Abstract
Creatine (Cr) plays a central role in energy provision through a reaction catalyzed by phosphorylcreatine kinase. Furthermore, this amine enhances both gene expression and satellite cell activation involved in hypertrophic response. Recent findings have indicated that Cr supplementation has a therapeutic role in several diseases characterized by atrophic conditions, weakness, and metabolic disturbances (i.e., in the muscle, bone, lung, and brain). Accordingly, there has been an evidence indicating that Cr supplementation is capable of attenuating the degenerative state in some muscle disorders (i.e., Duchenne and inflammatory myopathies), central nervous diseases (i.e., Parkinson’s, Huntington’s, and Alzheimer’s), and bone and metabolic disturbances (i.e., osteoporosis and type II diabetes). In light of this, Cr supplementation could be used as a therapeutic tool for the elderly. The aim of this review is to summarize the main studies conducted in this field and to highlight the scientific and clinical perspectives of this promising therapeutic supplement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
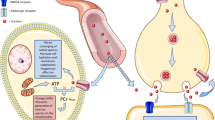
Creatine (Cr), a natural substance in the human body, is partly synthesized by kidneys, pancreas, and liver (approximately, 1–2 g/day), as well as ingested from food (approximately, 1–5 g/day), especially meat and fish, and thereafter mainly transported (about 95%) to the skeletal muscles, brain, and testes (Wyss and Kaddurah-Daouk 2000). Cr plays an important role in rapid energy provision during muscle contraction involving the transfer of N-phosphoryl group from phosphorylcreatine (PCr) to ADP to regenerate ATP through a reversible reaction catalyzed by phosphorylcreatine kinase (PCK). Moreover, Cr is responsible for energy transfer from mitochondria to cytosol. This function is only possible due to the presence of different PCK isoforms linking the sites of ATP generation (i.e., mitochondria; Mt-PCK) to those of ATP consumption (i.e., skeletal muscle and brain; MM-PCK and BB-PCK, respectively). Other relevant roles of the PCr–PCK system involve (a) the ATP/ADP ratio maintenance, which enhances mitochondrial respiration; (b) attenuation of ADP increase and, consequently, minimization of adenine nucleotide loss; (c) oxidative stress prevention via direct and indirect antioxidant action; (d) pH maintenance via H+ buffering; (e) glycolysis and glycogenolysis activation through Pi release, thereby integrating the carbohydrate and Cr degradation to provide energy at the onset of exercise. A reader interested in the Cr–PCK system is encouraged to consult the excellent reviews by Wallimann and Hemmer (1994), Wyss and Kaddurah-Daouk (2000), and Greenhaff (2001).
Since Professor Roger Harris and his colleagues have demonstrated that Cr loading is able to enhance muscle Cr and PCr content (Harris et al. 1992), Cr supplementation has been largely used to improve exercise capacity in healthy individuals and athletes. In fact, a growing body of evidence indicates that Cr exerts ergogenic effects, especially in supra-maximal short-term efforts, in which PCr–PCK plays an essential energetic role (Terjung et al. 2000). Furthermore, several well-controlled studies have unequivocally demonstrated that Cr supplementation is also capable of inducing strength and lean mass gains in healthy people (for details, see Nissen and Sharp 2003). Interestingly, it has been demonstrated that Cr-induced lean mass increase is not exclusively explained by water retention, as believed previously (Francaux and Poortmans 1999; Terjung et al. 2000). Instead, there is a strong evidence suggesting that Cr supplementation could promote overexpression of genes and proteins related to hypertrophy (Deldicque et al. 2008; Safdar et al. 2008), as well as satellite cell activation (Olsen et al. 2006). The aforementioned adaptations would lead to protein synthesis, thereby chronically inducing lean mass gains. Importantly, it has been demonstrated that Cr supplementation might also augment bone mineral density as a result of an increased muscle mass and/or a direct effect of Cr–PCK system on bone bioenergetics (Chilibeck et al. 2005; Gerber et al. 2005). Considering the beneficial adaptations observed in health people, several studies have investigated the effects of Cr supplementation in patients suffering from atrophy, muscle weakness, and metabolic dysfunction (i.e., muscular, bone, pulmonary, and cerebral). Recent findings have confirmed the potential therapeutic effects of Cr supplementation. The present review aims to summarize the studies involving the therapeutic use of Cr supplementation and to explore the perspectives of this promising scientific and clinical field.
Cr effects on muscle disorders
Various factors lead to necrosis, apoptosis, and autophagy in myopathies, including cytoskeletal disruption, elevation in reactive oxidative species (ROS) production, and electron transport chain dysfunction, increasing in protein degradation and intracellular calcium content rising (Tarnopolsky 2007). Interestingly, a growing body of knowledge suggests that Cr supplementation could counteract all of these dysfunctions.
Several studies have verified that Cr supplementation is able to prevent ROS production, especially in central nervous system (CNS) (see next sub-topic). In addition, attenuation has been observed in the release of intracellular calcium as a result of Cr supplementation (Wyss and Kaddurah-Daouk 2000). Although, at least in human adults, isotopic techniques have not yet validated any increase in protein skeletal muscle induced by this supplement under resting or exercise conditions (Louis et al. 2003b; Parise et al. 2001), recent studies have indicated that Cr supplementation can markedly reduce muscle catabolism (Menezes et al. 2007). Additionally, there is an evidence suggesting that Cr supplementation attenuates necrosis and improves mitochondrial respiration in mdx mice (a model that mimics Duchenne disease) (Passaquin et al. 2002).
The abovementioned beneficial effects of Cr provided the rationale for the first clinical trials testing Cr supplementation in myopathies. Tarnopolsky and Parise (1999) observed that muscle Cr and PCr content is reduced in patients with widespread muscle disorders, probably because of a defect in muscle Cr uptake. These findings reinforced the hypothesis that Cr supplementation could benefit patients with myopathies. Accordingly, the same group reported strength and weight gain in patients with muscle dystrophies, cytopathies, inflammatory myopathies, and peripheral neuropathy disorders (n = 21) supplemented with Cr for 11 days (Tarnopolsky and Martin 1999).
During the last decade, several randomized controlled trials (RCT) were conducted in order to test the effects of Cr supplementation in different myopathies (see Table 1). Nevertheless, the particularities of symptoms and physiopathology of each myopathy have hampered any general conclusions at this point. Considering this scenario, Kley et al. (2007) performed an interesting meta-analysis with the purpose of determining the efficacy of Cr supplementation in several muscle disorders. Twelve trials, involving 266 participants, met the selection criterion. The analyses revealed that CR is, in fact, capable of inducing strength and lean mass gains in patients with dystrophy. The most clinically relevant findings were seen in dystrophinopathies and myotonic dystrophy type 2. In contrast, no significant benefits were detected in myotonic dystrophy type 1 and facioscapulohumeral dystrophy. This systematic review reinforces the impossibility of data extrapolation among different myopathies, even for those that reserve some similar characteristics (i.e., dystrophies). Regarding metabolic myopathies, a slight attenuation in ATP consumption during exercise was shown. However, neither muscle mass nor strength improvements were observed as a consequence of Cr supplementation. In spite of this, the authors emphasize the low number of patients with metabolic myopathies enrolled in the analysis. Therefore, type II statistical error (to accept the null hypothesis when it is false) cannot be ruled out. Ultimately, it is worth highlighting that Cr use was associated with aggravated pain in patients with glycogenosis type V (McArdle disease); however, this was the only adverse effect attributed to Cr supplementation.
Recently, Chung et al. (2007) investigated whether Cr supplementation could enhance the effects of exercise training on muscle function in patients with inflammatory myopathy (i.e., dermatomyositis and polymyositis) who were clinically weak after conventional pharmacological treatment. The authors reported that patients supplemented with Cr for 6 months showed greater muscle function than their non-supplemented counterparts. These findings indicate the efficacy of Cr supplementation in patients with inflammatory myopathies, even under a chronic regime of corticoids.
Collectively, a critical literature review reveals that supplementary Cr could be a powerful therapeutic adjuvant to attenuate the symptoms caused by muscle disorders, especially in inflammatory myopathies and dystrophies. However, further RCTs are necessary. Nevertheless, Tarnopolsky (2007) stressed the rarity of some muscle disorders as a complex factor hampering the running of large clinical studies. In order to minimize this issue, Tarnopolsky (2007) suggests the adoption of crossover design, which avoids biological variability between subjects and then inflates statistical power. However, this design would potentially lead to two biases. First, as highlighted by Tarnopolsky (2007), “certain disorders such as amyotrophic lateral sclerosis (ALS) or polymyositis show either a rapid decline or improvement in function, respectively, and a parallel design is more appropriate.” Second, and perhaps most important, the crossover design requires a washout period. Generally, a 4–6 week washout period has been adopted in Cr supplementation studies. Nevertheless, evidence suggests that benefits of short-term Cr loading (i.e., 5 days) may persist for a longer (and unknown) period, mostly in meat eating individuals. In view of that, it is highly recommended that crossover studies measure muscle Cr content in order to guarantee that Cr returns to baseline values before placebo arm, avoiding type 2 errors.
Cr effects on bone and cartilage
For the development and repair process of bone and cartilage, cells require high energetic demand to survive, proliferate, differentiate, and synthesize the extracellular matrix (Gerber et al. 2005). This energy is provided by glycolysis and oxidative phosphorylation. Additionally, recent evidence suggests that the PCr–PCK system also plays an energetic role in these tissues. In fact, the presence of PCK isoforms in bone and cartilage during different stages of development corroborates this hypothesis (for details, see Wallimann and Hemmer 1994). Notoriously, stimuli able to induce bone mass development (i.e., insulin growth factor 1 and parathyroid hormone) also result in increased PCK activity (Somjen and Kaye 1994; Somjen et al. 1985). Similarly, it has been noted that chondrocyte hypertrophy is related to PCK overexpression (Hobson et al. 1999). Moreover, Funanage et al. (1992) demonstrated that β-guanidinopropionic acid administration (GPA, a Cr analog that competes for Cr uptake into the cells), which results in severe Cr and PCr content reductions and consequently in disturbances in PCK system, provokes endochondral disorder in vitro and in vivo. Taken together, these findings reveal the important role of the PCr–PCK system in non-excitable tissues, such as bone and cartilage.
Some studies with dystrophic patients under chronic corticoid treatment provided the preliminary evidence that Cr supplementation can yield beneficial adaptations to bone mass. In a study by Louis et al. (2003a), for example, young patients with Duchenne dystrophy supplemented with Cr showed increased bone mineral density (+3%) and reduced bone resorption (−30%). Chilibeck et al. (2005) investigated the effects of Cr supplementation combined with resistance training on bone mineral density in elderly people. The authors found that Cr-supplemented patients had superior increase in bone mineral density. Further, Candow et al. (2008) demonstrated that Cr supplementation reduces muscle protein degradation and bone resorption in older males undergoing resistance training. These findings enable us to conclude that the Cr supplementation exerts an additive effect to resistance training on bone mass.
At present, it has been intensively debated whether the Cr effects on bone metabolism are mediated by (1) a direct action of PCr–PCK system on bone bioenergetics (Gerber et al. 2005) or (2) an indirect effect of enhanced muscle mass and consequently inducing greater tension on bone at sites of muscle attachment (Chilibeck et al. 2005). In vitro experiments demonstrated that Cr exerts stimulatory effects on differentiation, metabolic activity, and mineralization of primary osteoblast-like cells (Gerber et al. 2005). Clearly, these findings cannot be attributed to Cr-induced muscle mass gain. Instead, this study corroborates the role of Cr bioenergetics on bone, possibly elevating PCr/Cr ratio and preserving the mitochondrial function and ultra-structure (Wallimann and Hemmer 1994). Surprisingly, no studies have tested the efficacy of Cr supplementation in individuals suffering from bone or cartilage degradation (i.e., osteoporosis and osteoarthritis). Likewise, it is tempting to speculate whether Cr supplementation could be used as an adjuvant therapy for bone fracture healing. RCT must address these interesting issues.
Cr effects on CNS
Although the brain constitutes only 2% of the body mass, it can be responsible for up to 20% of total energy consumption under resting conditions. Thus, it necessitates a remarkable ATP turnover in order to maintain membrane potentials as well as the signaling activities of the central and peripheral nervous system. The presence of PCK isoforms in the brain and spinal cord stresses the energetic role of PCr–PCK system on CNS (Andres et al. 2008). Accordingly, evidence indicates that brain Cr depletion is associated with several neural disorders seen in encephalomyopathies and mitochondrial myopathies (in‘t Zandt et al. 2004). Furthermore, some syndromes characterized by dysfunction of Cr synthesis or transport (i.e., Cr deficiency syndromes) are related to mental retardation, autism, speech delay, and brain atrophy (Item et al. 2001; Salomons et al. 2001; Stockler et al. 1994). These findings collectively reveal the potential therapeutic effect of Cr supplementation on CNS.
There is enough evidence showing the presence of PCK in pyramidal cells, which are involved in memory and learning process (Kaldis et al. 1996). In light of this, some investigations have addressed the effects of Cr supplementation on the cognition process. The results have indicated that this supplement is able to improve brain function in young subjects (Watanabe et al. 2002) as well as in elderly people (McMorris et al. 2007b). Likewise, benefits were observed in healthy subjects submitted to sleep deprivation (a condition that is associated with reduced brain Cr), suggesting that Cr supplementation may attenuate the cognitive impairment elicited by stress situations (McMorris et al. 2007a; McMorris et al. 2006). Using near infrared spectroscopy, Watanabe et al. (2002) demonstrated that CR supplementation enhances cerebral oxidation, which partially explains the reduced mental fatigue after a mathematical calculus sequence also observed in this study. Nonetheless, the full mechanisms by which Cr supplementation benefits cognition, as well as the conditions in which this supplement could be effective, remain to be elucidated.
Furthermore, a few psychiatric disorders have been successfully treated with Cr. It was shown that patients with disorders related to anxiety have reduced brain Cr content (Coplan et al. 2006). Hence, it is not surprising that Cr supplementation seems to be effective in relieving symptoms, attenuating depression, and improving the quality of sleep in individuals suffering from post-traumatic stress (Amital et al. 2006a). The same group also verified clinical benefits in a Cr-supplemented patient with depression and fibromyalgia (Amital et al. 2006b). On the other hand, Cr supplementation (5 g/d for 3 months) did not improve the clinical condition or the cognitive capacity in patients with schizophrenia.
Andres et al. (2008) stated that the benefits of Cr supplementation on the CNS may be extended to a vast spectrum of conditions, including disorders related to inborn error of metabolism, acute neurological disorders, and neurodegenerative diseases. Since these topics are comprehensively reviewed by the aforementioned authors, we will focus only on the main investigations in this field, highlighting the gaps in literature.
Cerebral Cr deficiency is associated with several inborn neuromuscular disorders. Not surprisingly, Cr supplementation is able to treat the syndromes characterized by defects in Cr synthesis (i.e., defects on S-adenosylmethionine transferase [GAMT] expression, essential for Cr synthesis), stabilizing cerebral Cr content and thereby improving motor behavior (Schulze 2003). In contrast, efforts using Cr supplementation and aiming to attenuate the neurological disturbances induced by deficiency in Cr transporter (CreaT) have been fruitless (Stockler et al. 1996). Additionally, it has been shown that guanidine compounds, including Cr, may affect GABAergic neurotransmission as (partial) agonists for GABAA receptors, suggesting a role of this compound as a central neuromodulator (De Deyn and Macdonald 1990; De Deyn and Wirshing 2001; Koga et al. 2005). For instance, Almeida et al. (2006) demonstrated that Cr is not only synthesized and taken up by central neurons, but also released in an action-potential dependent (exocytotic) manner, providing strong evidence for its role as a neuromodulator in the brain.
Galbraith et al. (2006) reported declined hypothalamic Cr concentration in rats treated with a powerful experimental anorectic compound, cobaltic protoporphyrin IX (CoPP). Further, intracerebroventricular administration of GPA resulted in decreased food intake and body weight. Taken together, these findings suggest that Cr concentrations in the brain may play a role in regulating food intake and body weight. In fact, it has been pointed out that regular Cr supplementation increases body mass through excess muscle water intake (Poortmans and Francaux 2008). However, how Cr plays a role in food intake and body weight regulation on CNS is far from clear. Likewise, whether Cr might be useful in some eating disorders (i.e., anorexia and bulimia) and excessive weight loss conditions (i.e., cancer and AIDS) remains uncertain.
Inborn errors in ammonia metabolism may also lead to cerebral Cr depletion (Cagnon and Braissant 2007). In vitro experiments revealed that Cr protects against axonal growth inhibition in brain cells challenged with ammonia (Braissant et al. 2002). To date, however, no clinical studies have been performed in this population.
Interestingly, a body of literature indicates that energetic disturbances induced by ischemia, cerebral vascular stroke, spinal cord injury, and cerebral trauma might be attenuated by Cr treatment (Adcock et al. 2002; Lensman et al. 2006; Ozkan et al. 2005; Scheff and Dhillon 2004). It has been speculated that this neuroprotective effect may be a result of Cr-induced vasodilatation response, although the complete underlying mechanisms are not clear. A RCT demonstrated that Cr-supplemented children and adolescents suffering from brain trauma showed greater clinical improvements (i.e., cognition function, behavior, personality aspects) than their non-supplemented counterparts (Sakellaris et al. 2006). Similar findings were obtained in animal models with spinal cord injury, although the benefits observed were less evident (Hausmann et al. 2002; Rabchevsky et al. 2003).
Perhaps more important are the promising effects of Cr supplementation on neurodegenerative disorders (Table 2), which are inborn or acquired diseases characterized by progressive cell loss from one or more regions of the nervous system. Despite the lack of certainty on the physiopathology of neurodegenerative mechanisms, it is known that energy depletion, oxidative stress, and mitochondrial dysfunction are factors associated with most of these disorders (Andres et al. 2008). Alzheimer’s disease is a common disorder that leads to dementia. Cerebral PCK expression seems to be reduced in patients with this disease (Aksenov et al. 1997). Importantly, it has been demonstrated that Cr exerts neuroprotective effects on neurons cultivated in neurotoxic media, induced by glutamate and β-amyloid protein (Brewer and Wallimann 2000). However, clinical trials are needed to assess whether Cr supplementation can be an effective strategy against Alzheimer’s disease.
ALS is another severe neurodegenerative disease that occurs with progressive motor neuron loss (Andres et al. 2008). In transgenic rats mimicking this disease, reductions in ATP content (Browne et al. 2006) and PCK activity were observed (Wendt et al. 2002). Furthermore, Cr supplementation seems to present neuroprotective properties in the same model, likely due to its direct antioxidant capacity or via the energetic role of the Cr–PCK system (Dupuis et al. 2004). Despite these promising findings in animal models, the first clinical trials did not reveal significant benefits in ALS patients (see Table 2). A large multi-center RCT is being conducted presently and certainly will help to clarify this issue (for details, see http://www.clinicaltrial.gov; identifier: NCT00069186).
Charcot-Marie-Tooth disease describes a group of disorders characterized by progressive sensorimotor neuropathy, which leads to long-term functional impairments (Andres et al. 2008). Recently, Smith et al. (2006) demonstrated that Cr supplementation, when combined with resistance training, promotes changes in myosin heavy chain (MHC) composition, although the design of this study did distinguish between the effects of Cr and those of resistance training.
Huntington’s disease is an autosomal dominantly inherited neurodegenerative disorder that clinically occurs with progressive movement loss and important cognitive and emotional dysfunction (Andres et al. 2008). There is evidence linking the severity of this disease to increased lactate concentration, decreased muscle PCr content, and mitochondrial defects (Grunewald and Beal 1999). Therefore, Cr supplementation may be a suitable method to prevent the gradual neuronal loss. Clinical studies have demonstrated that Cr supplementation reduces oxidative stress (Hersch et al. 2006) and glutamate concentration (Bender et al. 2005), as well as normalizing cerebral Cr content (Ryu et al. 2005). In addition, Tabrizi et al. (2005) found that Cr supplementation may also improve clinical status in some patients with Huntington’s disease. Notwithstanding the lack of larger RCT, Huntington’s disease seems to be a neurodegenerative disorder, sufferers of which could benefit from Cr supplementation.
Finally, it is important to highlight the advances in the studies involving Cr supplementation in patients with Parkinson’s events. This disease is clinically characterized by resting tremor, bradykinesia, rigidity, and postural imbalance (Andres et al. 2008). Evidence indicates that a defect in electron transport chain might be involved in the Parkinson’s physiopathology (Alam and Schmidt 2002; Schapira et al. 1990), which implies that therapeutic strategies focused on improvement in mitochondrial function may be promising in this pathology. In vitro experiments mimicking Parkinson’s disease revealed that Cr has protective effects against neurotoxic insults. However, the results of clinical studies remain controversial (Bender et al. 2006; Hass et al. 2007). Perhaps the best evidence on this issue will come at the conclusion of a large RCT enrolling about 1,700 patients, which is currently in progress (for details, see http://www.clinicaltrial.gov; identifier: NCT00449865).
The interest in the PCr–PCK system and, therefore, Cr supplementation effects on the CNS is relatively recent. The fact that exogenous Cr is able to cross the blood–brain barrier and increase the cerebral Cr concentration has generated great enthusiasm in the scientific community, since several neurological disorders are associated with reduced cerebral Cr content. Despite the promising findings in animal models, the results of several clinical studies have been considered somewhat disappointing. The apparent divergence may be partially explained by some methodological pitfalls. For example, Cr dosages in animal experiments are up to ten times greater than those in human trials (Andres et al. 2008). Moreover, it is important to note that there are major differences between species regarding Cr transport, metabolism, bioavailability, and physiological response, as previously pinpointed by elegant studies (Sewell and Harris 1995; Tarnopolsky et al. 2003). The capacity of transgenic animals to mimic neurological disorders in humans is also a matter of debate (Andres et al. 2008). Moreover, one may argue that the rather low permeability of the blood–brain barrier for Cr (Braissant and Henry 2008) might be one of the reasons for the lack of effect of Cr supplementation on CNS in many of the trials cited in this review. Therefore, it is reasonable to suggest that patients with Cr deficiency syndromes would have to be treated with very high doses of Cr on a long term basis (i.e., months or even years) in order to at least partially replenish cerebral Cr content (Battini et al. 2006; Schulze 2003). Once again, the interested reader is encouraged to consult the broad review by Andres et al. (2008).
Other therapeutic effects of Cr
Given that PCK isoforms are present in several organs, it is believed that the PCr–PCK system might play different physiological roles, depending on its location. In addition, the well-documented effects of Cr supplementation on bioenergetics, muscle mass, bone metabolism, and CNS could hypothetically confer benefits to heterogeneous populations. Table 3 depicts the putative therapeutic effects of Cr supplementation on several conditions.
It has been demonstrated that patients with heart failure have both diminished muscle Cr (Mancini et al. 1988) and cardiac ATP flux through PCK (Weiss et al. 2005). Accordingly, it has been speculated that Cr supplementation may increase energy provision by cardiomyocytes and thereby enhance heart contractile function, which would result in improved cardiac function in patients with congenital heart failure (CHF). Indeed, Gordon et al. (1995) demonstrated that Cr-supplemented patients with CHF exhibited increased muscle Cr and PCr content, which was related to the improvements in strength and aerobic capacity. However, no studies have verified the benefits on cardiac function as a result of Cr supplementation. Additionally, it is tempting to speculate that Cr supplementation can beneficially affect the skeletal muscle disorders previously reported in this population (Volaklis and Tokmakidis 2005). Also, considering the lack of long-term studies involving Cr supplementation on CHF patients, it is possible to assert that this field is open and needs to be further explored.
Moreover, studies have shown that Cr supplementation may promote improvement in carbohydrate and lipid metabolism. Earnest et al. (1996) found improved lipid profiles in hypercholesterolemic individuals supplemented with Cr. However, our group did not observe benefits in the lipid profiles of Cr-supplemented healthy subjects undergoing aerobic training (Gualano et al. 2008b). Apparently, pre-trial differences in lipoprotein concentrations between the samples may partially explain these seemingly contradictory findings. The mechanisms underlying the improvement in lipid profile also likely involve the effects of Cr on insulin sensitivity (see below), since it is known that changes in glycemia and/or insulinemia may directly affect blood lipoproteins.
Ferrante et al. (2000) revealed that Cr intake can ameliorate hyperglycemia, a typical condition observed in transgenic mice with Huntington’s disease, delaying the onset of diabetes. Supporting these findings, Op’t Eijnde et al. (2006) verified that Cr ingestion can reduce the insulinogenic index in an animal model of inherited type 2 diabetes. Recently, we demonstrated that Cr supplementation combined with aerobic training promote great improvement on glucose tolerance than aerobic training alone (Gualano et al. 2008a). Taken together, these results suggest that Cr might exert beneficial effects on glucose uptake mainly in insulin resistance conditions, although the exact mechanisms underlying these adaptations have yet to be clarified. Interestingly, Op‘t Eijnde et al. (2001) showed that Cr intake increases GLUT-4 protein content in line with glycogen accumulation during muscle disuse by immobilization and a subsequent period of training, suggesting a role for GLUT-4 in Cr-induced glucose uptake enhancement. We are currently investigating a possible therapeutic effect of Cr supplementation in patients with type II diabetes.
Atrophy is a strong and independent predictor of mortality in patients with chronic obstructive pulmonary disease (COPD) (Marquis et al. 2002). Furthermore, COPD patients present decreased muscle PCr content (Green et al. 2008). Thus, the use of Cr supplementation in this disease could be justified. Fuld et al. (2005) demonstrated that patients with CPOD supplemented with Cr have improved muscle mass, strength, aerobic capacity, and clinical condition compared to non-supplemented patients. However, two recent RCT did not reveal benefits in CPOD patients after Cr supplementation. The reasons for this discrepancy are unknown, and further studies are thus required to resolve this issue.
As discussed above, the anabolic effects of Cr supplementation guarantee its application to disorders of muscular etiology. Nonetheless, several diseases—independently of their physiopathology—occur with weakness and muscle mass waste due to the disease activity itself, chronic drug regime (i.e., corticoids), long-term bed rest, sedentary lifestyle, or malnutrition. This notion comprises acute lymphoblastic leukemia (ALL). Recently, it was shown that Cr supplementation was able to attenuate both body fat accumulation and body mass index in children with ALL during maintenance chemotherapy (Bourgeois et al. 2008). Long-term studies should be conducted to test the therapeutic effects of Cr supplementation (whether or not combined with exercise training) in ALL patients, preferentially using more clinically significant outcomes, such as quality of life and physical capacity.
The aging process is usually associated with a cluster of disturbances that includes sarcopenia, decreased muscle strength and power, physical inability, osteoporosis, cognitive function impairment, lipid profile disturbance, insulin resistance, mitochondrial dysfunction, and other functional and structural losses. Interestingly, a remarkable body of knowledge indicates that this adverse condition may be partially or even totally reverted by exercise training (for a review, see Pedersen and Saltin 2006). Hence, strategies for enhancing the effects of physical exercise are welcome. Considering the documented additive effects of Cr supplementation on resistance training, it is not surprising that Cr supplementation brings benefits to elderly people.
Following short-term Cr supplementation, senior subjects exhibit smaller increases in PCr than young subjects (Rawson et al. 2002). Therefore, it has been speculated that the Cr response may be minimized in elderly people. In fact, a meta-analysis performed in 2002 concluded that there was no available evidence supporting Cr use for elderly people (Dempsey et al. 2002). Since then, however, the number of studies involving supplementary Cr and elderly individuals considerably increased. While the elucidation of the mechanisms responsible for differences in Cr response between young and elderly subjects remains an open scientific question, the Cr-induced positive adaptations seen in aged people have an unequivocal relevance in clinical practice.
Stout et al. (2007) recently showed that short-term Cr supplementation (14 days) is sufficient to improve resistance to fatigue and strength in senior subjects. Brose et al. (2003) reported superior muscle mass and strength gains in Cr-supplemented and exercise-trained elderly individuals when compared to those who were only exercise-trained. McMorris et al. (2007b) observed marked improvement in cognitive function in Cr-supplemented elderly people. Chilibeck et al. (2005) verified that Cr supplementation promotes additive gains to resistance training on bone mineral density in elderly subjects. Taken together, these findings stress the Cr supplementation ability to prevent/attenuate several degenerative processes associated with aging. However, the long-term effects of Cr in older people remain unknown.
Recently, using an animal model, Bender et al. (2008) shed some light on this topic. This study demonstrated that mice supplemented with Cr presented a longer healthy life span (9%). Cr-fed mice showed a trend towards reduction in ROS and lower accumulation of the “aging pigment” lipofuscin in the brain. As well, genes related to neuronal growth, neuroprotection, and learning were overexpressed in Cr-fed mice. Overall, these findings led the authors to conclude that Cr supplementation is a promising nutritional strategy for increasing longevity and improving health during the aging process. Future studies need to investigate whether these results can be reproduced in humans.
Conclusions and perspectives
Considering the widespread effects of Cr supplementation, one may conclude that this molecule could be one of the most promising nutritional supplements in the therapeutic field. However, the reader should be aware that several therapeutic effects attributed to this supplement are still speculative. In other words, although in vitro and animal studies have provided strong rationality for clinical Cr benefits, the number of RCT is very limited. Currently, numerous large RCTs are being conducted to investigate the effects of Cr supplementation on broad diseases, including Parkinson’s, Huntington’s, depression, AIDS, osteoarthritis, osteoporosis, type II diabetes, etc. (for examples, see http://www.clinicaltrial.gov). Indeed, the available clinical information about Cr therapeutic application will greatly increase in the near future.
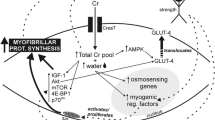
In parallel, it is important that “basic science” continues elucidating the mechanisms by which Cr supplementation exerts its effects. Using the microarray technique, Safdar et al. (2008) recently demonstrated that Cr supplementation for 10 days enhances the expression of genes related to osmotic regulation, satellite cell proliferation and differentiation, DNA repair and replication, RNA transcription control, and cell death. Recently developed molecular biology techniques (i.e., proteomic analyses) will allow us to identify proteins that are, in fact, expressed and activated by previously described genes as a result of Cr intake. Certainly, the knowledge transference from basic to more applied levels is a key step in the consolidation of Cr as a therapeutic supplement.
Some unresolved issues with respect to Cr supplementation protocol are particularly relevant in the therapeutic field. For example, it has been noted that Cr transport saturation occurs as a result of chronic Cr intake in rats (Guerrero-Ontiveros and Wallimann 1998). However, this observation has not yet been confirmed in humans. Thus, it is compulsory to evaluate whether “cycled” Cr supplementation provides greater benefits than continuous classical regimen or not.
Despite the large body of evidence indicating that Cr supplementation promotes increased strength and lean mass, a very interesting study showed that healthy subjects supplemented with Cr and submitted to 8-week resistance training had similar strength and lean mass gains as compared to those who underwent the same training and were supplemented with an isoenergetic and isonitrogenous diet (Tarnopolsky et al. 2001). These findings suggest that studies that include a carbohydrate as placebo could overestimate the effects of Cr supplementation on skeletal muscle. Future clinical studies must consider isoenergetic and isonitrogenous placebo in order to avoid this bias.
Finally, it is noteworthy to point out that Cr supplementation seems to be a safe supplement, as opposed to some allegations from several food agencies and expert organizations (such as: AFSSA, French Agency for Sanitary and Alimentary Security; FDA, Food and Drug Administration USA; ACSM, American College of Sports Medicine). The major controversial point refers to renal function, since a few case reports have attributed renal dysfunction to Cr supplementation (Kuehl et al. 1998; Pritchard and Kalra 1998; Thorsteinsdottir et al. 2006). However, our groups (Gualano et al. 2008c; Poortmans 1998; Poortmans and Francaux 2008 Poortmans et al. 1997; Poortmans and Francaux 1999, 2000; Poortmans et al. 2005; Poortmans and Francaux 2008) and others (Kreider et al. 2003; Robinson et al. 2000) have repeatedly demonstrated that Cr supplementation does not induce renal disease in healthy individuals. Nonetheless, we prudently recommended the periodical assessment of renal parameters (i.e., every 3 months), especially in individuals with (or at risk for) renal function impairment for whom there is a lack of safety data (i.e., elderly, cancer, type II diabetes, etc.). Since the possible adverse effects of Cr supplementation are beyond the scope of this review, the interested reader is encouraged to see the comprehensive reviews by Poortmans and Francaux 2000, 2008.
Even if emergent therapeutic effects of Cr supplementation have provoked great enthusiasm in the scientific community, further disorder-specific RCTs must be applied to fully appreciate the therapeutic role of Cr supplementation.
References
Adcock KH, Nedelcu J, Loenneker T, Martin E, Wallimann T, Wagner BP (2002) Neuroprotection of creatine supplementation in neonatal rats with transient cerebral hypoxia-ischemia. Dev Neurosci 24:382–388. doi:10.1159/000069043
Aksenov MY, Aksenova MV, Payne RM, Smith CD, Markesbery WR, Carney JM (1997) The expression of creatine kinase isoenzymes in neocortex of patients with neurodegenerative disorders: Alzheimer’s and Pick’s disease. Exp Neurol 146:458–465. doi:10.1006/exnr.1997.6550
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136:317–324. doi:10.1016/S0166-4328(02)00180-8
Almeida LS, Salomons GS, Hogenboom F, Jakobs C, Schoffelmeer AN (2006) Exocytotic release of creatine in rat brain. Synapse 60:118–123. doi:10.1002/syn.20280
Amital D, Vishne T, Roitman S, Kotler M, Levine J (2006a) Open study of creatine monohydrate in treatment-resistant posttraumatic stress disorder. J Clin Psychiatry 67:836–837
Amital D, Vishne T, Rubinow A, Levine J (2006b) Observed effects of creatine monohydrate in a patient with depression and fibromyalgia. Am J Psychiatry 163:1840–1841. doi:10.1176/appi.ajp.163.10.1840-b
Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR (2008) Functions and effects of creatine in the central nervous system. Brain Res Bull 76:329–343. doi:10.1016/j.brainresbull.2008.02.035
Andrews R, Greenhaff P, Curtis S, Perry A, Cowley AJ (1998) The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur Heart J 19:617–622. doi:10.1053/euhj.1997.0767
Battini R, Alessandri MG, Leuzzi V, Moro F, Tosetti M, Bianchi MC, Cioni G (2006) Arginine:glycine amidinotransferase (AGAT) deficiency in a newborn: early treatment can prevent phenotypic expression of the disease. J Pediatr 148:828–830. doi:10.1016/j.jpeds.2006.01.043
Bender A, Auer DP, Merl T, Reilmann R, Saemann P, Yassouridis A, Bender J, Weindl A, Dose M, Gasser T, Klopstock T (2005) Creatine supplementation lowers brain glutamate levels in Huntington’s disease. J Neurol 252:36–41. doi:10.1007/s00415-005-0595-4
Bender A, Koch W, Elstner M, Schombacher Y, Bender J, Moeschl M, Gekeler F, Muller-Myhsok B, Gasser T, Tatsch K, Klopstock T (2006) Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology 67:1262–1264. doi:10.1212/01.wnl.0000238518.34389.12
Bender A, Beckers J, Schneider I, Holter SM, Haack T, Ruthsatz T, Vogt-Weisenhorn DM, Becker L, Genius J, Rujescu D, Irmler M, Mijalski T, Mader M, Quintanilla-Martinez L, Fuchs H, Gailus-Durner V, de Angelis MH, Wurst W, Schmidt J, Klopstock T (2008) Creatine improves health and survival of mice. Neurobiol Aging 29:1404–1411. doi:10.1016/j.neurobiolaging.2007.03.001
Bourgeois JM, Nagel K, Pearce E, Wright M, Barr RD, Tarnopolsky MA (2008) Creatine monohydrate attenuates body fat accumulation in children with acute lymphoblastic leukemia during maintenance chemotherapy. Pediatr Blood Cancer 51:183–187. doi:10.1002/pbc.21571
Braissant O, Henry H (2008) AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: a review. J Inherit Metab Dis (Epub ahead of print)
Braissant O, Henry H, Villard AM, Zurich MG, Loup M, Eilers B, Parlascino G, Matter E, Boulat O, Honegger P, Bachmann C (2002) Ammonium-induced impairment of axonal growth is prevented through glial creatine. J Neurosci 22:9810–9820
Brewer GJ, Wallimann TW (2000) Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem 74:1968–1978. doi:10.1046/j.1471-4159.2000.0741968.x
Brose A, Parise G, Tarnopolsky MA (2003) Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J Gerontol A Biol Sci Med Sci 58:11–19
Browne SE, Yang L, DiMauro JP, Fuller SW, Licata SC, Beal MF (2006) Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Neurobiol Dis 22:599–610. doi:10.1016/j.nbd.2006.01.001
Cagnon L, Braissant O (2007) Hyperammonemia-induced toxicity for the developing central nervous system. Brain Res Rev 56:183–197. doi:10.1016/j.brainresrev.2007.06.026
Candow DG, Little JP, Chilibeck PD, Abeysekara S, Zello GA, Kazachkov M, Cornish SM, Yu PH (2008) Low-dose creatine combined with protein during resistance training in older men. Med Sci Sports Exerc (Epub ahead of print)
Chilibeck PD, Chrusch MJ, Chad KE, Shawn Davison K, Burke DG (2005) Creatine monohydrate and resistance training increase bone mineral content and density in older men. J Nutr Health Aging 9:352–353
Chung YL, Alexanderson H, Pipitone N, Morrison C, Dastmalchi M, Stahl-Hallengren C, Richards S, Thomas EL, Hamilton G, Bell JD, Lundberg IE, Scott DL (2007) Creatine supplements in patients with idiopathic inflammatory myopathies who are clinically weak after conventional pharmacologic treatment: 6-month, double-blind, randomized, placebo-controlled trial. Arthritis Rheum 57:694–702. doi:10.1002/art.22687
Coplan JD, Mathew SJ, Mao X, Smith EL, Hof PR, Coplan PM, Rosenblum LA, Gorman JM, Shungu DC (2006) Decreased choline and creatine concentrations in centrum semiovale in patients with generalized anxiety disorder: relationship to IQ and early trauma. Psychiatry Res 147:27–39. doi:10.1016/j.pscychresns.2005.12.011
De Deyn PP, Macdonald RL (1990) Guanidino compounds that are increased in cerebrospinal fluid and brain of uremic patients inhibit GABA and glycine responses on mouse neurons in cell culture. Ann Neurol 28:627–633. doi:10.1002/ana.410280505
De Deyn PP, Wirshing WC (2001) Scales to assess efficacy and safety of pharmacologic agents in the treatment of behavioral and psychological symptoms of dementia. J Clin Psychiatry 62(Suppl 21):19–22
Deacon SJ, Vincent EE, Greenhaff PL, Fox J, Steiner MC, Singh SJ, Morgan MD (2008) Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178:233–239. doi:10.1164/rccm.200710-1508OC
Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M (2008) Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol 104:371–378. doi:10.1152/japplphysiol.00873.2007
Dempsey RL, Mazzone MF, Meurer LN (2002) Does oral creatine supplementation improve strength? A meta-analysis. J Fam Pract 51:945–951
Drory VE, Gross D (2002) No effect of creatine on respiratory distress in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 3:43–46. doi:10.1080/146608202317576534
Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP (2004) Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci USA 101:11159–11164. doi:10.1073/pnas.0402026101
Earnest CP, Almada AL, Mitchell TL (1996) High-performance capillary electrophoresis-pure creatine monohydrate reduces blood lipids in men and women. Clin Sci (Lond) 91:113–118
Escolar DM, Buyse G, Henricson E, Leshner R, Florence J, Mayhew J, Tesi-Rocha C, Gorni K, Pasquali L, Patel KM, McCarter R, Huang J, Mayhew T, Bertorini T, Carlo J, Connolly AM, Clemens PR, Goemans N, Iannaccone ST, Igarashi M, Nevo Y, Pestronk A, Subramony SH, Vedanarayanan VV, Wessel H (2005) CINRG randomized controlled trial of creatine and glutamine in Duchenne muscular dystrophy. Ann Neurol 58:151–155. doi:10.1002/ana.20523
Faager G, Soderlund K, Skold CM, Rundgren S, Tollback A, Jakobsson P (2006) Creatine supplementation and physical training in patients with COPD: a double blind, placebo-controlled study. Int J Chron Obstruct Pulmon Dis 1:445–453. doi:10.2147/copd.2006.1.4.445
Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF (2000) Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J Neurosci 20:4389–4397
Francaux M, Poortmans JR (1999) Effects of training and creatine supplement on muscle strength and body mass. Eur J Appl Physiol Occup Physiol 80:165–168. doi:10.1007/s004210050575
Fuld JP, Kilduff LP, Neder JA, Pitsiladis Y, Lean ME, Ward SA, Cotton MM (2005) Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 60:531–537. doi:10.1136/thx.2004.030452
Funanage VL, Carango P, Shapiro IM, Tokuoka T, Tuan RS (1992) Creatine kinase activity is required for mineral deposition and matrix synthesis in endochondral growth cartilage. Bone Miner 17:228–236. doi:10.1016/0169-6009(92)90742-V
Galbraith RA, Furukawa M, Li M (2006) Possible role of creatine concentrations in the brain in regulating appetite and weight. Brain Res 1101:85–91. doi:10.1016/j.brainres.2006.05.032
Gerber I, ap Gwynn I, Alini M, Wallimann T (2005) Stimulatory effects of creatine on metabolic activity, differentiation and mineralization of primary osteoblast-like cells in monolayer and micromass cell cultures. Eur Cell Mater 10:8–22
Gordon A, Hultman E, Kaijser L, Kristjansson S, Rolf CJ, Nyquist O, Sylven C (1995) Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc Res 30:413–418
Green HJ, Burnett ME, D’Arsigny CL, O’Donnell DE, Ouyang J, Webb KA (2008) Altered metabolic and transporter characteristics of vastus lateralis in chronic obstructive pulmonary disease. J Appl Physiol 105:879–886. doi:10.1152/japplphysiol.90458.2008
Greenhaff PL (2001) The creatine–phosphocreatine system: there’s more than one song in its repertoire. J Physiol 537:657. doi:10.1113/jphysiol.2001.013478
Groeneveld GJ, Veldink JH, van der Tweel I, Kalmijn S, Beijer C, de Visser M, Wokke JH, Franssen H, van den Berg LH (2003) A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Ann Neurol 53:437–445. doi:10.1002/ana.10554
Grunewald T, Beal MF (1999) Bioenergetics in Huntington’s disease. Ann N Y Acad Sci 893:203–213. doi:10.1111/j.1749-6632.1999.tb07827.x
Gualano B, Novaes RB, Artioli GG, Freire TO, Coelho DF, Scagliusi FB, Rogeri PS, Roschel H, Ugrinowitsch C, Lancha AH Jr (2008a) Effects of creatine supplementation on glucose tolerance and insulin sensitivity in sedentary healthy males undergoing aerobic training. Amino Acids 34:245–250. doi:10.1007/s00726-007-0508-1
Gualano B, Ugrinowitsch C, Artioli GG, Benatti FB, Scagliusi FB, Harris RC, Lancha AH Jr (2008b) Does creatine supplementation improve the plasma lipid profile in healthy male subjects undergoing aerobic training? J Int Soc Sports Nutr 5:16. doi:10.1186/1550-2783-5-16
Gualano B, Ugrinowitsch C, Novaes RB, Artioli GG, Shimizu MH, Seguro AC, Harris RC, Lancha AH Jr (2008c) Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trial. Eur J Appl Physiol 103:33–40. doi:10.1007/s00421-007-0669-3
Guerrero-Ontiveros ML, Wallimann T (1998) Creatine supplementation in health and disease. Effects of chronic creatine ingestion in vivo: down-regulation of the expression of creatine transporter isoforms in skeletal muscle. Mol Cell Biochem 184:427–437. doi:10.1023/A:1006895414925
Harris RC, Soderlund K, Hultman E (1992) Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 83:367–374
Hass CJ, Collins MA, Juncos JL (2007) Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: a randomized trial. Neurorehabil Neural Repair 21:107–115. doi:10.1177/1545968306293449
Hausmann ON, Fouad K, Wallimann T, Schwab ME (2002) Protective effects of oral creatine supplementation on spinal cord injury in rats. Spinal Cord 40:449–456. doi:10.1038/sj.sc.3101330
Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, Como P, Zimmerman C, Lin M, Zhang L, Ulug AM, Beal MF, Matson W, Bogdanov M, Ebbel E, Zaleta A, Kaneko Y, Jenkins B, Hevelone N, Zhang H, Yu H, Schoenfeld D, Ferrante R, Rosas HD (2006) Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology 66:250–252. doi:10.1212/01.wnl.0000194318.74946.b6
Hobson GM, Funanage VL, Elsemore J, Yagami M, Rajpurohit R, Perriard JC, Hickok NJ, Shapiro IM, Tuan RS (1999) Developmental expression of creatine kinase isoenzymes in chicken growth cartilage. J Bone Miner Res 14:747–756. doi:10.1359/jbmr.1999.14.5.747
in‘t Zandt HJ, Renema WK, Streijger F, Jost C, Klomp DW, Oerlemans F, Van der Zee CE, Wieringa B, Heerschap A (2004) Cerebral creatine kinase deficiency influences metabolite levels and morphology in the mouse brain: a quantitative in vivo 1H and 31P magnetic resonance study. J Neurochem 90:1321–1330
Item CB, Stockler-Ipsiroglu S, Stromberger C, Muhl A, Alessandri MG, Bianchi MC, Tosetti M, Fornai F, Cioni G (2001) Arginine:glycine amidinotransferase deficiency: the third inborn error of creatine metabolism in humans. Am J Hum Genet 69:1127–1133
Kaldis P, Hemmer W, Zanolla E, Holtzman D, Wallimann T (1996) ‘Hot spots’ of creatine kinase localization in brain: cerebellum, hippocampus and choroid plexus. Dev Neurosci 18:542–554
Kley RA, Vorgerd M, Tarnopolsky MA (2007) Creatine for treating muscle disorders. Cochrane Database Syst Rev CD004760
Klopstock T, Querner V, Schmidt F, Gekeler F, Walter M, Hartard M, Henning M, Gasser T, Pongratz D, Straube A, Dieterich M, Muller-Felber W (2000) A placebo-controlled crossover trial of creatine in mitochondrial diseases. Neurology 55:1748–1751
Koga Y, Takahashi H, Oikawa D, Tachibana T, Denbow DM, Furuse M (2005) Brain creatine functions to attenuate acute stress responses through GABAnergic system in chicks. Neuroscience 132:65–71
Kornblum C, Schroder R, Muller K, Vorgerd M, Eggers J, Bogdanow M, Papassotiropoulos A, Fabian K, Klockgether T, Zange J (2005) Creatine has no beneficial effect on skeletal muscle energy metabolism in patients with single mitochondrial DNA deletions: a placebo-controlled, double-blind 31P-MRS crossover study. Eur J Neurol 12:300–309
Kreider RB, Melton C, Rasmussen CJ, Greenwood M, Lancaster S, Cantler EC, Milnor P, Almada AL (2003) Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol Cell Biochem 244:95–104
Kuehl K, Goldberg L, Elliot D (1998) Renal insufficiency after creatine supplementation in a college football athlete (abstract). Med Sci Sports Exerc 30:S235
Kuethe F, Krack A, Richartz BM, Figulla HR (2006) Creatine supplementation improves muscle strength in patients with congestive heart failure. Pharmazie 61:218–222
Lensman M, Korzhevskii DE, Mourovets VO, Kostkin VB, Izvarina N, Perasso L, Gandolfo C, Otellin VA, Polenov SA, Balestrino M (2006) Intracerebroventricular administration of creatine protects against damage by global cerebral ischemia in rat. Brain Res 1114:187–194
Louis M, Lebacq J, Poortmans JR, Belpaire-Dethiou MC, Devogelaer JP, Van Hecke P, Goubel F, Francaux M (2003a) Beneficial effects of creatine supplementation in dystrophic patients. Muscle Nerve 27:604–610
Louis M, Poortmans JR, Francaux M, Berre J, Boisseau N, Brassine E, Cuthbertson DJ, Smith K, Babraj JA, Waddell T, Rennie MJ (2003b) No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol Endocrinol Metab 285:E1089–E1094
Mancini DM, Ferraro N, Tuchler M, Chance B, Wilson JR (1988) Detection of abnormal calf muscle metabolism in patients with heart failure using phosphorus-31 nuclear magnetic resonance. Am J Cardiol 62:1234–1240
Marquis K, Debigare R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F (2002) Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166:809–813
Mazzini L, Balzarini C, Colombo R, Mora G, Pastore I, De Ambrogio R, Caligari M (2001) Effects of creatine supplementation on exercise performance and muscular strength in amyotrophic lateral sclerosis: preliminary results. J Neurol Sci 191:139–144
McMorris T, Harris RC, Swain J, Corbett J, Collard K, Dyson RJ, Dye L, Hodgson C, Draper N (2006) Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology (Berl) 185:93–103
McMorris T, Harris RC, Howard AN, Langridge G, Hall B, Corbett J, Dicks M, Hodgson C (2007a) Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol Behav 90:21–28
McMorris T, Mielcarz G, Harris RC, Swain JP, Howard A (2007b) Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14:517–528
Menezes LG, Sobreira C, Neder L, Rodrigues-Junior AL, Martinez JA (2007) Creatine supplementation attenuates corticosteroid-induced muscle wasting and impairment of exercise performance in rats. J Appl Physiol 102:698–703
NET-PD N (2006) A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 66:664–671
Nissen SL, Sharp RL (2003) Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol 94(2):651–659
Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M (2006) Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol 573:525–534
Op‘t Eijnde B, Richter EA, Henquin JC, Kiens B, Hespel P (2001) Effect of creatine supplementation on creatine and glycogen content in rat skeletal muscle. Acta Physiol Scand 171:169–176
Op’t Eijnde B, Jijakli H, Hespel P, Malaisse WJ (2006) Creatine supplementation increases soleus muscle creatine content and lowers the insulinogenic index in an animal model of inherited type 2 diabetes. Int J Mol Med 17:1077–1084
Ozkan O, Duman O, Haspolat S, Ozgentas HE, Dikici MB, Gurer I, Gungor HA, Guzide Gokhan A (2005) Effect of systemic creatine monohydrate supplementation on denervated muscle during reinnervation: experimental study in the rat. J Reconstr Microsurg 21:573–579
Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA (2001) Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol 91:1041–1047
Passaquin AC, Renard M, Kay L, Challet C, Mokhtarian A, Wallimann T, Ruegg UT (2002) Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in mdx mice. Neuromuscul Disord 12:174–182
Pedersen BK, Saltin B (2006) Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 16(Suppl 1):3–63
Poortmans JR (1998) Renal dysfunction accompanying oral creatine supplements: reply. Lancet 352:234
Poortmans JR, Francaux M (1999) Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med Sci Sports Exerc 31:1108–1110
Poortmans JR, Francaux M (2000) Adverse effects of creatine supplementation: fact or fiction? Sports Med 30:155–170
Poortmans JR, Francaux M (2008) Creatine consumption in health. In: Stout JR, Antonio J, Kalman D (eds) Essentials of creatine in sports and health. Humana Press, Totawa, pp 127–172
Poortmans JR, Auquier H, Renaut V, Durussel A, Saugy M, Brisson GR (1997) Effect of short-term creatine supplementation on renal responses in men. Eur J Appl Physiol Occup Physiol 76:566–567
Poortmans JR, Kumps A, Duez P, Fofonka A, Carpentier A, Francaux M (2005) Effect of oral creatine supplementation on urinary methylamine, formaldehyde, and formate. Med Sci Sports Exerc 37:1717–1720
Pritchard NR, Kalra PA (1998) Renal dysfunction accompanying oral creatine supplements. Lancet 351:1252–1253
Rabchevsky AG, Sullivan PG, Fugaccia I, Scheff SW (2003) Creatine diet supplement for spinal cord injury: influences on functional recovery and tissue sparing in rats. J Neurotrauma 20:659–669
Rawson ES, Clarkson PM, Price TB, Miles MP (2002) Differential response of muscle phosphocreatine to creatine supplementation in young and old subjects. Acta Physiol Scand 174:57–65
Robinson TM, Sewell DA, Casey A, Steenge G, Greenhaff PL (2000) Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. Br J Sports Med 34:284–288
Ryu H, Rosas HD, Hersch SM, Ferrante RJ (2005) The therapeutic role of creatine in Huntington’s disease. Pharmacol Ther 108:193–207
Safdar A, Yardley NJ, Snow R, Melov S, Tarnopolsky MA (2008) Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Genomics 32:219–228
Sakellaris G, Kotsiou M, Tamiolaki M, Kalostos G, Tsapaki E, Spanaki M, Spilioti M, Charissis G, Evangeliou A (2006) Prevention of complications related to traumatic brain injury in children and adolescents with creatine administration: an open label randomized pilot study. J Trauma 61:322–329
Salomons GS, van Dooren SJ, Verhoeven NM, Cecil KM, Ball WS, Degrauw TJ, Jakobs C (2001) X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet 68:1497–1500
Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54:823–827
Scheff SW, Dhillon HS (2004) Creatine-enhanced diet alters levels of lactate and free fatty acids after experimental brain injury. Neurochem Res 29:469–479
Schneider-Gold C, Beck M, Wessig C, George A, Kele H, Reiners K, Toyka KV (2003) Creatine monohydrate in DM2/PROMM: a double-blind placebo-controlled clinical study. Proximal myotonic myopathy. Neurology 60:500–502
Schulze A (2003) Creatine deficiency syndromes. Mol Cell Biochem 244:143–150
Sewell DA, Harris RC (1995) Effect of creatine supplementation in the thoroughbred horse. Equine Vet J 18:239–242
Shefner JM, Cudkowicz ME, Schoenfeld D, Conrad T, Taft J, Chilton M, Urbinelli L, Qureshi M, Zhang H, Pestronk A, Caress J, Donofrio P, Sorenson E, Bradley W, Lomen-Hoerth C, Pioro E, Rezania K, Ross M, Pascuzzi R, Heiman-Patterson T, Tandan R, Mitsumoto H, Rothstein J, Smith-Palmer T, MacDonald D, Burke D (2004) A clinical trial of creatine in ALS. Neurology 63:1656–1661
Smith CA, Chetlin RD, Gutmann L, Yeater RA, Alway SE (2006) Effects of exercise and creatine on myosin heavy chain isoform composition in patients with Charcot-Marie-Tooth disease. Muscle Nerve 34:586–594
Somjen D, Kaye AM (1994) Stimulation by insulin-like growth factor-I of creatine kinase activity in skeletal-derived cells and tissues of male and female rats. J Endocrinol 143:251–259
Somjen D, Kaye AM, Rodan GA, Binderman I (1985) Regulation of creatine kinase activity in rat osteogenic sarcoma cell clones by parathyroid hormone, prostaglandin E2, and vitamin D metabolites. Calcif Tissue Int 37:635–638
Stockler S, Holzbach U, Hanefeld F, Marquardt I, Helms G, Requart M, Hanicke W, Frahm J (1994) Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res 36:409–413
Stockler S, Hanefeld F, Frahm J (1996) Creatine replacement therapy in guanidinoacetate methyltransferase deficiency, a novel inborn error of metabolism. Lancet 348:789–790
Stout JR, Sue Graves B, Cramer JT, Goldstein ER, Costa PB, Smith AE, Walter AA (2007) Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64–86 years). J Nutr Health Aging 11:459–464
Tabrizi SJ, Blamire AM, Manners DN, Rajagopalan B, Styles P, Schapira AH, Warner TT (2003) Creatine therapy for Huntington’s disease: clinical and MRS findings in a 1-year pilot study. Neurology 61:141–142
Tabrizi SJ, Blamire AM, Manners DN, Rajagopalan B, Styles P, Schapira AH, Warner TT (2005) High-dose creatine therapy for Huntington disease: a 2-year clinical and MRS study. Neurology 64:1655–1656
Tarnopolsky MA (2007) Clinical use of creatine in neuromuscular and neurometabolic disorders. Subcell Biochem 46:183–204
Tarnopolsky M, Martin J (1999) Creatine monohydrate increases strength in patients with neuromuscular disease. Neurology 52:854–857
Tarnopolsky MA, Parise G (1999) Direct measurement of high-energy phosphate compounds in patients with neuromuscular disease. Muscle Nerve 22:1228–1233
Tarnopolsky MA, Roy BD, MacDonald JR (1997) A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve 20:1502–1509
Tarnopolsky MA, Parise G, Yardley NJ, Ballantyne CS, Olatinji S, Phillips SM (2001) Creatine-dextrose and protein-dextrose induce similar strength gains during training. Med Sci Sports Exerc 33:2044–2052
Tarnopolsky MA, Bourgeois JM, Snow R, Keys S, Roy BD, Kwiecien JM, Turnbull J (2003) Histological assessment of intermediate- and long-term creatine monohydrate supplementation in mice and rats. Am J Physiol Regul Integr Comp Physiol 285:R762–R769
Tarnopolsky M, Mahoney D, Thompson T, Naylor H, Doherty TJ (2004a) Creatine monohydrate supplementation does not increase muscle strength, lean body mass, or muscle phosphocreatine in patients with myotonic dystrophy type 1. Muscle Nerve 29:51–58
Tarnopolsky MA, Mahoney DJ, Vajsar J, Rodriguez C, Doherty TJ, Roy BD, Biggar D (2004b) Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology 62:1771–1777
Terjung RL, Clarkson P, Eichner ER, Greenhaff PL, Hespel PJ, Israel RG, Kraemer WJ, Meyer RA, Spriet LL, Tarnopolsky MA, Wagenmakers AJ, Williams MH (2000) American College of Sports Medicine roundtable. The physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc 32:706–717
Thorsteinsdottir B, Grande JP, Garovic VD (2006) Acute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrate. J Ren Nutr 16:341–345
Verbessem P, Lemiere J, Eijnde BO, Swinnen S, Vanhees L, Van Leemputte M, Hespel P, Dom R (2003) Creatine supplementation in Huntington’s disease: a placebo-controlled pilot trial. Neurology 61:925–930
Volaklis KA, Tokmakidis SP (2005) Resistance exercise training in patients with heart failure. Sports Med 35:1085–1103
Vorgerd M, Grehl T, Jager M, Muller K, Freitag G, Patzold T, Bruns N, Fabian K, Tegenthoff M, Mortier W, Luttmann A, Zange J, Malin JP (2000) Creatine therapy in myophosphorylase deficiency (McArdle disease): a placebo-controlled crossover trial. Arch Neurol 57:956–963
Vorgerd M, Zange J, Kley R, Grehl T, Husing A, Jager M, Muller K, Schroder R, Mortier W, Fabian K, Malin JP, Luttmann A (2002) Effect of high-dose creatine therapy on symptoms of exercise intolerance in McArdle disease: double-blind, placebo-controlled crossover study. Arch Neurol 59:97–101
Wallimann T, Hemmer W (1994) Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem 133–134:193–220
Walter MC, Lochmuller H, Reilich P, Klopstock T, Huber R, Hartard M, Hennig M, Pongratz D, Muller-Felber W (2000) Creatine monohydrate in muscular dystrophies: a double-blind, placebo-controlled clinical study. Neurology 54:1848–1850
Walter MC, Reilich P, Lochmuller H, Kohnen R, Schlotter B, Hautmann H, Dunkl E, Pongratz D, Muller-Felber W (2002) Creatine monohydrate in myotonic dystrophy: a double-blind, placebo-controlled clinical study. J Neurol 249:1717–1722
Watanabe A, Kato N, Kato T (2002) Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci Res 42:279–285
Weiss RG, Gerstenblith G, Bottomley PA (2005) ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci USA 102:808–813
Wendt S, Dedeoglu A, Speer O, Wallimann T, Beal MF, Andreassen OA (2002) Reduced creatine kinase activity in transgenic amyotrophic lateral sclerosis mice. Free Radic Biol Med 32:920–926
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80:1107–1213
Acknowledgments
Bruno Gualano is grateful to Conselho Nacional de Pesquisa e Desenvolvimento (CNPq). All authors contributed equally to this manuscript.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gualano, B., Artioli, G.G., Poortmans, J.R. et al. Exploring the therapeutic role of creatine supplementation. Amino Acids 38, 31–44 (2010). https://doi.org/10.1007/s00726-009-0263-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-009-0263-6