Abstract
Hypertensive patients with normal glucose tolerance (NGT) but 1-h post-load plasma glucose ≥ 155 mg/dl (1-h high), during an oral glucose tolerance test (OGTT), show higher insulin resistance and multiple target organ damages. Experimental and clinical studies demonstrate that silybin presents anti-inflammatory and metabolic effects, improving insulin resistance and endothelial dysfunction. This study aims to evaluate the effects of the complex silybin–vitamin E and phospholipids on inflammatory, metabolic and vascular parameters in NGT 1-h high hypertensive patients. This is a pilot, single arm, interventional, longitudinal study enrolling 50 Caucasian NGT 1-h high hypertensive outpatients, 27 men and 23 women, age range 42–60 years (mean + SD = 52 ± 7). After 6 months of silybin intake, there is a significant improvement in metabolic profile. The glucose response during OGTT significantly improves (AUCglucose0–120 309.6 ± 63.4 at baseline vs 254.6 ± 35.5 at the follow-up, ∆ = − 55, 95% CI from − 67 to − 43, p < 0.0001), so as insulin response (AUCinsulin0–120 238.2 ± 99.1 vs 159.3 ± 44.9, ∆ = − 78.9, 95% CI from − 100.0 to − 57.8, p < 0.0001), in accordance with the increase of insulin sensitivity index Matsuda. Silybin intake is associated with a significant reduction of both clinical and central systolic blood pressure, with betterment in clinical and central pulse pressure and reduction of arterial stiffness parameters. In conclusion, this study demonstrates that silybin may improve the metabolic aspect and vascular damage in NGT 1-h high hypertensive patients who are at higher metabolic and cardiovascular risk. Thus, in these patients, silybin might strengthen the effect of antihypertensive drugs giving further cardiovascular protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin resistance (IR) represents a common pathophysiological mechanism of type 2 diabetes and arterial hypertension, both associated with the appearance and progression of cardiovascular disease (CVD) [1, 2].
Recently, we reported that a value of 1-h post-load plasma glucose ≥ 155 mg/dL (1-h high), during an oral glucose tolerance test (OGTT), is able to identify subjects with normal glucose tolerance (NGT) but who are at high risk of incident type 2 diabetes [3]; in accordance with this, 1-h high NGT hypertensive patients show an unfavorable metabolic profile characterized by higher IR and subclinical inflammation and multiple target organ damages, similar to that observed in individuals with impaired glucose tolerance (IGT) [4,5,6,7,8].
Experimental and clinical studies demonstrate that silybin, the main active component extracted from the milk thistle, presents important anti-inflammatory, antifibrotic and metabolic effects, particularly, in the liver [9, 10]. In patients with non-alcoholic fatty liver disease (NAFLD), the complex silybin–vitamin E-phospholipids may improve all metabolic syndrome parameters, reducing IR and ameliorating glucose metabolism and liver histology [11, 12]. Given the common pathophysiological pathways shared by both cardiovascular and metabolic diseases, it is plausible that silybin may be protective also for tissues other than the liver, and in different clinical settings. Thus, silybin shows markedly improved IR and endothelial dysfunction in an animal model of diabetes and obesity [13]. On the basis of these considerations, the aim of this study is to evaluate the effects of the complex silybin–vitamin E and phospholipids (®Realsil) on inflammatory, metabolic and vascular parameters in a group of never-treated 1-h high NGT hypertensive patients.
Methods
Study population
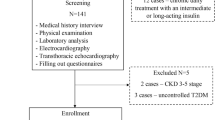
This pilot, single arm, interventional, longitudinal study consists of 50 Caucasian never-treated hypertensive outpatients, 27 men and 23 women, age range 42–60 years (mean + SD = 52 ± 7), who have an NGT with 1-h post-load plasma glucose ≥ 155 mg/dl, during OGTT. All patients underwent physical examination and review of their medical history. Causes of secondary hypertension were excluded by appropriate clinical and biochemical tests. Other exclusion criteria were represented by history or clinical evidence of ischemic or valvular heart disease, congestive heart failure, peripheral vascular and chronic gastrointestinal diseases associated with malabsorption, chronic pancreatitis, history of any malignant or autoimmune disease, alcohol or drug abuse, liver or kidney failure, treatments able to modify glucose metabolism and smoking.
All subjects underwent anthropometrical evaluation by measuring weight, height, body mass index (BMI) and waist. After 12-h fasting, a 75 g OGTT was performed with 0, 30-, 60-, 90- and 120-min sampling for plasma glucose and insulin. Glucose tolerance status was defined on the basis of OGTT using the World Health Organization (WHO) criteria.
Laboratory determinations
Glucose, triglyceride, total and high density lipoprotein cholesterol (HDL-C) concentrations were determined by enzymatic methods (Roche, Basel, Switzerland). Plasma insulin concentration was determined with a chemiluminescence-based assay (Immulite, Siemens, Italy). The minimum detectable concentration was 2 mIU/mL and the maximal inter-assay coefficient of variation was 5.5%. Total serum insulin like growth factor (IGF)-1 concentrations were measured by a chemiluminescent immunoassay (Nichols Institute Diagnostic, San Juan Capistrano, CA). The minimum detectable concentration was 0.03 mg/l, and the maximal inter-assay coefficient of variation was 7%. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured using the α-ketoglutarate reaction, and high-sensitivity C-reactive protein (hs-CRP) was measured by automated instrument (CardioPhase_hs-CRP, Siemens, Italy). The intra-assay coefficient of variation for hs-CRP was < 6%. Serum creatinine and uric acid (UA) were measured in the routine laboratory by an automated technique based on the measurement of Jaffe chromogen and by the URICASE/POD (Boehringer Mannheim, Mannheim, Germany) method implemented in an autoanalyzer.
Insulin sensitivity
The homeostasis model assessment (HOMA) index was calculated as [fasting insulin (µU/mL) × fasting glucose (mmol/l)]/22.5. Insulin sensitivity was evaluated using the Matsuda index (insulin sensitivity index [ISI]), calculated as follows: 10,000/square root of [fasting glucose (mmol/l) × fasting insulin (mU/l)] × [mean glucose × mean insulin during OGTT]. The Matsuda index is strongly related to euglycemic–hyperinsulinemic clamp, which represents the gold standard test for measuring insulin sensitivity [14, 15]. The trapezoidal method was used to calculate glucose and insulin area under the curve (AUC) during the OGTT [16]. Renal function was evaluated by calculation of the estimated glomerular filtration rate (e-GFR), using the CKD-Epi equation [17].
Blood pressure measurements
Readings of clinic blood pressure (BP) were obtained in the left arm of the supine patients, after 5 min of quiet rest, with a mercury sphygmomanometer. Systolic BP (SBP) and diastolic BP (DBP) were recorded at the first appearance (phase I) and the disappearance (phase V) of Korotkoff sounds. Baseline BP values were the average of the last two of the three consecutive measurements obtained at intervals of 3 min, on three separate occasions at least 2 weeks apart. Patients with a clinic SBP ≥ 140 mmHg or DBP ≥ 90 mmHg were defined as hypertensive, according to current guidelines [18]. For the study protocol, only patients with essential arterial hypertension of mild degree were considered (SBP ≥ 140 mmHg and < 160 mmHg and/or DBP ≥ 90 and < 100 mmHg).
Arterial stiffness and central BP measurements
The evaluation of the arterial stiffness was performed by the analysis of the shape and speed of the peripheral and central pressure wave. All studies were performed in the supine position, in a quiet room with a constant temperature between 22 and 24 °C, after abstaining from cigarette smoking and food and alcohol intake in the 12 h preceding the study. These measurements were obtained by a validated system (SphygmocorTM; AtCor Medical, Sydney, Australia) that employs high-fidelity applanation tonometry (Millar) and appropriate computer software for the analysis of pressure wave (SphygmocorTM) [6]. Pressure calibration was obtained through automatically, non-invasively recorded supine brachial artery BP of the dominant arm after a 30-min rest (Dinamap Compact T; Johnson & Johnson Medical Ltd, Newport, UK). BP was measured five times over 10 min, and the mean of the last three measurements was taken for calibration. Pressure wave recording was performed at the radial artery of the dominant arm with the wrist softly hyperextended, and it is the average of single pressure waves recorded consecutively for 8 s. Pressure wave recordings were accepted only if variation of peak and bottom pressures of single pressure waves was < 5%. The central pressure wave was automatically derived from the radial pressures by a built-in generalized transfer function. In addition, pressure wave measurement was also obtained at the right carotid artery, as it is well known that central AI may be more accurately derived from this vascular site [19]. Central waveforms were further analyzed to identify the time to peak/shoulder of the first (T1) and second (T2) pressure wave components during systole. The pressure at the peak/shoulder of T1 was identified as outgoing pressure wave height (P1), and the pressure at the peak/shoulder of T2 was identified as the reflected pressure wave height (P2), either absolutely or as percent of ejection duration. AP was defined as the difference between P2 and P1, and AI as [AP/pulse pressure (PP)] × 100. Aortic pulse wave velocity (PWV) was determined from the carotid and femoral pressure waveforms. Carotid to femoral transit time (DT) was computed from the foot-to-foot time difference between carotid and femoral waveforms. The distance between the surface markings of the sternal notch and femoral artery was used to estimate the path length between the carotid and femoral arteries (L), and PWV was computed as L/DT.
Modality of intervention
After enrollment, all patients were encouraged to make lifestyle changes according to the current guidelines [20]; moreover, it was also recommended to take silybin conjugated to vitamin E and phosphatidylcholine that shows higher intestinal absorption and bioavailability (Indena, IBI-Lorenzini spa Italy: Realsil®) as granules for oral solution at a dose of 3 g bid. Each subject was re-evaluated monthly by performing a clinical examination to consider the necessity for antihypertensive treatment. Finally, inflammatory, metabolic and hemodynamic parameters were assessed after 6 months of treatment. In particular, at the end of the follow-up period, a new OGTT was performed, to test the effects of silybin on glucose tolerance and IR status.
The protocol was approved by the local Ethics Committee (Comitato Etico Azienda Ospedaliera “Mater Domini”) and informed written consent was obtained from all participants. All the investigations were performed in accordance with the principles of the 1975 Declaration of Helsinki (clinicaltrials.gov: NCT03538327).
Statistical analysis
Continuous data are expressed as mean ± SD. For all variables, comparisons between baseline (T0) and post-treatment values (T6) were performed using paired Student’s t test. The changes in all biomarkers in response to silybin intake were expressed as mean and 95% CI, according to the CONSORT statement [21]. Simple linear regression analysis was performed to assess the relationship between variation in arterial stiffness indices (PWV, AI, AP), expressed as Δ of variation between baseline and follow-up (ΔT0–6) and the variation of metabolic and inflammatory covariates that significantly improved after the treatment (expressed as ΔT0–6). Thus, variables reaching statistical significance and gender, as dichotomic variable, were inserted in a stepwise multivariate linear regression model to assess the magnitude of their individual effect on ΔPWV, ΔAI and ΔAP, with regard to the relationship of a variable every eight patients.
In the regression analysis, to test the independent effect of glucose and insulin responses on vascular parameters, we included only ΔAUC0–120 of glucose and insulin. We did not include ΔMatsuda to avoid a possible colinearity. Differences were assumed to be significant at p < 0.05. All comparisons were performed using SPSS 20.0 statistical software for Windows (SPSS, Inc., Chicago, IL).
Results
Study population
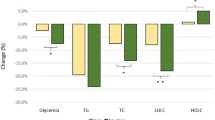
The main clinical, anthropometric and biochemical parameters of the study population at baseline and after the period of treatment are reported in Table 1. After 6 months of silybin intake, there is a significant improvement in metabolic profile; in particular, a significant reduction in total cholesterol and triglyceride and an increase in HDL-cholesterol levels are observed. In addition, fasting glucose and insulin significantly decrease after silybin treatment with reduction of the HOMA index. Of interest, as shown in Fig. 1, the glucose response during OGTT is significantly reduced after silybin (AUCglucose0–120 309.6 ± 63.4 vs 254.6 ± 35.5, ∆ = − 55, 95% CI from − 67 to − 43, p < 0.0001), as well as insulin response (AUCinsulin0–120 238.2 ± 99.1 vs 159.3 ± 44.9, ∆ = − 78.9, 95% CI from − 100.0 to − 57.8, p < 0.0001); in accordance with this, the Matsuda index significantly increases (Table 1). In addition, silybin treatment is associated with an increase in IGF-1 levels, and the improvement of renal function is demonstrated by a significant increase of 4 ml/min/1.73 m2 in e-GFR. Finally, also hs-CRP, UA and liver enzymes are significantly reduced by silybin treatment, confirming a general improvement of both metabolic and inflammatory status, without any significant change in the anthropometric parameters.
In Table 2, we report the clinical and central hemodynamic parameters, before and after silybin treatment. Interestingly, silybin intake is significantly associated with a reduction of both clinical and central SBP, with consequent betterment in clinical and central PP values. Similarly, we observed a significant reduction in PWV and the other related arterial stiffness parameters, i.e., AI and AP.
Correlational analysis on arterial stiffness parameters
A linear regression analysis was performed to test the correlation between change in arterial stiffness indices (PWV, AI, AP), expressed as Δ of variation between baseline and follow-up (ΔT0–6) and the different metabolic and inflammatory covariates. As shown in Table 3, ΔPWV is significantly correlated with ΔAUC insulin0–120 and ΔAUCglucose0–120. There is a significant correlation between ΔAI and the variation of AUC insulin0–120, AUCglucose0–120 and triglyceride. Finally, ΔAP is significantly correlated with ΔAUC insulin0–120 and ΔIGF-1. Thus, variables reaching statistical significance and gender, as dichotomic variable, were added in a stepwise multivariate linear regression model to assess the magnitude of their individual effect on ΔPWV, ΔAI and ΔAP. The variation of AUC insulin0–120 is the strongest predictor for all three vascular function parameters. In particular, it justifies a 30.9, 29.4 and 13.2% of variation for ΔPWV, ΔAI and ΔAP, respectively. The change in AUCglucose0–120 adds another 6.7% for ΔPWV variation and ΔIGF-1 accounts for another 10.9% for ΔAP.
Discussion
In this study, we demonstrate that a 6-month treatment with an oral complex containing silybin, phosphatidylcholine and vitamin E induces, in a group of newly diagnosed hypertensive patients, a significant improvement in the metabolic and inflammatory profile. In particular, silybin treatment is able to reduce both fasting glucose and insulin levels as well as glucose and insulin AUC during OGTT, with the increase of the Matsuda index, confirming its positive impact on IR status. As a consequence of insulin sensitivity improvement, IGF-1 levels significantly increase while hs-CRP levels decrease. Clinically relevant, all these changes occur independently of the body weight modification; in fact, both BMI and waist circumference did not change significantly.
This study is the first evidence of the positive metabolic effects exerted by silybin, on NGT hypertensive patients with high 1-h post-load plasma glucose who are at high risk for incident type 2 diabetes and target organ damage [3,4,5,6,7]. In fact, in previous studies, the metabolic effects of silybin were assessed in patients with NAFLD, where silybin treatment was associated with a significant improvement of IR, liver function tests and liver histology [11, 12]. The positive effect of silybin on insulin signalling may be partly explained by the attenuation of the insulin receptor substrate-1 (IRS-1)/phosphoinositide 3-kinase (PI3K)/Akt pathway, as demonstrated by experimental in vitro and in vivo studies [10, 22, 23]. In particular, in skeletal muscle cells, silybin may prevent palmitate-induced IR promoting IRS-1 tyrosine phosphorylation, and inhibiting the negative effect of serine phosphorylation [22]. Moreover, in an NAFLD animal model, the addition of silybin improves IR and hepatic histology, while being able to reduce resistin expression in cultured rat liver cells. Similarly, in our patients, we also observe a significant reduction of both AST and ALT levels.
Because all these actions are able to be nullified by a PI-3K specific blocker, it is plausible that silybin operates by this pathway [23]. Thus, on this evidence, it is possible to affirm that the positive effects of silybin on IR pathophysiological mechanisms may be clinically relevant because the improvement of insulin sensitivity reduces the inflammatory burden and oxidative stress that promote different pathogenetic pathways, leading not only to glucose intolerance, but also in the development of subclinical target organ damage that represents an independent predictor of subsequent cardiovascular events [24,25,26].
At the same time, we also demonstrate a significant improvement of all the parameters related to vascular stiffness due to the reduction of AUC insulin0–120 after silybin treatment, which is the strongest predictor of these changes, justifying in particular a 30.9% of PWV variation. These findings confer an important biological plausibility to our results, because an increase in PWV is associated with the rise in both central SBP and PP, with a consequent growth of cardiac systolic work and decrease of coronary perfusion pressure, all conditions promoting CV events [27, 28]. Apart from the improvement of IR, the positive effect of silybin on vascular stiffness may be justified by other mechanisms. In particular, silybin has demonstrated, in a mouse model of diabetes, reduced plasma and aorta asymmetric dimethyl-l-arginine levels [13], an endogenous competitive inhibitor of endothelial nitric oxide synthase that reduces nitric oxide bioavailability in humans [29]. In addition, silybin is able to inhibit radical accumulation by an important scavenger activity, interfering with both lipid membrane peroxidation and stabilization of mitochondrial membrane [9, 30]. In particular, in rats with NAFLD, silybin treatment is more effective than that with rosiglitazone in increasing the levels of superoxide dismutase and glutathione. Moreover, silybin enhances the gene and protein expression of adiponectin, but inhibits that of resistin thus improving insulin sensitivity. Finally, silybin is effective in stabilizing mitochondrial membrane fluidity with reduction of free radicals [30]. Therefore, it is plausible that the positive effect on IR and mitochondrial function and the inhibition of oxidative stress may represent the crucial mechanisms for the vascular protective effect of silybin and these actions may justify the reduction of arterial stiffness as demonstrated in our study.
It is likely that the improvement of vascular compliance may justify the reduction of both SBP and PP as observed in our patients, conferring a clinical relevance because, as reported in a recent meta-analysis, including 123 studies with over 600,000 participants, a 10 mmHg reduction in SBP reduces the risk of major CV events by 20%, coronary heart disease by 17% and stroke by 27% [31]. The vascular benefit after treatment is also evidenced by the improvement in renal function as demonstrated by a significant reduction of creatinine and increase of e-GFR.
Obviously, this is a pilot study obtained on a small sample, and these data should be confirmed in a randomized controlled multicenter study, including a larger number of patients. In addition, the results were observed in NGT hypertensives with high 1-h post-load plasma glucose, and it may not be valid in other settings of patients. Another important limitation of the study is the lack of oxidative stress evaluation after intake of silybin. This is a crucial point because the reduction of oxidative stress may account for the improvement in arterial stiffness. However, the reduction of oxidative stress, even if not measured, can be hypothesized considering the betterment of insulin sensitivity and inflammatory burden observed in patients of the study after silybin intake.
Finally, the strengths are represented by the complete metabolic assessment. In fact, IR was assessed with OGTT-derived indices, considering glycemic and insulinemic responses. Moreover, vascular stiffness was measured by carotid–femoral PWV that represents the gold standard test.
In conclusion, this study demonstrates that silybin may improve the metabolic aspect and vascular damage in NGT hypertensive patients with 1 h-post-load plasma glucose ≥ 155 mg/dl, characterized by higher metabolic and cardiovascular risk. Taken together, in hypertensive insulin-resistant patients, silybin might strengthen the effect of antihypertensive drugs through the improvement of the metabolic condition, giving further vascular protection.
References
Ferrannini E, Cushman WC (2012) Diabetes and hypertension: the bad companions. Lancet 380:601–610. https://doi.org/10.1016/S0140-6736(12)60987-8
Muniyappa R, Montagnani M, Koh KK, Quon MJ (2007) Cardiovascular actions of insulin. Endocr Rev 28:463–491. https://doi.org/10.1210/er.2007-0006
Fiorentino TV, Marini MA, Andreozzi F, Arturi F, Succurro E, Perticone M, Sciacqua A, Hribal ML, Perticone F, Sesti G (2015) One-hour post load hyperglycemia is a stronger predictor of type 2 diabetes than impaired fasting glucose. J Clin Endocrinol Metab 100:3744–3751. https://doi.org/10.1210/jc.2015-2573
Sciacqua A, Miceli S, Carullo G, Greco L, Succurro E, Arturi F, Sesti G, Perticone F (2011) One-hour post load plasma glucose levels and left ventricular mass in hypertensive patients. Diabetes Care 34:1–6. https://doi.org/10.2337/dc11-0155
Sciacqua A, Miceli S, Greco L, Arturi F, Naccarato P, Mazzaferro D, Tassone EJ, Turano L, Martino F, Sesti G, Perticone F (2011) One-hour postload plasma glucose levels and diastolic function in hypertensive patients. Diabetes Care 34:2291–2296. https://doi.org/10.2337/dc11-0879
Sciacqua A, Maio R, Miceli S, Pascale A, Carullo G, Grillo N, Arturi F, Sesti G, Perticone F (2012) Association between one-hour post-load plasma glucose levels and vascular stiffness in essential hypertension. PLoS One 7:e44470. https://doi.org/10.1371/journal.pone.0044470
Perticone F, Sciacqua A, Perticone M, Miceli S, Maio R, Tassone JE, Arturi F, Sesti G (2013) Phenotypic characterization of normotolerant hypertensive patients. Int J Cardiol 165:322–326. https://doi.org/10.1016/j.ijcard.2011.08.076
Perticone F, Sciacqua A, Perticone M, Arturi F, Scarpino PE, Quero M, Sesti G (2012) Diabetes Care 35:153–157. https://doi.org/10.2337/dc11-1727
Loguercio C, Festi D (2011) Silybin and the liver: from basic research to clinical practice. World J Gastroenterol 17:2288–2301. https://doi.org/10.3748/wjg.v17.i18.2288
Trappoliere M, Caligiuri A, Schmid M, Bertolani C, Failli P, Vizzutti F, Novo E, di Manzano C, Marra F, Loguercio C, Pinzani M (2009) Silybin, a component of silymarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J Hepatol 50:1102–1111. https://doi.org/10.1016/j.jhep.2009.02.023
Federico A, Trappoliere M, Tuccillo C, de Sio I, Di Leva A, Del Vecchio Blanco C, Loguercio C (2006) A new silybin–vitamin E–phospholipid complex improves insulin resistance and liver damage in patients with non-alcoholic fatty liver disease: preliminary observations. Gut 55:901–902. https://doi.org/10.1136/gut.2006.091967
Loguercio C, Andreone P, Brisc C, Brisc MC, Bugianesi E, Chiaramonte M, Cursaro C, Danila M, de Sio I, Floreani A, Freni MA, Grieco A, Groppo M, Lazzari R, Lobello S, Lorefice E, Margotti M, Miele L, Milani S, Okolicsanyi L, Palasciano G, Portincasa P, Saltarelli P, Smedile A, Somalvico F, Spadaro A, Sporea I, Sorrentino P, Vecchione R, Tuccillo C, Del Vecchio Blanco C, Federico A (2012) Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med 52:1658–1665. https://doi.org/10.1016/j.freeradbiomed.2012.02.008
Li Volti G, Salomone S, Sorrenti V, Mangiameli A, Urso V, Siarkos I, Galvano F, Salamone F (2011) Effect of silibinin on endothelial dysfunction and ADMA levels in obese diabetic mice. Cardiovasc Diabetol. 10:62. https://doi.org/10.1186/1475-2840-10-62
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA (2007) Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 30:89–94. https://doi.org/10.2337/dc06-1519
Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L (1990) Blood glucose area under the curve, methodological aspects. Diabetes Care 13:172–175
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
ESH/ESC guidelines for the management of arterial hypertension (2013) The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34:2159–2219. https://doi.org/10.1093/eurheartj/eht151
Chen CH, Ting CT, Nussbacher A, Nevo E, Kass DA, Pak P, Wang SP, Chang MS, Yin FC (1996) Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension 27:168–175
European Guidelines on cardiovascular disease prevention in clinical practice (2016) The sixth joint task force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EA). Eur Heart J 37:2315–2381
Bolignano D, Mattace-Raso F, Torino C, D’Arrigo G, Abd ElHafeez S, Provenzano F, Zoccali C, Tripepi G (2013) The quality of reporting in clinical research: the CONSORT and STROBE initiatives. Aging Clin Exp Res 25:9–15. https://doi.org/10.1007/s40520-013-007-z
Li HB, Yang YR, Mo ZJ, Ding Y, Jiang WJ (2015) Silibinin improves palmitate-induced insulin resistance in C2C12 myotubes by attenuating IRS-1/PI3 K/Akt pathway inhibition. Braz J Med Biol Res 48:440–446. https://doi.org/10.1590/1414-431X20144238
Zhang Y, Hai J, Cao M, Zhang Y, Pei S, Wang J, Zhang Q (2013) Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/Akt pathway. Int Immunopharmacol 17:714–720. https://doi.org/10.1016/j.intimp.2013.08.019
Ceravolo R, Maio R, Cuda G, Scozzafava A, Sciacqua A, Vatrano M, Bellieni G, D’Angelo G, Schipani FA, Sesti G, Perticone F (2003) Relation of fasting insulin related to insertion/deletion polymorphism of angiotensin-converting enzyme-gene and cardiac mass in never-treated patients with systemic hypertension. Am J Cardiol 92:1234–1237
Nerpin E, Risérus U, Ingelsson E, Sundström J, Jobs M, Larsson A, Basu S, Arnlöv J (2008) Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care 31:1550–1555. https://doi.org/10.2337/dc08-0369
Sciacqua A, Scozzafava A, Pujia A, Maio R, Borrello F, Andreozzi F, Vatrano M, Cassano S, Perticone M, Sesti G, Perticone F (2005) Interaction between vascular dysfunction and cardiac mass increases the risk of cardiovascular outcomes in essential hypertension. Eur Heart J 26:921–927. https://doi.org/10.1093/eurheartj/ehi112
Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H (2009) Role of pulse pressure amplification in arterial hypertension: experts’ opinion and review of the data. Hypertension 54:375–383. https://doi.org/10.1161/HYPERTENSIONAHA.109.134379
Ceravolo R, Maio R, Pujia A, Sciacqua A, Ventura G, Costa MC, Sesti G, Perticone F (2003) Pulse pressure and endothelial dysfunction in never-treated hypertensive patients. J Am Coll Cardiol 41:1753–1758
Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, Tripepi G, Sesti G, Zoccali C (2005) Asymmetric dimethylarginine, l-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol 46:518–523. https://doi.org/10.1016/j.jacc.2005.04.040
Yao J, Zhi M, Minhu C (2011) Effect of silybin on high-fat-induced fatty liver in rats. Braz J Med Biol Res 44:652–659
Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K (2016) Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 387:957–967. https://doi.org/10.1016/S0140-6736(15)01225-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statements on human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sciacqua, A., Perticone, M., Tripepi, G. et al. Metabolic and vascular effects of silybin in hypertensive patients with high 1-h post-load plasma glucose. Intern Emerg Med 14, 77–84 (2019). https://doi.org/10.1007/s11739-018-1951-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-018-1951-6