(36):34268–34276. doi:10.1074/jbc.M300043200
Gawrieh S, Chalasani N (2015) Pharmacotherapy for nonalcoholic fatty liver disease. Semin Liver Dis 35(3):338–348. doi:10.1055/s-0035-1562951
Ghoshal AK, Ahluwalia M, Farber E (1983) The rapid induction of liver cell death in rats fed a choline-deficient methionine-low diet. Am J Pathol 113(3):309–314
Gualano B, Artioli GG, Poortmans JR, Lancha Junior AH (2010) Exploring the therapeutic role of creatine supplementation. Amino Acids 38(1):31–44. doi:10.1007/s00726-009-0263-6
Gualano B, Pinto AL, Perondi MB, Roschel H, Sallum AM, Hayashi AP, Solis MY, Silva CA (2011) Therapeutic effects of exercise training in patients with pediatric rheumatic diseases. Revista Brasileira de Reumatologia 51(5):490–496
Hardy T, Anstee QM, Day CP (2015) Nonalcoholic fatty liver disease: new treatments. Curr Opin Gastroenterol 31(3):175–183. doi:10.1097/MOG.0000000000000175
Harris RC, Soderlund K, Hultman E (1992) Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci 83(3):367–374
Jacobs RL, Lingrell S, Zhao Y, Francis GA, Vance DE (2008) Hepatic CTP:phosphocholine cytidylyltransferase-alpha is a critical predictor of plasma high density lipoprotein and very low density lipoprotein. J Biol Chem 283(4):2147–2155. doi:10.1074/jbc.M706628200
Jacobs RL, Zhao Y, Koonen DP, Sletten T, Su B, Lingrell S, Cao G, Peake DA, Kuo MS, Proctor SD, Kennedy BP, Dyck JR, Vance DE (2010) Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem 285(29):22403–22413. doi:10.1074/jbc.M110.108514
Jay MA, Ren J (2007) Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr Diabet Rev 3(1):33–39
Johnson NA, George J (2010) Fitness versus fatness: moving beyond weight loss in nonalcoholic fatty liver disease. Hepatology 52(1):370–381. doi:10.1002/hep.23711
Kawano Y, Cohen DE (2013) Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol 48(4):434–441. doi:10.1007/s00535-013-0758-5
Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, Kajimura S, Gygi SP, Spiegelman BM (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 22(3):643–655. doi:10.1016/j.cell.2015.09.035
Kharbanda KK, Mailliard ME, Baldwin CR, Beckenhauer HC, Sorrell MF, Tuma DJ (2007) Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol 46(2):314–321. doi:10.1016/j.jhep.2006.08.024
Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, Petersen KF, Samuel VT, Shulman GI (2011) Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA 108(39):16381–16385. doi:10.1073/pnas.1113359108
Kwon do Y, Jung YS, Kim SJ, Park HK, Park JH, Kim YC (2009) Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J Nutr 139(1):63–68. doi:10.3945/jn.108.094771
Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E (2006) Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabet Care 29(6):1337–1344. doi:10.2337/dc05-2565
Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Unalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, Nonalcoholic Steatohepatitis Clinical Research N (2011) Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. Jama 305(16):1659–1668. doi:10.1001/jama.2011.520
Lewis GF, Carpentier A, Adeli K, Giacca A (2002) Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 23(2):201–229. doi:10.1210/edrv.23.2.0461
Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, Vance DE (2006) The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab 3(5):321–331. doi:10.1016/j.cmet.2006.03.007
Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B Jr, Reitman ML, Gonzalez FJ (2003) Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Investig 111(5):737–747. doi:10.1172/JCI17223
Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Faga E, Silli B, Pagano G (2003) Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 37(4):909–916. doi:10.1053/jhep.2003.50132
Musso G, Gambino R, Cassader M, Pagano G (2010) A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 52(1):79–104. doi:10.1002/hep.23623
Noureddin M, Mato JM, Lu SC (2015) Nonalcoholic fatty liver disease: update on pathogenesis, diagnosis, treatment and the role of S-adenosylmethionine. Exp Biol Med 240(6):809–820. doi:10.1177/1535370215579161
Pastori D, Polimeni L, Baratta F, Pani A, Del Ben M, Angelico F (2015) The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis 47(1):4–11. doi:10.1016/j.dld.2014.07.170
Pontikos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D (1998) Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J 17:1688–1699
Postic C, Girard J (2008) Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Investig 118(3):829–838. doi:10.1172/JCI34275
Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ, Nash CRN (2010) The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 52(3):894–903. doi:10.1002/hep.23759
Schwenger KJ, Allard JP (2014) Clinical approaches to non-alcoholic fatty liver disease. World J Gastroenterol WJG 20(7):1712–1723. doi:10.3748/wjg.v20.i7.1712
Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L, Moyses HE, Calder PC, Byrne CD, On behalf of the WSI (2014) Effects of purified eicosapentaenoic and docosahexaenoic acids in non-alcoholic fatty liver disease: Results from the *WELCOME study. Hepatology. doi:10.1002/hep.27289
Selhub J (2002) Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging 6(1):39–42
Song J, da Costa KA, Fischer LM, Kohlmeier M, Kwock L, Wang S, Zeisel SH (2005) Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J Off Publ Fed Am Soc Exp Biol 19(10):1266–1271. doi:10.1096/fj.04-3580com
Stead LM, Au KP, Jacobs RL, Brosnan ME, Brosnan JT (2001) Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am J Physiol Endocrinol Metab 281(5):E1095–E1100
Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL (2006) Is it time to reevaluate methyl balance in humans? Am J Clin Nutr 83(1):5–10
Stockebrand M, Sauter K, Neu A, Isbrandt D, Choe CU (2013) Differential regulation of AMPK activation in leptin- and creatine-deficient mice. FASEB J Off Publ Fed Am Soc Exp Biol 27(10):4147–4156. doi:10.1096/fj.12-225136
Tailleux A, Wouters K, Staels B (2012) Roles of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta 1821 5:809–818. doi:10.1016/j.bbalip.2011.10.016
Takasawa K, Kubota N, Terauchi Y, Kadowaki T (2008) Impact of increased PPARγ activity in adipocytes in vivo on adiposity, insulin sensitivity and the effects of rosiglitazone treatment. Endocr J 55(4):767–776
Tilg H, Moschen AR (2010) Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52(5):1836–1846. doi:10.1002/hep.24001
Vance DE (2013) Physiological roles of phosphatidylethanolamine N-methyltransferase. Biochim Biophys Acta 1831(3):626–632. doi:10.1016/j.bbalip.2012.07.017
Viollet B, Guigas B, Leclerc J, Hébrard S, Lantier L, Mounier R, Andreelli F, Foretz M (2009) AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 196(1):81–98. doi:10.1111/j.1748-1716.2009.01970.x
Vos MB, Lavine JE (2013) Dietary fructose in nonalcoholic fatty liver disease. Hepatology 57(6):2525–2531. doi:10.1002/hep.26299
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40(5):1271–1296. doi:10.1007/s00726-011-0877-3
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80(3):1107–1213
Zhang Y, Cui Y, Wang XL, Shang X, Qi ZG, Xue J, Zhao X, Deng M, Xie ML (2015) PPARα/γ agonists and antagonists differently affect hepatic lipid metabolism, oxidative stress and inflammatory cytokine production in steatohepatitic rats. Cytokine 75(1):127–135. doi:10.1016/j.cyto.2015.05.031
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108(8):1167–1174
Acknowledgments
Deminice R is suported by Brazilian Grant from Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (Capes: 88881.068035/2014-01); de Castro GS is supported by Science without Borders Program—Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (246567/2013-9). Brosnan JT and Brosnan ME are supported by grant #97851 from the Canadian Institutes for Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that there is no potential conflict of interests regarding this article. Authors declare that this manuscript is a review and did not involve any humans and/or animal research.
Additional information
Handling Editor: T. Wallimann and R. Harris.
Rights and permissions
About this article
Cite this article
Deminice, R., de Castro, G.S., Brosnan, M.E. et al. Creatine supplementation as a possible new therapeutic approach for fatty liver disease: early findings. Amino Acids 48, 1983–1991 (2016). https://doi.org/10.1007/s00726-016-2183-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2183-6