Abstract
β-Alanine (BA) supplementation has become an ergogenic aid amongst competitive athletes to augment intramuscular carnosine content, leading to higher buffer capacity and exercise performance. We investigated 27 regularly trained young males and females who were randomly allocated either to placebo (PL) or BA ingestion for 8 weeks. Every single day, BA or PL (4.0–5.6 g day−1) supplements were ingested by participants and associated with a strong plyometric high-intensity training (two sessions per week during the 8 weeks). Before and after training, maximal jump heights were recorded during squat jump (SJ) and countermovement jump (CMJ) and an index of fatigue was recorded as a mean height of 45 consecutive CMJ. Blood lactate was measured at rest, after completing the fatigue test and every 5 min thereafter up to 30 min recovery. After plyometric training, SJ and CMJ were increased, respectively, by 8.8 and 6.4 % in PL group and 9.9 and 11.0 % in BA group (p < 0.01, no difference between groups). Blood lactate reached a maximal value of 9.4 ± 1.6 mmol l−1 in PL group, and 10.3 ± 1.3 mmol l−1 in BA group, with a slight better performance in the fatigue test (+8.6 %, p ≤ 0.01) for BA group as compared to PL group. To conclude, 2-month β-alanine supplementation resulted in a slight improvement of explosive force after 45 maximal consecutive jumps in young athletes. However, the practical adequacy of supplementation remains questionable in an active and healthy population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β-Alanine supplementation (Harris et al. 2006) has become of growing interest amongst competitive athletes participating in a range of different sports, such as rowing (Hobson et al. 2013), road cycling (Howe et al. 2013; Bex et al. 2014; Gross et al. 2014a, b; Saunders et al. 2014), kayaking, swimming (Chung et al. 2012), running (Ducker et al. 2013), resistance training (Kendric et al. 2008; Kresta et al. 2014), and sprinting (Derave et al. 2007), with or without suitable effect. β-Alanine is a non-proteinogenic amino acid synthesized within the liver from the irreversible degradation of thymine, cytosine, and uracil (McMurray and Hackney 2005), and then transported to muscle cells to be combined with l-histidine to form carnosine (Sale et al. 2013). The latter has multiple functions (Caruso et al. 2012) but its primary role in muscle fibres is to maintain an intracellular buffering capacity due to its pK (a) of 6.83 (Harris and Sale 2012).
Several reviews pointed out that oral β-alanine and/or carnosine supplementation enhances exercise performance in healthy conditions (Culbertson et al. 2010; Caruso et al. 2012; Hoffman et al. 2012; Bellinger 2014; Quesnele et al. 2014) and might have potential therapeutic role in ageing, neurological diseases, diabetes, and cancer (Sale et al. 2013). Most studies and reviews gave some evidence that supplementation with β-alanine may increase athletic performance; however, one review cautioned the use of β-alanine supplementation as an ergogenic aid until there is sufficient evidence confirming its safety (Quesnele et al. 2014).
Those studies used classical investigations to appreciate the effect of β-alanine intake on exercise performance, such as muscle buffer capacity, delay in the onset of muscular fatigue, and facilitated recovery. However, Bellinger estimated that scientific confirmation is required for repeated maximal effort (Bellinger 2014). Therefore, we decided to investigate this specificity using squat jump (SJ) and countermovement jump (CMJ) measurements (Bosco et al. 1983; Garcia-Lopez et al. 2005; Bellinger 2014) to assess the strength of extensor muscles of the lower limbs, with and without β-alanine supplementation in human subjects.
Methods
Study population
Twenty-seven white young subjects (students of physical education) were enrolled in this study: 13 subjects in placebo group (PL) (six men and seven women, age 21.4 ± 2.1 years, mean ± SD, weight 63.7 ± 8.5 kg, height 174.7 ± 11.6 cm); 14 subjects in β-alanine group (BA) (six men and eight women, age 22.1 ± 2.1 years, weight 63.6 ± 10.0 kg, height 168.8 ± 9.4 cm). All subjects were healthy and physically active. None of them were subjected to allergy or intolerant to the placebo or β-alanine supplementation. The informed written consent of the subjects was recorded after full explanation of the whole investigation. This study was approved by the Medical Ethical Committee of the Academic Hospital and this project was conducted in accordance with the guidelines of the declaration of Helsinki of 1975 as revised in 1976.
Placebo and β-alanine double-blind supplementation was randomly distributed, and administered during 8 weeks. The total daily doses of 5.6 g were split into single doses of 800 mg ingested every 2 h, starting at 08.00 hours. Each single dose contained either microcrystalline cellulose (PL) or β-alanine (BA) enrobed in small digestible capsules. This wide daily distribution avoided the appearance of potential symptoms of paraesthesia or irritation of the skin (Sale et al. 2013).
Exercise protocol
The exercise protocol was carried out in the morning the day before the beginning of the supplementation and 3 days before the end of the 8-week supplementation.
The exercise test was based on vertical jump tests largely used to assess different forms of explosive strength in the extensor muscles of the lower limbs (Bosco et al. 1983; Sebert et al. 1990; Bobbert et al. 1996; Garcia-Lopez et al. 2005; Bobbert 2014). The subjects performed five maximal squat jumps (SJ), then five countermovement jumps (CMJ). The average of the three intermediate heights was used for statistical analyses. Then, subjects underwent a fatigue test consisting of 45 consecutive CMJ followed by 30-min rest. An “Optojump” instrument system (Microgate, Italy), consisting of two parallel bars disposed opposite to each others and connected to a computer, was used to quantify the flight time during vertical jump estimating the height of the jump. This system has been validated by Glatthorn et al. (2011) using a force plate.
Blood lactate was collected from warmed ear samples before the fatigue test, by its end and every 5 min during the recovery period (30 min), using the “Lactate Scout analyser” (Senslab, Germany), a biosensor enzymatic amperometry. This test strip uses l-lactate oxidase to catalyse the oxidation of l-lactate.
Training was carried out during 8 weeks, two sessions per week, and consisted in repeated plyometric exercises of the lower limbs performed during 30–50 s each (five exercises per session, repeated twice).
Statistics
Conventional statistical methods were used to calculate means, standard deviation and standard error of the mean. Prior comparing each independent variable, the normality of the data was confirmed with the Kolmogorov–Smirnov test. Covariance analyses (ANCOVA) were used to calculate regression and angular coefficient. Jump heights (in cm), the mean height of the 45 consecutive CMJ (in cm), and the evolution of post-exercise values over 30 min were recorded before and after exercise training in PL and BA groups. Gains between pre- and post-tests (in %) were investigated in PL and BA groups. A repeated measures ANOVA was used to compare the variations amongst the means of blood lactate followed by Dunnett’s multiple comparisons for post hoc analyses with the pre-exercise value as a control when the value was less than 0.05. A probability of p ≤ 0.05 was considered significant for all conditions.
Results
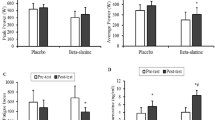
Squat jump (SJ) and countermovement jump (CMJ) values, recorded in placebo (PL) and β-alanine (BA) groups, before and after supplementation are reported in Fig. 1. After training, an increase in jump height (in cm) was observed not only for SJ in both PL group (+8.8 ± 2.3 %; p < 0.01) and BA group (+9.9 ± 2.2 %; p < 0.001) but also for CMJ (6.4 ± 1.4 and 11.0 ± 2.1 %, respectively, for PL and BA groups; p < 0.001), without any statistical differences between the two groups.
Comparison in % of SJ and CMJ height after training increased. The increase was significantly different in the both group (respectively 8.8 ± 2.3 and 6.4 ± 1.4 % for SJ and CMJ in PL group and 9.9 ± 2.2 and 11.0 ± 2.1 % in BA group). No significant differences between the two groups were observed. **, ***Significant differences with the pre-training condition at, respectively, p < 0.01 and p < 0.001
The fatigue test (mean height of the consecutive 45 CMJ) did not show any statistical differences in pre-test values between PL and BA groups. The BA group showed an improved average performance (+8.6 ± 2.0 %, p < 0.01) after supplementation (Fig. 2). Moreover, the decline in jump heights during the fatigue test was reduced in a lesser extent in the BA group (3.9 %; p ≤ 0.05; Fig. 3).
Comparison of the negative linear regression in CMJ heights over time after training in both groups. The equations were, respectively, for PL and BA groups: y = −1.226 + 94.04; r 2 = 0.79; p < 0.001 and y = −1.158 + 94.83; r 2 = 0.80; p < 0.001. The slope of the regression obtained after training was significantly reduced for the BA group in comparison to PL group (p < 0.05)
Blood lactate increased by the end of the 45 CMJ in both groups (PL 9.4 ± 1.6 mmol L−1, BA 10.3 ± 1.3 mm L−1, NS between groups) and decreased progressively during the 30 min post-exercise with no differences in pre- and post-test values (Fig. 4).
The distribution of the blood lactate before and after training in Pl (a) and BA (b) groups. **Significant difference between rest and post-exercise conditions (at the end of the fatigue test and every 5 min during the recovery period) at p < 0.01. However, there is no significant difference between Pl and BA groups before and after training
Discussion
The present study shows a reduced statistical decline in performance during the 45 maximal CMJ (+8.6 %) in males and females submitted to high-intensity training during 2 months whilst consuming 5.6 g β-alanine (BA) every day, without any statistical differences between groups (Fig. 2). Previous publications already suggested possible benefits of β-alanine supplementation in high-intensity trained athletes (Howe et al. 2013; Sale et al. 2013; Painelli et al. 2014), whilst other studies were less convincing (Culbertson et al. 2010; Kresta et al. 2014; Quesnele et al. 2014). Moreover, a recent study observed an increase of 7 ± 3 % in power output after three maximal CMJ in professional alpine skiers (Gross et al. 2014a, b). Due to a wide daily distribution of the supplementation, our subjects did not experience any side effect disturbances, as often observed after BA absorption (Harris et al. 2006; Décombaz et al. 2012).
Our results showed that a 2-month training increased single jump height both in placebo and β-alanine supplementation groups, as observed recently by Gross et al. (2014a, b) on professional alpine skiers. Nevertheless, maximal blood lactate concentrations and blood lactate decline during the recovery period (30 min) did not show any statistical differences between placebo and β-alanine consumers. Thus, we could argue that during the 45-jump test anaerobic glycolysis and lactate accumulation were not connected to any major disturbance of buffering capacity, accumulation of lactate, or improved mitochondrial coupling. A possible explanation is that BA supplementation increased carnosine muscle content in subjects and that the proton-sequestering property of the carnosine molecule reduced the drop in muscle pH induced by the anaerobic glycolysis, improving performance (Harris et al. 2006).
To conclude, 2-month β-alanine supplementation resulted in a slight improvement of explosive force after 45 maximal consecutive jumps in young athletes. However, the practical adequacy of supplementation remains questionable in an active and healthy population.
References
Bellinger PM (2014) β-Alanine supplementation for the athletic performance: an update. J Strength Cond Res 28:1751–1770
Bex T, Chung W, Baguet A, Stegen S, Stautemas S, Achten E, Derave W (2014) Muscle carnosine loading by beta-alanine supplementation is more pronounced in trained vs untrained muscles. J Appl Physiol 116:204–209
Bobbert ME (2014) Effect of unloading and loading on power in simulated countermovement and squat jumps. Med Sci Sports Exerc 46:1176–1184
Bobbert ME, Gerritsen KG, Litjens MC, Van Soest AJ (1996) Why is countermovement jump height greater than squat jump height? Med Sci Sports Exerc 28:1402–1412
Bosco CP, Luhtanen P, Komi PV (1983) A simple method for measurement of mechanical power of jumping. Eur J Appl Physiol 50:273–282
Caruso J, Charles J, Unruh K, Giebel R, Learmonth L, Potter W (2012) Ergogenic effects of β-alanine and carnosine: proposed future research to quantify their efficency. Nutrients 4:585–601
Chung W, Shaw G, Anderson ME, Pyne DB, Saunders PU, Bishop DJ, Burke LM (2012) Effect of 10 week beta-alanine supplementation on competition and training performance in elite swimmers. Nutrients 4:1441–1453
Culbertson JY, Kreider RB, Greenwood M, Cooke M (2010) Effects of beta-alanine on muscle carnosine and exercise performance: a review of the current literature. Nutrients 2:75–98
Décombaz J, Beaumont M, Vuichoud J, Bouisset F, Stellingwerff T (2012) Effect of slow-release β-alanine tablets on absorption kinetics and paresthesia. Amino Acids 43:67–76
Derave W, Özdemir MS, Harris RC, Pottier A, Reyngoudt H, Koppo K, Achten E (2007) β-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J Appl Physiol 103:1728–1736
Ducker KJ, Dawson B, Wallman KE (2013) Effect of beta-alanine supplementation on 800 m running performance. Int J Sport Nutr Exerc Metab 23:554–561
Garcia-Lopez J, Peleteiro J, Rodgriguez-Marroyo JA, Morante JC, Herrero JA, Villa JG (2005) The validation of a new method that measures contact and fligh times during vertical jump. Int J Sports Med 26:294–302
Glatthorn J, Gouge S, Nussbaumer S, Stauffacher S, Impellizzeri FM, Maffiuletti NA (2011) Validity and reliability of Optojump photoelectric cells for estimation vertical jump height. J Strength Cond Res 25:556–560
Gross M, Bieri K, Hoppeler H, Norman B, Vogt M (2014a) Beta-alanine supplementation improves jumping power and affects severe-intensity performance in professional alpine skier. Int J Sport Nutr Exerc Metab 24:665–673
Gross M, Boesch C, Bolliger CS, Norman B, Gustafsson T, Hoppeler H, Vogt H (2014b) Effects of beta-alanine supplementation and interval training on physiological determinants of severe performance. Eur J Appl Physiol 114:221–234
Harris RC, Sale C (2012) Beta-alanine supplementation in high-intensity exercise. Med Sport Sci 59:1–17
Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Wise JA (2006) The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30:279–289
Hobson RM, Harris RC, Martin D, Smith P, Macklin B, Gualano B, Sale C (2013) Effect of beta-alanine with and without sodium bicarbonate on 2000 m rowing performance. Int J Sport Nutr Exerc Metab 23:480–487
Hoffman JR, Emerson NS, Stout JR (2012) β-Alanine supplementation. Curr Sports Med Rep 11:189–195
Howe ST, Bellinger PM, Driller MW, Shing CM, Fell JW (2013) The effect of beta-alanine supplementation on isokinetic force and cycling performance in highly trained cyclists. Int J Sport Nutr Exerc Metab 23:562–570
Kendric IP, Harris RC, Kim HJ, Kim CK, Dang VH, Lam MT (2008) Effect of 10 weeks resistance training combined with β-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids 34:547–554
Kresta JY, Oliver JM, Jagim AR, Fluckey S, Kelly K, Meininger C, Mertens-Talcott SU, Rasmussen C, Kreider RB (2014) Effects of 28 days of beta-alanine and creatine supplementation on muscle carnosine, body composition and exercise performance in recreationally active females. J Int Soc Sports Nutr 11:55–69
McMurray RG, Hackney AC (2005) Interactions of metabolic hormones, adipose tissue and exercise. Sports Med 35:393–412
Painelli VdS, Saunders B, Sale C, Harris RC, Solis MY, Roschel H, Gualano B, Artioli GG, Jr Lancha AH (2014) Influence of training status on high-intensity intermittent performance in response to β-alanine supplementation. Amino Acids 46:1207–1215
Quesnele JJ, Laframboise MA, Wong JJ, Kim P, Wells GD (2014) The effects of beta-alanine supplementation on performance: a systematic review of the literature. Int J Sport Nutr Exerc Metab 24:14–27
Sale C, Artioli GG, Gualano B, Saunders B, Hobson RM, Harris RC (2013) Carnosine: from exercise performance to health. Amino Acids 44:1477–1491
Sebert P, Barthelemy L, Dietman Y, Douguet C, Boulay JA (1990) A simple device for measuring a vertical jump: description and results. Eur J Appl Physiol 61:271–273
Acknowledgments
We are indebted to Hedelab (Belgium) for the two food supplementations (placebo and β-alanine).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
Statement of human rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinski declaration and its later amendments or comparable ethical standards. Statement on the welfare of animals: This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: G. Lubec.
Rights and permissions
About this article
Cite this article
Carpentier, A., Olbrechts, N., Vieillevoye, S. et al. β-Alanine supplementation slightly enhances repeated plyometric performance after high-intensity training in humans. Amino Acids 47, 1479–1483 (2015). https://doi.org/10.1007/s00726-015-1981-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-1981-6