Abstract
Dietary supplementation with N-acetylcysteine (NAC) has been reported to improve intestinal health and treat gastrointestinal diseases. However, the underlying mechanisms are not fully understood. According to previous reports, NAC was thought to exert its effect through glutathione synthesis. This study tested the hypothesis that NAC enhances enterocyte growth and protein synthesis independently of cellular glutathione synthesis. Intestinal porcine epithelial cells were cultured for 3 days in Dulbecco’s modified Eagle medium containing 0 or 100 μM NAC. To determine a possible role for GSH (the reduced form of glutathione) in mediating the effect of NAC on cell growth and protein synthesis, additional experiments were conducted using culture medium containing 100 μM GSH, 100 μM GSH ethyl ester (GSHee), diethylmaleate (a GSH-depletion agent; 10 μM), or a GSH-synthesis inhibitor (buthionine sulfoximine, BSO; 20 μM). NAC increased cell proliferation, GSH concentration, and protein synthesis, while inhibiting proteolysis. GSHee enhanced cell proliferation and GSH concentration without affecting protein synthesis but inhibited proteolysis. Conversely, BSO or diethylmaleate reduced cell proliferation and GSH concentration without affecting protein synthesis, while promoting protein degradation. At the signaling level, NAC augmented the protein abundance of total mTOR, phosphorylated mTOR, and phosphorylated 70S6 kinase as well as mRNA levels for mTOR and p70S6 kinase in IPEC-1 cells. Collectively, these results indicate that NAC upregulates expression of mTOR signaling proteins to stimulate protein synthesis in enterocytes independently of GSH generation. Our findings provide a hitherto unrecognized biochemical mechanism for beneficial effects of NAC in intestinal cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
N-acetylcysteine (NAC), an aminothiol and synthetic precursor of intracellular cysteine and GSH, has been used in clinical practice for several decades (Samuni et al. 2013). For example, NAC is effective in treating various inflammatory diseases, such as gastrointestinal inflammation, cardiac injury, acute respiratory distress syndrome, bronchitis, AIDS, nephropathy, and psychiatric disorders (Samuni et al. 2013). NAC is chemically stable in physiological fluid and is readily transported by cells (Lai et al. 2012; Lee et al. 2013). Therefore, NAC provides a direct and convenient means to increase the intracellular concentration of glutathione (GSH, the reduced form of glutathione) in many tissues and cell types (Yang et al. 2012).
NAC can directly react with oxidative metabolites and protect cells from oxidative damage and indirectly exert antioxidative effects via the synthesis of GSH (Wu et al. 2004; Lai et al. 2012). Hence, it has generally been assumed that the efficacy of NAC in treating clinical diseases is due to its antioxidant/radical scavenger properties (Kim et al. 2001). However, through modulating levels of various target genes and/or proteins, NAC has also been reported to regulate cell cycle and apoptosis, including the inhibition of proliferation of normal human cells and transformed cells (Samuni et al. 2013), prevention of apoptosis of neuronal cells and fibroblasts (Cossarizza et al. 1995), and enhancement of survival of PC12 and endothelial cells (Barajas-Espinosa et al. 2014). Apparently, there are discrepancies among these studies with regard to effects of NAC on cell growth and apoptosis because the mechanisms responsible for the biological function of NAC are incompletely understood.
In bowel diseases, NAC is an adjuvant agent in the treatment of necrotizing enterocolitis and Crohn’s disease (Ozdemir et al. 2012; Romagnoli et al. 2013). These studies concluded that the beneficial effects of NAC on intestinal health were due largely to the increased GSH content. Although GSH is known to be important for intestinal function (Mårtensson et al. 1990; Wu et al. 2004), emerging evidence indicates that NAC may protect intestinal cells from oxidative injury and apoptosis (Hou et al. 2012; Zhu et al. 2013). At present, there are no studies regarding effects of NAC on protein turnover (synthesis and degradation) in any cell type, although we demonstrated that NAC enhanced intestinal mucosal growth and improved intestinal function under lipopolysaccharide or acetic acid challenges (Hou et al. 2012; Wang et al. 2013).
According to published reports, NAC has long been thought to exert positive effects in intestinal cells mainly through GSH synthesis and antioxidative reactions (Barajas-Espinosa et al. 2014; Kim et al. 2001). However, alternative mechanisms for the biological function of NAC are possible but largely unknown. In view of the foregoing, we hypothesize that NAC enhances cell growth and protein synthesis independently of intracellular GSH synthesis. The purpose of this study is to test this hypothesis, and the findings will provide an important clue for understanding cellular and molecular mechanisms whereby NAC exerts beneficial effects on human and animal intestinal health.
Materials and methods
Reagents
N-Acetylcysteine (NAC), diethylmaleate (DEM), buthionine sulfoximine (BSO), glutathione (GSH), and glutathione ethyl ester (GSHee) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s F12 Ham medium (DMEM-F12), fetal bovine serum (FBS), and antibiotics were purchased from invitrogen (Grand Island, NY, USA). Epidermal growth factor and selenium were obtained from BD Biosciences (Bedford, MA, USA). Plastic culture plates were manufactured by Corning Inc. (Corning, NY, USA). Unless indicated, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Experimental design
Several experiments were performed with IPEC-1 cells, which is a stable cell line of enterocytes isolated from newborn pigs (Haynes et al. 2009). First, effect of NAC (100 μM) on cell growth was determined. Second, the role of GSH on regulation of NAC on cell growth was determined using a GSH-depletion agent (DEM), a GSH-synthesis inhibitor (BSO), or GSH ethyl ester (GSHee). Third, effects of NAC, BSO, DEM, and GSHee on intracellular concentrations of cysteine and GSH were determined. Fourth, effects of NAC, BSO, and GSHee on intracellular protein synthesis and degradation were determined. Last, effects of NAC on mRNA levels and protein abundances for key proteins involved in mTOR signaling were determined.
Cell culture
IPEC-1 cells were cultured in the presence of DMEM-F12 according to previous studies (Haynes et al. 2009; Yi et al. 2015). Briefly, cells were grown in serial passages in uncoated plastic culture flasks (75 cm2) in DMEM-F12 containing 17.5 mM d-glucose, 2, 0.7 mM arginine, 15 mM HEPES (pH = 7.4), 5 % FBS, epidermal growth factor (5 μg/L), insulin (5 μg/ml), transferrin (5 μg/ml), selenium (5 μg/ml), penicillin (50 μg/ml), streptomycin (4 μg/ml), and 0.25 μg/ml amphotericin B (Fungizone®). Medium was changed everyday. All cell cultures were carried out at 37 °C in a 5 % CO2 incubator. At confluence, cells were passaged using trypsinization.
Determination of cell growth
At confluence, IPEC-1 cells were trypsinized, washed thoroughly with DMEM-F12, and then seeded in 24-well cell culture plates with approximately 4 × 104 cells per well and maintained at 37 °C in a 5 % CO2 incubator (Yi et al. 2015). After overnight culture, the cells were washed twice with phosphate buffer saline (PBS) and then cultured in 1 ml of custom DMEM containing 5 % FBS, 5 mM d-glucose, 0.1 mM sodium pyruvate (Tan et al. 2010), and 0 or 100 μM NAC. The physiological concentrations (μM) of other amino acids in the custom DMEM were as follows: l-alanine 350; l-arginine·HCl, 100; l-asparagine, 50; l-aspartic acid, 20; l-cystine·2HCl, 75; l-glutamine, 500; l-glutamic acid, 75; glycine, 250; l-histidine·HCl·H2O, 100; l-isoleucine, 150; l-leucine, 200; l-lysine·HCl, 200; l-methionine, 75; l-phenylalanine, 100; l-proline, 200; l-serine, 200; l-threonine, 200; l-tryptophan, 75; l-tyrosine·2Na·2H2O, 100; l-valine, 250; and taurine, 100 (Xi et al. 2012). The medium was changed every day. Cell growth was determined by counting the number of cells in each well of the plates using automated cell counter (TC20™, Bio-Rad Laboratories, Inc., CA, USA). The optimum time and NAC dose for cell growth were determined on the basis of cell number after 0, 1, 2, 3, and 4 day culture.
To determine effects of BSO, DEM, GSH, or GSHee on cell growth, IPEC-1 cells were cultured for 3 days in custom DMEM with addition of (1) BSO (10 μM) (Markovic et al. 2009); (2) DEM (20 μM) (Ruiz et al. 2003); (3) NAC (100 μM) + BSO (10 μM); (4) NAC (100 μM) + DEM (20 μM); (5) 100 μM GSH; or (6) 100 μM GSHee. Cell growth was determined as described above.
Determination of GSH and cysteine
Cellular concentrations of GSH and cysteine were determined by high-performance liquid chromatography (HPLC) according to the methods of Wang et al. (2014) with some modifications. Briefly, after 3 day culture, the medium was removed and cells (~5×106) were washed three times with PBS (pH = 7.4). An aliquot (0.2 ml) of buffer (12 mM iodoacetic acid, 1.5 M HClO4) was added to the Petri dish, and the whole solution was transferred to a 1.5-ml tube (Iantomasi et al. 1999). Cells were homogenized with a small plastic homogenizer, and the whole solution was centrifuged at 10,000g for 1 min. The supernatant fluid (150 μl) was transferred to a new 1.5-ml tube and mixed with 2 M K2CO3. After centrifugation (10,000g, 1 min), an aliquot of the supernatant fluid (50 μl) was stored at −20 °C until derivatization with iodoacetic acid (25 mM) (Jones et al. 2004). The HPLC system consisted of a model 2475 multi λ fluorescence detector (excitation and emission wavelength were set at 220 and 450 nm, respectively; gain was set at 1), a Supelco C18 column (4.6 mm × 15 cm, 3 μm; Sigma-Aldrich, St. Louis, MO, USA), a Supelco C18 guard column (4.6 mm × 5 cm, 20–40 μm; Sigma-Aldrich, St. Louis, MO, USA), and a Model 600E Powerline multisolvent delivery system with 100 μl heads, a Model 717 plus WISP Autosampler. In HPLC workstation, the autosampler was programmed to mix 25 μl of sample (or standard) with 25 μl of the o-phthaldialdehyde solution (50 mg o-phthaldialdehyde was dissolved in 1.25 ml methanol and added with 11.2 ml of 40 mM sodium borate buffer, 50 μl of 2-mercaptoethanol, and 0.4 ml of Brij-35) for 1 min, and the derivatized solution was then injected into the HPLC column without any delay. GSH and cysteine concentrations in cells are expressed as nmol/106 cells.
Determination of protein synthesis and degradation
Protein synthesis in IPEC-1 cells was determined according to the method of Xi et al. (2012) and Kong et al. (2012). The cells (~3×105) were seeded in six-well culture plates with 2 ml of DMEM-F12 containing 5 % FBS, 1 % antibiotics (P/S), and 0.05 % insulin. After 48-h culture, the medium was removed and the cells were washed twice with PBS (pH = 7.4). The cells were then cultured with 2 ml custom DMEM containing 5 % FBS, 1 % antibiotics (P/S), 0.05 % insulin, and either GSH (100 μM), GSHee (100 μM), BSO (10 μM), DEM (20 μM), NAC (100 μM), BSO (10 μM) + NAC (100 μM), or DEM (20 μM) + NAC (100 μM) according to the experimental design. Each treatment was assigned eight replicate wells.
After 21-h culture, the medium was removed, and cells were then cultured for 3 h in 2-ml custom DMEM containing 5 % FBS, 1 % antibiotics (P/S), 0.05 % insulin, and 1 mM l-phenylalanine (Phe) plus 0.8 μCi l-[ring-2, 4-3H]phenylalanine, and 0 or 100 µM NAC or other added substance, as described previously. At the end of a 3-h culture period, the medium was removed and cells were rapidly washed three times with 2 ml PBS (pH = 7.4). Afterwards, 2 ml 10 % trichloroacetic acid (TCA) was added to each well, and then IPEC-1 cells were scraped from the bottom of the well (Wu and Thompson 1990). The well was washed twice with 2 ml 10 % TCA, and the whole solution was collected in 15-ml tubes and centrifuged at 3000g for 10 min. The supernatant fluid was discarded, and the pellets were washed twice with 5 ml of 10 % TCA and dried in air at 37 °C (30 min). An aliquot (0.5 ml) of 1 M NaOH was added to each cell pellet, and the solution was placed at 37 °C for several hours until the pellet was dissolved. Part of the solution (0.4 ml) was transferred to a 20-ml scintillation vial containing 20 ml Hionic Fluor scintillation cocktail (PerkinElmer, MA, USA) to determine protein-bound 3H-phenylalanine (Xi et al. 2012). 3H-phenylalanine radioactivity was determined using a liquid scintillation counter after standing overnight at room temperature. An aliquot (0.1 ml) of the cell solution was stored at −20 °C for protein assay using the bicinchoninic acid (BCA) method (Smith et al. 1985). Specific activity of 3H-phenylalanine in medium was used to calculate protein synthesis in cells (Tan et al. 2010). The rates of protein synthesis in IPEC-1 cells were expressed as nmol Phe/mg protein/3 h (Wu and Thompson 1990).
Rates of protein degradation in IPEC-1 cells were determined, as described previously (Tan et al. 2010). Briefly, cells were cultured for 3 days in custom-made DMEM containing 5 % FBS, and 0 or 100 µM NAC or other added substance, as described previously. Starting from day 4, cells were cultured for 24 h in 2 ml of custom-made DMEM containing 5 % FBS, 0.1 mmol/l l-Phe plus 6.3 MBq of l-[ring-2, 4-3H] Phe, and 0 or 100 µM NAC or other added substance. After a 24-h period of culture to label cellular proteins, cells were washed three times with 3 ml of DMEM to deplete intracellular- and extracellular-free [3H]Phe. The cells were then cultured for 3 h in 2 ml of DMEM containing 5 % FBS, 1 mmol/l l-Phe, and 0 or 100 µM to measure the release of [3H]Phe from labeled protein as an indicator of proteolysis.
Real-time PCR (RT-PCR) analysis of gene expression
Total RNA extraction was performed according to our previous study (Yi et al. 2015). After 3-day incubation, total RNA in 0 and 100 μM NAC groups was extracted and purified using the TRIzol Reagent protocol (Invitrogen, Carlsbad, CA). Total RNA was quantified using the NanoDrop® ND-2000 UV–VIS spectrophotometer (Thermo Scientific, Wilmington, DE, USA) at an OD of 260 nm. The purity of RNA was assessed by determining OD260/OD280 ratios. All of the samples had an OD260/OD280 ratio above 1.8, corresponding to 90–100 % pure nucleic acids. Meanwhile, RNA integrity in each sample was determined using 1 % denatured agarose gel electrophoresis. RNA was used for RT-PCR analysis when it had a 28 S/18 S rRNA ratio ≥1.8 (Hou et al. 2013). Total RNA was reverse-transcribed using a PrimeScript® RT reagent kit with gDNA Eraser (Takara, Dalian, China) according to the manufacturer’s instruction. cDNA was synthesized and stored at −20 °C until use.
To amplify cDNA fragments, primer pairs (Table 1) were used for RT-PCR. To minimize amplification of potentially contaminating genomic DNA, the primers were designed to span introns and intron–exon boundaries. The RT-PCR was performed using the SYBR® Premix Ex Taq™ (Takara, Dalian, China) on an Applied Biosystems 7500 Fast Real-Time PCR System (Foster City, CA). The total volume of PCR reaction system was 50 μl. Briefly, the reaction mixture contained 0.2 µM of each primer, 25 µl of SYBR® Premix Ex Taq™ (2×), and 4 µl of cDNA in a 50-µl reaction volume. All PCRs were performed in triplicate on a 96-well RT-PCR plate (Applied Biosystems) under the following conditions (two-step amplification): 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 31 s. A subsequent melting curve (95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s) with continuous fluorescence measurements and final cooling to room temperature was processed. The specificity of the RT-PCR reactions was assessed by analyzing the melting curves of the products and size verification of the amplicons (Meurens et al. 2009). The delta cycle threshold (CT) method was used to analyze the relative expression of the target gene (Fu et al. 2010). To ensure the sensitivity and accuracy of the results obtained by RT-PCR, data were normalized geometric averaging of three internal reference genes: ribosomal protein L19 (RPL19), hydroxymethylbilane synthase (HMBS), and hypoxanthine phosphoribosyltransferase 1 (HPRT1) (Nygard et al. 2007; Bruel et al. 2010).
Western blot analysis of protein abundance
After a 3-day period of culture, cells were analyzed for total protein and specific proteins using the Western blotting technique (Li et al. 2008; Yao et al. 2012). Briefly, cells in 6-well culture plates were gently washed with Dulbecco’s phosphate-buffered saline and viable cells were counted using an automated cell counter. Cells were collected and lysed in 1 ml buffer (20 mM HEPES, pH 7.4, 2 mM EGTA, 50 mM NaF, 100 mM KCl, 0.2 mM EDTA, 50 mM β-glycerophosphate, 1 mM dithiothreitol, 0.1 mM PMSF, 1 mM benzamidine, and 0.5 mM sodium vanadate) with a Polytron homogenizer and centrifuged at 12,000g for 15 min at 4 °C. The supernatant fluid was aliquoted into micro-centrifuge tubes, to which 2 × SDS sample buffer was added in a 1:1 ratio. The samples were boiled and cooled on ice before use for Western blotting. Proteins were separated by electrophoresis on a 10 % polyacrylamide gel, and then electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane. Skim-milk powder in TBST buffer (1 × Tris-buffered saline including 0.1 % Tween 20) was used to blot the membrane for 1 h at room temperature. Membranes were incubated overnight at 4 °C with one of the following primary antibodies: mTOR (cell signaling, 1:1000), phosphorylated mTOR (Ser2448) (cell signaling, 1:1000), 4E-BP1 (cell signaling, 1:1000), phosphorylated 4E-BP1 (Thr70) (cell signaling, 1:1000), p70S6 kinase (cell signaling, 1:1000), phosphorylated p70S6 kinase (Thr389) (cell signaling, 1:1000), or β-actin (Sigma Chemicals, 1:5000). Thereafter, the membranes were washed with TBS-T and incubated for 1 h at room temperature with anti-goat (mouse) immunoglobulin G horseradish peroxidase conjugated secondary antibody (Beijing ZhongShan Golden Bridge Biological Technology Co., Ltd, China; 1:5000 dilution). After being washed with TBST, blots on the membrane were developed using an Enhanced Chemiluminescence Western blotting kit (ECL-plus, Amersham Biosciences, Sweden), visualized, and quantified using an imaging system (Alpha Innotech FluorChem FC2, CA, USA). Abundances of all proteins of interest were normalized to those for β-actin.
Statistical analysis
All values are expressed as mean ± SEM. Data were performed by one-way analysis of variance. The normality and constant variance for experimental data were tested by the Levene’s test (Wei et al. 2012). Differences among treatment groups were determined using Duncan’s multiple range tests. However, differences between means of two groups (e.g., mRNA levels and protein abundances of 0 and 100 μM NAC groups) were determined by the Student’s paired t test. All statistical analyses were performed using SPSS 13.0 software (Chicago, IL, USA). Probability values <0.05 were taken to indicate statistical significance.
Results
Effects of NAC, BSO, DEM, GSH, and GSHee on IPEC-1 cell growth
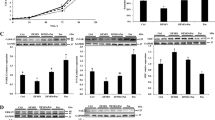
Effects of NAC, BSO, DEM, GSH, and GSHee on IPEC-1 cell number are illustrated in Figs. 1 and 2. Compared with the control group, addition of 100 μM NAC to the culture medium DMEM for 3 days increased (P < 0.01) cell numbers. Additionally, cell numbers in the 100 μM NAC group were 65, 64, 55, and 55 % greater than those in the 0 μM NAC group on days 1, 2, 3, and 4, respectively (Fig. 1). However, on days 1, 2, 3, and 4, there was no difference in cell numbers among different concentrations of NAC (Fig. 1).
NAC enhances IPEC-1 growth. IPEC-1 cells were cultured in medium supplemented with 0, 100, 200, 500, or 1000 μM NAC on days 0, 1, 2, 3, and 4. Data are mean ± SEM, n = 8. On each day of culture, the numbers of cells in the 100, 200, 500, or 1000 μM NAC groups did not differ (P > 0.05) but were greater (P < 0.01) than those in the control (0 μM NAC) group
Effects of glutathione synthesis and its inhibition on IPEC-1 growth. IPEC-1 cells were cultured in medium containing 100 μM NAC, 10 μM BSO, 20 μM DEM, 100 μM GSH, 100 μM GSHee, 100 μM NAC plus 10 μM BSO, or 100 μM NAC plus 20 μM DEM for 3 days. Both NAC and GSHee enhanced, but DEM and BSO inhibited IPEC-1 growth. Data are mean ± SEM, n = 8. a–e Means sharing different letters differ (P < 0.05). BSO buthionine sulfoximine, DEM diethylmaleate, GSH reduced glutathione, GSHee glutathione ethyl ester
On the other hand, cell numbers were decreased (P < 0.05) by 10 μM BSO or 20 μM DEM for 72 h (3 days), as compared with the control group. Moreover, compared with the BSO group, adding 100 μM NAC to culture medium in the presence of 10 μM BSO increased (P < 0.01) IPEC-1 cell numbers by 68 %. Similarly, simultaneous addition of 100 μM NAC and 20 μM DEM enhanced (P < 0.05) cell numbers by 33 % in comparison with the DEM group. Interestingly, the addition of 100 μM GSH to culture medium did not affect (P > 0.05) cell numbers, as compared with the 0 μM NAC group (Fig. 2). However, cell numbers in the 100 μM GSHee group were 20 % higher (P < 0.05), compared with the 0 μM NAC group.
Effects of NAC, GSH, and GSHee on the concentrations of GSH and cysteine in IPEC-1 cells
Effects of NAC, GSH, and GSHee on the concentrations of GSH and cysteine in IPEC-1 cells are summarized in Fig. 3. After culture with 100 μM NAC for 72 h, IPEC-1 cells exhibited higher concentration of GSH (25.2 nmol/106 cells, P < 0.05), as compared with other groups. Simultaneous addition of NAC plus BSO or NAC plus DEM to the culture medium decreased (P < 0.05) the GSH concentration (6.5 and 5.1 nmol/106 cells, respectively) in comparison with the control group (10.5 nmol/106 cells). Interestingly, the addition of 100 μM GSH did not affect the cellular GSH level (10.2 nmol/106 cells), but the addition of 100 μM GSHee increased (P < 0.05) the concentration of intracellular GSH (16 nmol/106 cells), as compared with the control group (10.5 nmol/106 cells) (Fig. 3).
Effects of glutathione synthesis and its inhibition on intracellular concentrations of GSH and cysteine in IPEC-1 cells. Cells were cultured in medium supplemented with 100 μM NAC, 100 μM NAC plus 10 μM BSO, 100 μM NAC plus 20 μM DEM, 100 μM GSH, or 100 μM GSHee for 3 days. NAC increased the concentrations of GSH and cysteine in IPEC-1 cells. GSHee, rather GSH, elevated the concentrations of GSH and cysteine in IPEC-1 cells. Data are mean ± SEM, n = 8. A–D and a–d means sharing different letters differ (P < 0.05). BSO buthionine sulfoximine, DEM diethylmaleate, GSH reduced glutathione, GSHee glutathione ethyl ester
There were no differences in cellular cysteine levels between the NAC and the NAC + BSO groups (19.4 vs. 20.7 nmol/106 cells, P > 0.05) or between the GSH and the control groups (10.1 vs. 10.7 nmol/106 cells, P > 0.05) (Fig. 3). However, compared with the control group, simultaneous addition of 100 μM NAC and 20 μM DEM to the culture medium decreased (7.1 vs. 10.1 nmol/106 cells, P < 0.05) cysteine concentrations in IPEC-1 cells. Additionally, intracellular cysteine concentrations in the 100 μM GSHee group (15.4 nmol/106 cells) were higher (P < 0.05) than those in the control group, but lower (P < 0.05) than the NAC and NAC + BSO groups (Fig. 3).
Effects of NAC, BSO, and GSHee on protein synthesis and degradation in IPEC-1 cells
The addition of 100 µM GSH, 100 µM GSHee, 10 µM BSO, or 20 µM DEM to the culture medium did not affect, but 100 µM NAC increased (P < 0.05), the rate of protein synthesis in IPEC-1 cells (Table 2). The addition of 100 µM GSH to the culture medium had no effect on the rate of intracellular protein degradation, but 100 µM GSHee or NAC decreased (P < 0.05) the rate of protein degradation. NAC attenuated (P < 0.05) the reduction in the rate of proteolysis brought about by BSO or DEM (Table 2). Additionally, increasing concentrations of GSHee from 0 to 1000 μM in culture medium did not affect protein synthesis in IPEC-1 cells (Fig. 4).
Effects of GSHee on protein synthesis in IPEC-1 cells. IPEC-1 cells were cultured in medium supplemented with 0 to 1000 μM glutathione ethyl ester (GSHee). GSHee did not affect the protein synthesis of IPEC-1 cells. Data are mean ± SEM, n = 8. Means did not differ among the different treatment groups (P > 0.05)
Effects of NAC on gene expression in IPEC-1 cells
The addition of 100 μM NAC for 72-h affected expression of genes encoding for key proteins involved in mTOR signaling in IPEC-1 cells (Fig. 5). Specifically, compared with the 0 μM NAC group, the addition of 100 μM NAC increased (P < 0.05) the mRNA levels for mTOR (+34 %) and p70S6 kinase (+22 %). However, there was no difference (P > 0.05) in expression of the genes for 4E-BP1 between the 0 μM NAC and 100 μM NAC groups.
Relative abundances of mRNAs for mTOR, 4E-BP1, and p70S6 kinase in IPEC-1 cells. Cells were cultured in custom DMEM supplemented with 0 or 100 μM NAC for 3 days. NAC increased the mRNA levels for mTOR and p70S6 kinase. All mRNA levels in 0 mM NAC group were regarded as 1. Data are mean ± SEM, n = 8. *P < 0.05
Effects of NAC on abundances of total and phosphorylated mTOR, 4E-BP1, and p70S6 kinase proteins in IPEC-1 cells
Abundances of total and phosphorylated mTOR, 4E-BP1, and p70S6 kinase proteins in IPEC-1 cells are shown in Fig. 6. Compared with the 0 μM NAC group, the addition of 100 μM NAC increased (P < 0.05) the abundance of total mTOR and p70S6 kinase proteins (+55 and +137 %, respectively), as well as the ratios of phosphorylated mTOR to total mTOR and phosphorylated p70S6 kinase to total p70S6 kinase in IPEC-1 cells (+89 and +44 %, respectively). However, there were no differences in the abundance of total 4E-BP1 protein or the ratio of phosphorylated 4E-BP1 protein to total 4E-BP1 protein between the 0 μM NAC and 100 μM NAC groups (Fig. 6).
Abundances of mTOR, 4E-BP1, and p70S6 kinase proteins in IPEC-1 cells. Cells were cultured in medium supplemented with 0 or 100 μM NAC for 3 days. NAC increased the protein abundance of total and phosphorylated mTOR and p70S6 kinase in IPEC-1 cells. Data are mean ± SEM, n = 6. a, b Means sharing different letters differ (P < 0.05). mTOR mammalian target of rapamycin, 4E-BP1 4E-binding protein-1
Discussion
NAC is widely used to protect cells from oxidative injury in medicine and to improve gut function under stress conditions in animal production (Hou et al. 2015). In addition, NAC has been reported to protect the intestine from apoptosis (Mayer and Noble 1994; Shen et al. 2001). Synthesis of GSH from NAC-derived cysteine has long been considered to account for the beneficial effects of NAC on cells (Barajas-Espinosa et al. 2014; Kim et al. 2001). In recent years, we found that dietary supplementation with a small amount of NAC relative to dietary provision of cysteine enhanced small-intestinal mucosal mass in LPS-challenged young pigs (Hou et al. 2012, 2013), suggesting that NAC may have hitherto unrecognized effects other than simply serving as a precursor of cysteine for GSH synthesis in cells. To our knowledge, this is the first study to report that NAC stimulates protein synthesis and inhibits proteolysis in cells.
Results of our study indicated that NAC at 100 μM increased the growth of IPEC-1 cells, compared with the control (Fig. 1). Cell number was not affected by NAC levels ranging from 200 to 1000 μM, in comparison with the 100 μM NAC group (Fig. 1). In contrast, there were reports that NAC inhibited the proliferation of normal human cells and transformed cells (Samuni et al. 2013), and arrested the cell cycle in NIH 3T3 cells (Huang et al. 1995), 308 papilloma cells (Liu et al. 1999), and hepatic stellate cells (Kim et al. 2001).Of particular note, extracellular NAC concentrations used in these previous studies ranged from 1 to 60 mM, which were 10- to 60-times those used in the present study (0.1–1 mM) that could be achieved with dietary NAC supplementation in animals. Thus, at low concentrations (<1 mM), NAC stimulates cell growth. It is possible that the effect of NAC on cell growth may be dependent on cell type.
Because cell proliferation depends on the balance between protein synthesis and degradation (Wu 2009), these parameters were determined in the present study. We found that protein synthesis in IPEC-1 cells was increased and protein degradation was inhibited by the addition of 100 μM NAC to the culture medium containing a physiological concentration of cysteine (Table 2). In a cell-free protein system, Shingaki and Nimura (2011) reported that cysteine, rather than cystine, contributed to protein synthesis. These authors concluded that cysteine could be a convenient and useful measure to support protein expression in cells. Three possible mechanisms may be responsible for effects of NAC on enhancing cell protein synthesis. First, through N-deacetylation, NAC can increase cellular concentrations of cysteine (Fig. 3) and provide a substrate for protein synthesis (Wu et al. 2014). Second, increased GSH concentration brought about by the addition of NAC (Fig. 3) may stimulate protein synthesis. However, this suggestion is not substantiated by our findings that protein synthesis in IPEC-1 cells was independent of an increase in intracellular cysteine brought about by the addition of GSHee (Fig. 3; Table 2). Third, NAC affects protein synthesis and growth via several signaling pathways involving mTOR, ERK, and EGF (Kim et al. 2001; Hou et al. 2013).
mTOR is a master regulator of protein synthesis (Suryawan et al. 2009). Thus, expression of mTOR and its two key downstream targets (4E-BP1 and p70S6 kinase) was determined in the present study. Our results indicate that NAC enhanced the abundances of total and phosphorylated proteins for mTOR and p70S6 kinase in IPEC-1 cells (Fig. 6). Similarly, Yang et al. (2012) reported that NAC increased the amounts of phosphorylated mTOR and Akt proteins in RGC-5 cells but these authors did not measure protein synthesis. The underlying mechanism whereby NAC affects the expression of these proteins is still unknown. Nevertheless, NAC can increase the mRNA levels for mTOR and p70S6 kinase (Fig. 5), indicating that NAC may regulate the mTOR signaling pathway at both gene transcription and protein phosphorylation levels.
Another salient finding of the present study is that elevated intracellular GSH by GSHee addition did not affect protein synthesis in IPEC-1 cells. Interestingly, increasing concentrations of GSHee from 0 to 500 or 1000 μM did not affect protein synthesis (Fig. 4). Therefore, it can be surmised that the stimulatory effect of NAC on intracellular protein synthesis is independent of GSH synthesis or concentration in IPEC-1 cells. However, administration of BSO or DEM (a GSH-depleting agent) could reduce cell numbers, whereas simultaneous administration of NAC plus either BSO or DEM could increase cell numbers (Fig. 2). These results can be explained by the findings that NAC or GSHee inhibited protein degradation in IPEC-1 cells (Table 2).
As reported for NIH 3T3 fibroblasts (Iantomasi et al. 1999), cell growth is positively associated with intracellular GSH status in IPEC-1 cells. GSH derived from endogenous synthesis (e.g., NAC) or exogenous addition (e.g., GSH ethyl ester) can regulate cellular events, including cell proliferation and apoptosis, signal transduction, and gene expression (Wu et al. 2004; Markovic et al. 2009). However, it is apparent that exogenous GSH at 100 µM was not effective in augmenting GSH concentration in IPEC-1 cells. Likewise, Levy et al. (1993) reported that GSH was not efficiently transported into most animal cells, and Mårtensson et al. (1990) found that GSHee was more effective than GSH itself in increasing cellular levels of GSH. However, results of our study indicate that GSHee did not affect the rate of protein synthesis in IPEC-1 cells (Table 2).
As noted previously, NAC is readily be absorbed by the small-intestinal mucosa (Hou et al. 2012) and increases cellular cysteine concentrations (Fig. 3). BSO administration did not influence intracellular cysteine level but reduced GSH synthesis and availability. In contrast, DEM, an alkylating agent, can convert GSH and cysteine to oxidized glutathione and cystine, respectively (Markovic et al. 2009). Interestingly, cells cultured with GSHee exhibited a higher concentration of intracellular cysteine than the control group (Fig. 3), indicating that intracellular GSH may be partially hydrolyzed to form cysteine. It is known that two cytosolic enzymes, γ-glutamyl transpeptidase and dipeptidase, are responsible for catalyzing the hydrolysis of GSH to cysteinyl-glycine and cysteine, respectively (Wu et al. 2004). Consequently, both NAC and GSHee are able to elevate the concentrations of intracellular cysteine and GSH. NAC and GSHee increased the proliferation of IPEC-1 cells via different mechanisms. Specifically, NAC activated the mTOR cell signaling pathway to enhance protein synthesis and inhibit protein degradation, whereas GSHee had no effect on protein synthesis but inhibited protein degradation (Table 2).
In summary, our results indicate that NAC enhances protein synthesis and inhibits proteolysis in intestinal epithelial cells, thereby resulting in increased cell growth. NAC exerts its effect on protein synthesis independently of GSH synthesis and availability, but dependently on activation of the mTOR signaling pathway. Collectively, these novel findings help elucidate cellular and molecular mechanisms for beneficial effects of NAC on intestinal health, and have important implications for the use of NAC in nutrition and clinical practice.
Abbreviations
- BSO:
-
Buthionine sulfoximine
- DEM:
-
Diethylmaleate
- GSH:
-
Glutathione
- GSHee:
-
Glutathione ethyl esters
- HMBS:
-
Hydroxymethylbilane synthase
- HPRT1:
-
Hypoxanthine phosphoribosyltransferase 1
- mTOR:
-
Mammalian target of rapamycin
- RPL19:
-
Ribosomal protein L19
- 4E-BP1:
-
4E-binding protein-1
References
Barajas-Espinosa A, Basye A, Jesse E et al (2014) Redox activation of DUSP4 by N-acetylcysteine protects endothelial cells from Cd2+-induced apoptosis. Free Radic Biol Med 74:188–199
Bruel T, Guibon R, Melo S et al (2010) Epithelial induction of porcine suppressor of cytokine signaling 2 (SOCS2) gene expression in response to Entamoeba histolytica. Dev Comp Immunol 34:562–571
Cossarizza A, Franceschi C, Monti D et al (1995) Protective effect of N-acetylcysteine in tumor necrosis factor-alpha-induced apoptosis in U937 cells—the role of mitochondria. Exp Cell Res 220:232–240
Fu WJ, Stromberg AJ, Viele K et al (2010) Statistics and bioinformatics in nutritional sciences: analysis of complex data in the era of systems biology. J Nutr Biochem 21:561–572
Haynes TE, Li P, Li X et al (2009) l-Glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37:131–142
Hou Y, Wang L, Zhang W et al (2012) Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 43:1233–1242
Hou Y, Wang L, Yi D et al (2013) N-acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids 45:513–522
Hou YQ, Wang L, Yi D, Wu G (2015) N-acetylcysteine and intestinal health: a focus on its mechanism of action. Front Biosci 20:872–891
Huang TS, Duyster J, Wang JY (1995) Biological response to phorbol ester determined by alternative G1 pathways. Proc Natl Acad Sci 92:4793–4797
Iantomasi T, Favilli F, Degl’Innocenti D et al (1999) Increased glutathione synthesis associated with platelet-derived growth factor stimulation of NIH3T3 fibroblasts. Biochim Biophys Acta 1452:303–312
Jones DP, Go YM, Anderson CL et al (2004) Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J 18:1246–1248
Kim KY, Rhim T, Choi I et al (2001) N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J Biol Chem 276:40591–40598
Kong X, Tan B, Yin Y et al (2012) L-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem 23:1178–1183
Lai ZW, Hanczko R, Bonilla E et al (2012) N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 64:2937–2946
Lee MF, Chan CY, Hung HC et al (2013) N-acetylcysteine (NAC) inhibits cell growth by mediating the EGFR/Akt/HMG box-containing protein 1 (HBP1) signaling pathway in invasive oral cancer. Oral Oncol 49:129–135
Levy EJ, Anderson ME, Meister A (1993) Transport of glutathione diethyl ester into human cells. Proc Natl Acad Sci 90:9171–9917
Li P, Kim SW, Li X et al (2008) Dietary supplementation with cholesterol and docosahexaenoic acid increases the activity of the arginine-nitric oxide pathway in tissues of young pigs. Nitric Oxide 19:259–265
Liu M, Wikonkal NM, Brash DE (1999) Induction of cyclin-dependent kinase inhibitors and G1 prolongation by the chemopreventive agent N-acetylcysteine. Carcinogenesis 20:1869–1872
Markovic J, Mora NJ, Broseta AM et al (2009) The depletion of nuclear glutathione impairs cell proliferation in 3T3 fibroblasts. PLoS One 4:e6413
Mårtensson J, Jain A, Meister A (1990) Glutathione is required for intestinal function. Proc Natl Acad Sci 87:1715–1719
Mayer M, Noble M (1994) N-acetyl-l-cysteine is a pluripotent protector against cell-death and enhancer of trophic factor-mediated cell-survival in-vitro. Proc Natl Acad Sci 91:7496–7500
Meurens F, Berri M, Auray G et al (2009) Early immune response following Salmonella enterica subspecies enterica serovar Typhimurium infection in porcine jejunal gut loops. Vet Res 40:5
Nygard A, Jøgensen CB, Cirera S et al (2007) Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol Biol 8:67
Ozdemir R, Yurttutan S, Sari FN et al (2012) Antioxidant effects of N-acetylcysteine in a neonatal rat model of necrotizing enterocolitis. J Pediatr Surg 47:1625–1627
Romagnoli C, Marcucci T, Picariello L et al (2013) Role of N-acetylcysteine and GSH redox system on total and active MMP-2 in intestinal myofibroblasts of Crohn’s disease patients. Int J Colorectal Dis 28:915–924
Ruiz E, Siow RC, Bartlett SR et al (2003) Vitamin C inhibits diethylmaleate-induced l-cystine transport in human vascular smooth muscle cells. Free Radic Biol Med 34:103–110
Samuni Y, Goldstein S, Dean OM et al (2013) The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta 1830:4117–4129
Shen HM, Yang CF, Ding WX et al (2001) Superoxide radical-initiated apoptotic signalling pathway in selenite-treated HepG(2) cells: mitochondria serve as the main target. Free Radic Biol Med 30:9–21
Shingaki T, Nimura N (2011) Improvement of translation efficiency in an Escherichia coli cell-free protein system using cysteine. Protein Expr Purif 77:193–197
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Suryawan A, O’Connor PMJ, Bush JA et al (2009) Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids 37:105–110
Tan B, Yin Y, Kong X et al (2010) l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
Wang Q, Hou Y, Yi D et al (2013) Protective effects of N-acetylcysteine on acetic acid-induced colitis in a porcine model. BMC Gastroenterol 13:133
Wang W, Dai Z, Wu Z et al (2014) Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 46:2037–2045
Wei JW, Carroll RJ, Harden KK et al (2012) Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42:2031–2035
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G, Thompson JR (1990) The effect of glutamine on protein turnover in chick skeletal muscle in vitro. Biochem J 265:593–598
Wu G, Fang YZ, Yang S et al (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492
Wu G, Bazer FW, Dai ZL et al (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Xi P, Jiang Z, Dai Z et al (2012) Regulation of protein turnover by l-glutamine in porcine intestinal epithelial cells. J Nutr Biochem 23:1012–1017
Yang L, Tan P, Zhou W et al (2012) N-acetylcysteine protects against hypoxia mimetic-induced autophagy by targeting the HIF-1α pathway in retinal ganglion cells. Cell Mol Neurobiol 32:1275–1285
Yao K, Yin Y, Li X et al (2012) Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids 42:491–2500
Yi D, Hou Y, Wang L et al (2015) L-Glutamine enhances enterocyte growth via activation of the mTOR signaling pathway independently of AMPK. Amino Acids 47:65–78
Zhu L, Cai X, Guo Q et al (2013) Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways’ response to oxidative stress in weaned piglets. Br J Nutr 110:1938–1947
Acknowledgments
This research was jointly supported by the National Natural Science Foundation of China (Nos. 31372319, 31572416, 31402084), the Hubei Provincial Key Project for Scientific and Technical Innovation (2014ABA022), Hubei Provincial Research and Development Program (No. 2010BB023), Natural Science Foundation of Hubei Province (Nos. 2013CFA097, 2012FFB04805, 2011CDA131), the Hubei Hundred Talent Program, Agriculture and Food Research Initiative Competitive Grants of (2014-67015-21770) of the USDA National Institute of Food and Agriculture, and Texas A&M AgriLife Research (H-82000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yi, D., Hou, Y., Wang, L. et al. N-acetylcysteine stimulates protein synthesis in enterocytes independently of glutathione synthesis. Amino Acids 48, 523–533 (2016). https://doi.org/10.1007/s00726-015-2105-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2105-z