Abstract
The high efficiency of protein deposition during the neonatal period is driven by high rates of protein synthesis, which are maximally stimulated after feeding. In the current study, we examined the individual roles of amino acids and insulin in the regulation of protein synthesis in peripheral and visceral tissues of the neonate by performing pancreatic glucose–amino acid clamps in overnight-fasted 7-day-old pigs. We infused pigs (n = 8–12/group) with insulin at 0, 10, 22, and 110 ng kg−0.66 min−1 to achieve ~0, 2, 6 and 30 μU ml−1 insulin so as to simulate below fasting, fasting, intermediate, and fed insulin levels, respectively. At each insulin dose, amino acids were maintained at the fasting or fed level. In conjunction with the highest insulin dose, amino acids were also allowed to fall below the fasting level. Tissue protein synthesis was measured using a flooding dose of l-[4-3H] phenylalanine. Both insulin and amino acids increased fractional rates of protein synthesis in longissimus dorsi, gastrocnemius, masseter, and diaphragm muscles. Insulin, but not amino acids, increased protein synthesis in the skin. Amino acids, but not insulin, increased protein synthesis in the liver, pancreas, spleen, and lung and tended to increase protein synthesis in the jejunum and kidney. Neither insulin nor amino acids altered protein synthesis in the stomach. The results suggest that the stimulation of protein synthesis by feeding in most tissues of the neonate is regulated by the post-prandial rise in amino acids. However, the feeding-induced stimulation of protein synthesis in skeletal muscles is independently mediated by insulin as well as amino acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rate of protein deposition is more rapid during the neonatal period than at any other stage of postnatal life and is driven by high rates of protein synthesis (Denne and Kalhan 1987; Davis et al. 1989). During the neonatal period, more rapid gains in protein mass occur in skeletal muscle than in the body as a whole (Young 1970). The synthesis of skeletal muscle protein is high immediately after birth and declines more rapidly than in other tissues of the body during the first month of life (Davis et al. 1993a, 1996). A marked change in the composition of muscle also occurs during the neonatal period and is accompanied by a significant reduction in ribosome number and a specific accumulation of myofibril proteins (Davis et al. 1989; Fiorotto et al. 2000).
Neonates use dietary amino acids efficiently for growth (Davis et al. 1993b) because they can increase protein synthesis in response to feeding to a greater extent than mature animals (Davis et al. 1993a, 1996). The feeding-induced stimulation of protein synthesis occurs in all measured tissues in the neonatal pig and rat, and the greatest increase occurs in skeletal muscle, particularly in those muscles that contain predominately fast-twitch muscle fibers (Burrin et al. 1997; Davis et al. 1997). We have previously shown that raising amino acids from fasting levels to those typical of the fed state increases protein synthesis in skeletal muscle to rates similar to that of fed neonatal pigs, when insulin and glucose are maintained at fasting levels (Davis et al. 2002). Furthermore, raising insulin from the fasting to the fed level, in the presence of fasting amino acid and glucose levels, also increases muscle protein synthesis (Davis et al. 2001; Wray-Cahen et al. 1998). These responses to insulin and amino acids were greater in muscles that contain primarily fast-twitch glycolytic fibers than in those composed of primarily slow oxidative fibers. Although we further showed that insulin and amino acids independently stimulate protein synthesis in fast-twitch glycolytic muscle (O’Connor et al. 2003), it was not determined whether insulin and amino acids interact at low doses to stimulate protein synthesis in muscles of different fiber types.
In most visceral tissues of the neonate, including liver, raising amino acids to fed levels increases protein synthesis, whereas fed insulin levels do not elicit a response (Davis et al. 2001, 2002). We previously demonstrated that the stimulatory effect of amino acids on protein synthesis in the liver is independent of insulin in that the effect of amino acids occurs at different levels of insulin within the physiological range and even in the absence of insulin (O’Connor et al. 2004). However, it was not determined whether the stimulation of protein synthesis in other visceral tissues by amino acids is independent of insulin.
Although a number of studies have examined the effects of amino acids and insulin on protein synthesis in skeletal muscle (Garlick et al. 1983; O’Connor et al. 2003), there are surprisingly few reports of the effect of amino acids and insulin in other tissues (Charlton et al. 2000; Jefferson et al. 1983). Because whole-body protein synthesis rates represent the compilation of the rates of synthesis in all tissues and organs of the body, and rates of protein synthesis in visceral tissues considerably exceed those in peripheral tissues, examination of the effect of amino acids and insulin on protein synthesis in visceral tissues is important. In the current study, we utilized pancreatic glucose–amino acid clamps to determine the independent effects of amino acids and insulin on the regulation of protein synthesis in muscles of different fiber types, as well as in other peripheral and visceral tissues.
Methods
Animals
Multiparous sows (n = 11, crossbred Yorkshire × Landrace × Hampshire × Duroc; Agriculture Headquarters, Texas Department of Criminal Justice, Huntsville, TX) were housed in lactation crates in individual, environmentally controlled rooms, maintained on a commercial diet (5084, PMI Feeds, Richmond, IN), and provided water ad libitum throughout the lactation period. After farrowing, piglets remained with the sow but were not given supplemental creep feed. Piglets were studied at 5–8 days of age (2.1 ± 0.4 kg). Three to five days before the infusion study, pigs were anesthetized, and catheters were surgically inserted into a jugular vein and a carotid artery with sterile techniques, as described previously (Wray-Cahen et al. 1997). Piglets were returned to the sow until studied. The previously described protocol (O’Connor et al. 2003) was approved by the Animal Care and Use Committee of Baylor College of Medicine. The study was conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Pancreatic glucose–amino acid clamps
The clamp procedure has been previously described by O’Connor et al. (2003). Briefly, after an overnight fast, pigs were placed unanesthetized in a sling restraint system. The average basal concentration of blood glucose (YSI 2300 STAT Plus, Yellow Springs Instruments, Yellow Springs, OH) and plasma branched-chain amino acid (BCAA) concentrations (Beckett et al. 1996) to be used in the subsequent pancreatic glucose–amino acid clamp procedure were established during a 30-min basal period. The 2-h clamp was initiated with a primed (20 μg kg−1), continuous (100 μg kg−1 h−1) somatostatin (BACHEM, Torrance, CA) infusion. After a 10-min infusion of somatostatin, a continuous infusion of replacement glucagon (150 ng kg−1 h−1; Eli Lilly, Indianapolis, IN) was initiated and continued to the end of the clamp period. Insulin was infused at 0, 10, 22, or 110 ng kg−0.66 min−1 to achieve plasma insulin concentrations of ~0, 2, 6, or 30 μU ml−1 to simulate below fasting, fasting, intermediate, or fed insulin levels, respectively (Davis et al. 1996). At each dose of insulin, amino acids were clamped at either the fasting (500 nmol BCAA ml−1) or fed (1,000 nmol BCAA ml−1) level by using BCAA as an index for amino acid concentrations. BCAA concentrations were monitored every 5 min and the infusion rate of a balanced amino acid mixture was adjusted to maintain its concentration within 10% of the desired level (Davis et al. 2002). At the highest insulin dose only, amino acids were also allowed to fall below fasting levels (250 nmol BCAA ml−1) by omitting the amino acid clamp. Blood glucose concentrations were measured at 5-min intervals, and the dextrose infusion rate was adjusted to maintain blood glucose at a constant value. Blood samples also were taken at intervals for later determination of circulating insulin, glucagon, and individual essential and nonessential amino acid concentrations.
Plasma hormones and substrates
The concentrations of individual amino acids from frozen plasma samples obtained at 0 and 90 min after the start of the insulin infusions were measured with an HPLC method (PICO-TAG reverse-phase column, Waters, Milford, MA) as previously described (Davis et al. 1993a, b). With a porcine insulin radioimmunoassay kit (Linco, St. Louis, MO) that used porcine insulin antibody and human insulin standards, plasma radioimmunoreactive insulin concentrations were measured. Plasma radioimmunoreactive glucagon concentrations were measured using a porcine glucagon radioimmunoassay kit (Linco, St. Louis, MO) that used porcine glucagon antibody and human glucagon standards.
Tissue protein synthesis in vivo
Ninety minutes after the initiation of the clamp procedure, the fractional rate of protein synthesis was measured with a flooding dose (1.5 mmol kg−1 body weight, 1.0 mCi kg−1 body weight) of l-[4-3H] phenylalanine (Amersham Biosciences, Piscataway, NJ) as previously described (Garlick et al. 1980). Pigs were killed at 2 h and samples were obtained from the longissimus dorsi, gastrocnemius, masseter, and diaphragm muscles, left heart, skin, liver, pancreas, spleen, lung, jejunum, kidney, and stomach. Samples were immediately frozen in liquid nitrogen and stored at −70°C until analyzed, as previously described (Davis et al. 1996). The specific radioactivity of the protein hydrolysate, homogenate supernatant, and blood supernatant were determined as previously described (Davis et al. 1989). Previous studies have demonstrated that, after a flooding dose of tritiated phenylalanine is administrated, the specific radioactivity of tissue free phenylalanine is in equilibrium with the aminoacyl tRNA specific radioactivity, and therefore the tissue free phenylalanine is a valid measure of the tissue precursor pool specific radioactivity (Davis et al. 1999).
Calculations and statistics
The fractional rate of protein synthesis (K s , percentage of protein mass synthesized in a day) was calculated as
where S b (dpm min−1) is the specific radioactivity of the protein-bound phenylalanine and S a (dpm min−1) is the specific radioactivity of the tissue-free phenylalanine at the time of tissues collection and the linear regression of the blood specific radioactivity of the animal at 5, 15, and 30 min against time, and t is the time of labeling in minutes.
Analysis of variance (ANOVA; general linear modeling) was used to assess the effect of amino acids, insulin, and their interaction, and to determine whether there was a linear and/or quadratic relationship between BCAA and K s . If there was a significant interaction, Student’s t test was used to test for specific differences between groups. To determine the effectiveness of the clamp procedure, amino acid and insulin concentrations in each treatment group were compared with their basal concentrations by use of t tests. Probability values of P < 0.05 were considered statistically significant.
Results
Infusion, hormones, and substrates
Pancreatic glucose–amino acid clamps were performed in fasted 7-day-old pigs by infusion of somatostatin (to block insulin secretion), glucagon (at replacement levels), and glucose (as needed to maintain fasting levels). Insulin was infused at four doses to achieve levels that simulated (1) less than fasting (0 μU ml−1), (2) fasting (2 μU ml−1), (3) intermediate between fasting and fed (6 μU ml−1), or (4) fed (30 μU ml−1) conditions. Amino acids were clamped at fasting (500 nmol BCAA ml−1) or fed levels (1,000 nmol BCAA ml−1). At the highest insulin dose, amino acids were also allowed to fall to less than the fasting levels by omitting the amino acid clamp (~250 nmol BCAA ml−1) so that the dose–response effect of amino acids (~250, 500, and 1,000 BCAA nmol ml−1) could be examined. The targeted plasma insulin levels and amino acid levels were largely achieved in all treatment groups (Table 1) and were presented previously (O’Connor et al. 2003). Furthermore, the circulating glucose and glucagon concentrations were largely maintained at basal fasting levels during the infusion of somatostatin, glucagon, insulin, and/or amino acids (data not shown).
The effect of insulin and amino acids on protein synthesis
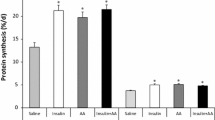
We examined the interaction of insulin and amino acids in the stimulation of protein synthesis in various tissues. For the purpose of comparison with muscles of different fiber types, we herein have included previously reported data on protein synthesis in the longissimus dorsi (LD), a muscle of primarily fast-twitch, glycolytic fibers (O’Connor et al. 2003). Both insulin and amino acids increased protein synthesis in the LD muscle (P < 0.01). There was a progressive increase in protein synthesis rates in the LD muscle as the level of insulin was increased (P < 0.005). Amino acids increased LD muscle protein synthesis (P < 0.05) at each dose of insulin except the highest dose (30 μU ml−1), where there was a tendency for amino acids to stimulate LD muscle protein synthesis (P = 0.06) (Table 2). Both insulin and amino acids also stimulated protein synthesis in the gastrocnemius, a muscle of mixed fiber types, the masseter muscle, which is composed of slow-twitch oxidative fibers, and the diaphragm, a unique muscle of mixed fiber composition that undergoes continuous contraction (P < 0.05). In the gastrocnemius muscle, there was a progressive increase in protein synthesis as the level of insulin was increased (P < 0.005). Amino acids increased protein synthesis in gastrocnemius muscle only at the highest dose of insulin (P < 0.05) (Table 2). In the masseter muscle, there was a progressive increase in protein synthesis as the level of insulin was increased up to 6 μU ml−1 (P < 0.005). Higher doses of insulin had no further effect on the rate of protein synthesis. Amino acids increased protein synthesis in the masseter (P < 0.05) only at lower dose of insulin (~0, 2 μU ml−1) (Table 2). In the diaphragm, insulin significantly increased the rate of protein synthesis (P < 0.05). Amino acids significantly increased the rate of protein synthesis (P < 0.05) at the highest insulin dose. Insulin increased protein synthesis in the heart at the two highest doses (6 and 30 μU ml−1) and at all doses (2, 6, and 30 μU ml−1) in the skin (P < 0.05; Table 2). In both the heart and skin, amino acids had no effect on the fractional rates of protein synthesis.
For the purpose of comparison with other visceral tissues, we herein report previously published data on liver protein synthesis (O’Connor et al. 2004). Table 2 shows that while insulin had no effect, amino acids increased the fractional rates of protein synthesis in the liver and pancreas (P < 0.05). Furthermore, examination of individual treatment effects revealed that liver protein synthesis was increased by insulin only in response to the highest insulin dose and in the presence of hyperaminoacidemia (P < 0.05). There was no effect of any dose of insulin in the pancreas (Table 2). By contrast, amino acids increased protein synthesis in the liver and pancreas at each dose of insulin (P < 0.05). In the spleen and the lung, insulin also had no effect on the fractional rates of protein synthesis but amino acids increased protein synthesis (P < 0.05). Examination of individual effects showed there was a significant effect of amino acids on spleen protein synthesis at the lowest dose of insulin (P < 0.05). In lung, amino acids increased the fractional rates of protein synthesis at the insulin level of ~0 and 30 μU ml−1 (P < 0.05; Table 2). Neither insulin nor amino acids has any effect on the fractional rates of protein synthesis in the stomach (Table 2). However, there was a tendency for amino acids to increase the overall rate of protein synthesis in the jejunum (P = 0.09) and the kidney (P = 0.13).
The dose–response effect of amino acids on protein synthesis
To determine whether there is a dose–response effect of amino acids on the fractional rates of protein synthesis in various tissues, insulin was infused to achieve the fed level only, while amino acids were at below fasting levels, the fasting level, or the fed level. In the LD and gastrocnemius, there was a progressive increase in protein synthesis as amino acids were raised from below fasting to fasting levels (P < 0.05) and from fasting to fed amino acids levels (P < 0.01; Table 3). In masseter, raising amino acids from below fasting levels to fasting levels significantly increased protein synthesis (P < 0.05) with no further increase observed at fed levels of amino acids (Table 3). Infusion of amino acids to achieve the plasma concentrations observed during feeding (1,000 BCAA nmol ml−1) significantly increased protein synthesis in the diaphragm (P < 0.05; Table 3), and tended to increase protein synthesis in the heart (P < 0.07). Amino acids had no effect on protein synthesis in the skin (Table 3).
To determine whether there is a dose–response effect of amino acids on protein synthesis in the visceral tissues of the neonate, fractional rates of protein synthesis were determined in lung, intestine, pancreas, liver, spleen, stomach, and kidney in neonatal pigs. For comparison, previously published data on liver protein synthesis are used herein (O’Connor et al. 2004). There was a progressive increase in protein synthesis in the pancreas and liver as amino acids were raised from below fasting to fasting levels (P < 0.05) and from fasting to fed amino acids levels (P < 0.01; Table 3). In the lung, the fractional rate of protein synthesis was increased only at the highest amino acid dose (Table 3). In the intestine, protein synthesis was influenced by the level of amino acids such that it increased as amino acids were raised from below fasting to fasting but fell modestly when amino acids were raised further (P < 0.05; Table 3). In contrast, the fractional rates of protein synthesis in the spleen, stomach, and kidney were unaffected by amino acid infusion (Table 3).
Discussion
In the current study, we set out to examine the individual effects of insulin and amino acid and their possible interaction in the regulation of protein synthesis in peripheral and visceral tissues of neonatal pigs. Since the neonate is made up of ~40% skeletal muscle as a percentage of body mass and skeletal muscle is a significant contributor to whole-body protein turnover (Preedy et al. 2001), understanding of how feeding affects skeletal muscle protein synthesis is important. We (Davis et al. 2001) and others (Garlick et al. 1983) have shown that the response of muscle protein synthesis to anabolic agents is greater in muscles of predominantly fast than in those of the slow fiber type. In this study, we compared the effect of amino acids and insulin on the different skeletal muscles: (1) longissimus dorsi muscle (contains primarily fast-twitch glycolytic fibers), (2) gastrocnemius muscle (comprised of mixed fiber type), (3) masseter muscle (contains primarily slow-twitch oxidative fibers) and (4) diaphragm (composed of mixed fibers but undergo continuous contraction). In agreement with our previous study (Davis et al. 2001), we found that insulin increased protein synthesis in all muscle types. The response to insulin was greater in longissimus dorsi compared to gastrocnemius, masseter, and diaphragm muscles suggesting that protein synthesis is more responsive to insulin in muscles containing primarily fast-twitch muscle fibers in the neonate. Interestingly, amino acids significantly increased longissimus dorsi muscle protein synthesis at almost all insulin levels (0, 2, and 6 μU ml−1) while in masseter muscle only at 0 and 2 μU ml−1 and in gastrocnemius and diaphragm muscles only at 30 μU ml−1. Furthermore, the dose–response effect of amino acids, in the presence of fed levels of insulin, showed a progressive increase in protein synthesis as amino acids were increased to fasting and fed levels in the longissimus dorsi and gastrocnemius muscles, whereas only fed levels of amino acids stimulated protein synthesis in the masseter and diaphragm. Together, this suggests that muscles containing primarily fast-twitch muscle fibers also are more sensitive to amino acids in the neonate.
Although, undoubtedly muscle protein synthesis during the neonatal period is highly sensitive to insulin and amino acids, some studies also indicate that muscle protein synthesis is also responsive to insulin and amino acids in growing but more mature animals. Using adolescent rats, Balage et al. (2001) observed that both insulin and amino acids are required for the stimulation of protein synthesis in response to feeding. Furthermore, Anthony et al. (2002) found that increases in serum insulin are permissive for the leucine-induced stimulation of muscle protein synthesis in young adult rats.
Skin accounts for a large proportion of whole-body protein synthesis (10–25%), and this proportion varies with stage of development (Obled and Arnal 1989). Previous studies have reported rates of protein synthesis in skin to be either higher or similar compared with muscle (Attaix et al. 1988; Biolo et al. 1994). In the current study, insulin but not amino acids increased the factional rate of protein synthesis in the skin. Furthermore, the rates of insulin-induced protein synthesis in the skin were comparable to those in skeletal muscle. Our data from the amino acid dose response study clearly suggest that amino acids have no effect of protein synthesis in the skin.
Previously, we showed that insulin stimulates protein synthesis in cardiac muscle of neonatal pigs (Davis et al. 2001). Furthermore, a study using diabetic rats demonstrated that insulin withdrawal reduces protein synthesis in heart muscle (Garlick et al. 1983). In the current study, insulin but not amino acids increased the fractional rate of protein synthesis. However, the amino acid dose response revealed that fed levels of amino acids tended to increase cardiac muscle protein synthesis. Together, the results suggest the feeding-induced stimulation of protein synthesis in the heart is primarily mediated by the post-prandial rise in insulin.
Since our previous studies indicated that feeding increased protein synthesis in all tissues of the neonatal pigs (Davis et al. 1996, 1997), we wished to determine whether insulin and amino acids independently regulate the protein synthetic response to feeding in visceral tissues of the neonate, as it does for skeletal muscle. In growing animals, liver protein synthesis rates can be modulated by changes in food intake (Burrin et al. 1992; Davis et al. 1996). Furthermore, studies in diabetic rats and in hepatocytes indicate that insulin regulates liver protein synthesis (Jefferson et al. 1983; Hsu et al. 1992). Consistent with our previous study (Davis et al. 2001), the results in the current study show that when amino acids were maintained at the fasting level, insulin had no effect on liver protein synthesis. However, our finding that protein synthesis was increased in response to the highest insulin dose in the presence of fed levels of amino acids only suggests that the possible role of insulin on liver protein synthesis after feeding should not be overlooked. We have previously demonstrated that amino acid infusion to the fed level, in the presence of fasting insulin levels, can reproduce the feeding-induced stimulation of liver protein synthesis in the neonatal pig (Davis et al. 2002). However, whether there was a potentially permissive effect of fasting insulin levels on amino-acid stimulated liver protein synthesis was not ruled out in our previous study (Davis et al. 2002). Here, we show the stimulatory effect of amino acids to be independent of insulin in that the effect of amino acids occurred at each dose of insulin, including the zero insulin dose, suggesting that neonatal liver is highly sensitive to the anabolic affect of amino acids. Moreover, there was a dose-dependent effect of amino acids on liver protein synthesis such that raising amino acids from below fasting to fasting levels increased liver protein synthesis with a further stimulatory effect at fed levels of amino acids.
Studies show that feeding stimulates protein synthesis in pancreas, spleen, and lung of the neonatal pig (Burrin et al. 1997; Davis et al. 1997). Recently, we found that insulin infusion in the presence of near-fasting levels of glucose and amino acids had no effect on protein synthesis in the pancreas and spleen of neonatal pigs (Davis et al. 2001). The results of the current study show that similar to liver, amino acids stimulate protein synthesis in the pancreas at each dose of insulin. Furthermore, a dose-dependent effect of amino acids were also seen such that raising the amino acid levels from below fasting to fasting levels increased protein synthesis in the pancreas with a further stimulatory effect at fed levels of amino acids. In contrast, in the spleen, amino acids increased protein synthesis only at the insulin level of nearly zero while in the lung, amino acids induced protein synthesis occurred at the lowest and the highest insulin levels. Amino acid dose response data revealed that in the spleen, amino acids had no effect while in the lung, raising amino acid levels from below fasting to fasting levels had no effect on protein synthesis. Conversely, further raising of amino acids to fed levels increased protein synthesis in lung. Although, the results of several studies show that feeding stimulates protein synthesis in the intestine, stomach, and kidney (Burrin et al. 1997; Davis et al. 1996), in the current study we found that amino acids only tended to increase the fractional rates of protein synthesis in the jejunum and the kidney. The amino acid dose response study showed that amino acids had no effect protein synthesis in kidney and stomach while raising amino acid levels from below fasting to fasting levels increased protein synthesis in the intestine with no further stimulatory effect at fed levels of amino acids. The findings that insulin failed to stimulate protein synthesis in the intestine and kidney is consistent with our previous study (Davis et al. 2001). One possible explanation for the lack of anabolic effect of amino acids on protein synthesis in the stomach and only modest effect in the intestine is that in the current study, amino acids were supplied parentally rather than enterally.
In conclusion, the current study was designed to determine the role of amino acids and insulin in the regulation of protein synthesis in numerous tissues in the neonatal pig. In previous studies, we showed that feeding increased protein synthesis in all neonatal pig tissues that we examined. In the current study, we further showed that there was a differential effect of amino acids and insulin on the regulation of protein synthesis in neonatal pig tissues. Although amino acids and insulin play critical roles in the regulation of the post-prandial increase in tissue protein synthesis in the neonate, other nutrients and/or growth factors/hormones also may be involved. Nevertheless, our data demonstrate the differential roles of amino acids and insulin in modulating the increase in tissue protein synthesis that supports growth in the neonate.
References
Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS (2002) Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab 282:E1092–E1101
Attaix D, Aurousseau E, Manghebati A, Arnal M (1988) Contribution of liver, skin, and skeletal muscle to whole-body protein synthesis in the young lamb. Br J Nutr 60:77–84
Balage M, Sinaud S, Prod’Homme M, Dardevet D, Vary T, Kimball SR, Jefferson LS, Grizard J (2001) Amino acids and insulin are both required to regulate assembly of the eIF4E IF4G complex in rat skeletal muscle. Am J Physiol Endocrinol Metab 281:E565–E574
Beckett PR, Hardin DS, Davis TA, Nguyen HV, Wray-Cahen D, Copeland KC (1996) Spectrophometric assay for measuring branched chain amino acid concentrations: application for measuring the sensitivity of protein metabolism to insulin. Anal Biochem 240:48–53
Biolo G, Gastaldelli M, Zhang XJ, Wolfe RR (1994) Protein synthesis and breakdown in skin and muscle: a leg model of amino acid kinetics. Am J Physiol Endocrinol Metab 267:E467–E474
Burrin DG, Davis TA, Fiorotto ML, Reeds PJ (1992) Hepatic protein synthesis in suckling rats: effects of stage of development and fasting. Pediatr Res 31:247–252
Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ (1997) Colostrum enhances the nutritional stimulation of vital organ protein synthesis in neonatal pigs. J Nutr 127:1284–1289
Charlton M, Ahlman B, Nair KS (2000) The effect of insulin on human small intestinal mucosal protein synthesis. Gastroenterology 188:299–306
Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ (1989) Protein turnover in skeletal muscle of suckling rats. Am J Physiol Regul Integr Comp Physiol 257:R1141–R1146
Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ (1993a) Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol Regul Integr Comp Physiol 265:R334–R340
Davis TA, Fiorotto ML, Reeds PJ (1993b) Amino acid compositions of body and milk protein change during the suckling period in rats. J Nutr 123:947–956
Davis TA, Burrin DG, Fiorotto ML, Nguyen HV (1996) Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab 270:E802–E809
Davis TA, Fiorotto ML, Burrin DG, Pond WG, Nguyen HV (1997) Intrauterine growth restriction does not alter response of protein synthesis to feeding in newborn pigs. Am J Physiol Endocrinol Metab 272:E877–E884
Davis TA, Fiorotto ML, Nguyen HV, Burrin DG (1999) Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol Endocrinol Metab 277:E103–E109
Davis TA, Fiorotto ML, Beckett PR, Burrin DG, Reeds PJ, Wray-Cahen D, Nguyen HV (2001) Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 280:E770–E779
Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O’Connor PMJ (2002) Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282:E880–E890
Denne SC, Kalhan SC (1987) Leucine metabolism in human newborns. Am J Physiol 253:E608–E615
Fiorotto ML, Davis TA, Reeds PJ (2000) Regulation of myofibrillar protein turnover during maturation in normal and undernourished rat pups. Am J Physiol Regul Integr Comp Physiol 278:R845–R854
Garlick PJ, McNurlan MA, Preedy VR (1980) A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J 192:719–723
Garlick PJ, Fern M, Preedy VR (1983) The effect of insulin infusion and food intake on muscle protein synthesis in postabsorptive rats. Biochem J 210:669–676
Hsu CJ, Kimball SR, Antonetti DA, Jefferson LS (1992) Effects of insulin on total RNA, poly(A) + RNA, and mRNA in primary cultures of rat hepatocytes. Am J Physiol Endocrinol Metab 263:E1106–E1112
Jefferson LS, Liao WSL, Peavy DE, Miller TB, Appel MC, Taylor JM (1983) Diabetes-induced alterations in liver protein synthesis. Changes in the relative abundance of mRNAs for albumin and other plasma proteins. J Biol Chem 258:1369–1375
Obled C, Arnal M (1992) Contribution of skin to whole-body protein synthesis in rats at different stages of maturity. J Nutr 122:2167–2173
O’Connor PM, Bush JA, Suryawan A, Nguyen HV, Davis TA (2003) Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 284:E110–E119
O’Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA (2004) Regulation of neonatal liver protein synthesis by insulin and amino acids in pigs. Am J Physiol Endocrinol Metab 286:E994–E1003
Preedy VR, Adachi J, Ueno Y, Ahmed S, Mantle D, Mullatti N, Rajendram R, Peters TJ (2001) Alcoholic skeletal muscle myopathy: definitions, features, contribution of neuropathy, impact and diagnosis. Eur J Neurol 8:677–687
Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA (1997) Insulin-stimulated amino acid utilization during glucose and amino acid clamps decreases with development. Am J Physiol Endocrinol Metab 273:E305–E314
Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA (1998) Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol Endocrinol Metab 275:E602–E609
Young VR (1970) The role of skeletal and cardiac muscle in the regulation of protein metabolism. In: Munro HN (ed) Mammalian protein metabolism, vol 4, Academic, New York, pp 585–674
Acknowledgments
We thank J. C. Stubblefield for care of animals and L. F. Weiser for secretarial assistance. This work is publication of the United States Department of Agriculture/Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine. This project has been funded in part by National Institutes of Health (NIH) grant AR-44474 and by the USDA/ARS under Cooperative Agreement no. 6250510000-33.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Rights and permissions
About this article
Cite this article
Suryawan, A., O’Connor, P.M.J., Bush, J.A. et al. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids 37, 97–104 (2009). https://doi.org/10.1007/s00726-008-0149-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0149-z