Abstract
Paraoxonase 1 (PON1), a component of high-density lipoprotein (HDL), is a calcium-dependent multifunctional enzyme that connects metabolisms of lipoproteins and homocysteine (Hcy). Both PON1 and Hcy have been implicated in human diseases, including atherosclerosis and neurodegeneration. The involvement of Hcy in disease could be mediated through its interactions with PON1. Due to its ability to reduce oxidative stress, PON1 contributes to atheroprotective functions of HDL in mice and humans. Although PON1 has the ability to hydrolyze a variety of substrates, only one of them—Hcy-thiolactone—is known to occur naturally. In humans and mice, Hcy-thiolactonase activity of PON1 protects against N-homocysteinylation, which is detrimental to protein structure and function. PON1 also protects against neurotoxicity associated with hyperhomocysteinemia in mouse models. The links between PON1 and Hcy in relation to pathological states such as coronary artery disease, stroke, diabetic mellitus, kidney failure and Alzheimer’s disease that emerge from recent studies are the topics of this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
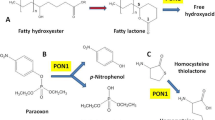
Paraoxonase 1 (PON1) exhibits a wide range of physiologically important activities, including homocysteine (Hcy)-thiolactone detoxification, drug metabolism, and detoxification of nerve agents. PON1 hydrolyzes the toxic oxon metabolites of organophosphorous insecticides, including parathion, diazinon, and chlorpyrifos, nerve agents, such as sarin and soman, aromatic esters, a variety of aromatic and aliphatic lactones (Draganov et al. 2005), as well as Hcy-thiolactone (Jakubowski 2000a). It has been extensively investigated in the context of human pathology, including cardiovascular disease, kidney disease, diabetes, and Alzheimer’s disease. PON1 is believed to have antiatherogenic properties; however, the mechanisms by which it protects against hardening of arteries are not well understood. A growing body of evidence links PON1 with Hcy, a risk factor for coronary artery disease (CAD) (Anderson et al. 2000). Hydrolysis of Hcy-thiolactone by PON1 prevents the accumulation of N-Hcy-protein in humans (Perla-Kaján and Jakubowski 2010; Jakubowski 2011, 2012) and protects against neurotoxicity associated with hypoerhomocysteinemia in mouse models (Borowczyk et al. 2012a). In this study, we review the recent advances in the PON1 research field with special emphasis on the links between PON1 and Hcy metabolism.
Structure and catalytic mechanism
Paraoxonase 1 is expressed in the liver (Kelso et al. 1994) and circulates in the blood in association with apolipoprotein (Apo) A1 in HDL particles (Blatter et al. 1993). Recent studies, however, indicate that PON1 is more widely distributed and present in other organs, including the brain (Marsillach et al. 2008). It belongs to the paraoxonase family consisting of three proteins: PON1, PON2 and PON3 that are all coded by genes located on the long arm of chromosome 7 (Humbert et al. 1993).
Human PON1 is a 45-kDa enzyme whose polypeptide chain consists of 354 amino acids (Hassett et al. 1991) and has two bound calcium ions, one playing a structural and the other a catalytic role (Harel et al. 2004). Early studies, which found that phenyl acetate is a mixed type inhibitor of the paraoxonase (POase) activity (Gan et al. 1991) while phenyl acetate and paraoxon are non-competitive inhibitors of the Hcy-thiolactonase (HTase) activity of PON1 (Jakubowski 2000a), suggested that different substrates bind at different active sites of the human PON1 enzyme. Recent structural studies (Ben-David et al. 2012) provide a mechanistic explanation for these observations.
Due to instability and tendency to form aggregates in the absence of detergents, native PON1 has been difficult to crystallize for structural studies. These limitations however were overcome by generating well-soluble rabbit PON1 variants in bacteria by directed evolution. According to the crystal structure determined at pH 4.5, the engineered PON1 variant is a six-bladed β-propeller, where each blade consists of four β-sheets. The top of the propeller is covered by three α helices, involved in the anchoring to the HDL particle (Harel et al. 2004). Recently, two other crystal structures of PON1 at pH 6.5 have been described, apo-protein and a complex with a lactone analog 2-hydroxyquinoline (2HQ). 2HQ inhibits all three activities of PON1 (lactonase, esterase, and phosphotriesterase) by binding to the catalytic calcium ion and displacing the phosphate ion that is present in both the apo structures. Comparison of different PON1 structures, namely apo at pH 4.5, apo at pH 6.5, and complexed with 2HQ at pH 6.5, disclose the existence of various conformations that result in different active site shapes and sizes, which account for the binding of different substrates to PON1’s active site. The authors identified three conformations of PON1: (1) closed, characterized by structured active-site loop (Lys70–Lys81) and Tyr71 pointing into the active site and represented by PON1 complexed with 2HQ; (2) open, observed at pH 4.5, where the active-site loop is flexible but Tyr71 is fixed and points outside the active site; (3) open, at pH 6.5 with the entire active-site loop being flexible (Ben-David et al. 2012).
Site-directed mutagenesis was used to identify PON1 active site residues and elucidate its catalytic mechanism. The postulated catalytic site includes the upper calcium atom, the phosphate ion, and the His115–His134 dyad. Activity assays indicate that His115 and His134 are responsible for the arylesterase (AREase) activity, but not for the POase activity of PON1. It was proposed that the two catalytic activities may be catalyzed by different active site residues (Harel et al. 2004). Indeed, certain Ala mutations of residues 70–81 showed significantly different effects of different activities. The most marked variation was observed for Ile74Ala which resulted in a large decrease of AREase and POase activities, while having no effect on the lactonase activity (Ben-David et al. 2012).
Computational docking was employed to reveal the binding modes of PON1 and putative reaction intermediates for different substrates: aliphatic lactones, aryl esters, and paraoxon. The differences of catalytic details proposed for PON1’s activities are summarized in Table 1 (Ben-David et al. 2012).
The data indicate that the lactonase activity takes place in closed conformation. The Tyr71 contacts the lactone ring, and the Ile74 contacts the alkyl side chain, but the structure of PON1 does not indicate a specific crevice for the alkyl side chain (Ben-David et al. 2012). Probably, this is why PON1 does not show a strong preference for the size of the alkyl side chain (Khersonsky and Tawfik 2005). The closed form is also likely to mediate the AREase activity. Phenyl acetate docks similarly to 2HQ, and Ile74 is involved in the catalysis. Moreover, it binds in a similar fashion to lactones and utilizes the same catalytic mechanism. Paraoxon on the other hand docks only to the open conformation.
The studies of (Ben-David et al. 2012) showing that different active-site residues or conformations are responsible for the hydrolysis of different substrates provide a possible explanation for the non-competitive inhibition by paraoxon or phenyl acetate of the HTase activity of PON1 (Jakubowski 2000a). Other inhibitors such as penicillamine and isoleucine (with d-stereoisomers being more potent than l-stereoisomers) are also non-competitive with respect to Hcy-thiolactone (Jakubowski 2000a), suggesting the presence of a separate specific amino acid-binding effector site(s) on the PON1 protein.
Polymorphism
There are several polymorphisms in the coding and non-coding regions of the PON1 gene, of which Q192R and L55M (Hassett et al. 1991; Adkins et al. 1993) are the most often studied.
Functional effects of the polymorphism at position 192 on PON1 activities are substrate dependent. For example, subjects with 192RR genotype have the highest mean POase activity, while diazoxonase, sarinase, and somanase have the lowest activities compared with other genotypes (192QR and 192QQ). All three genotypes give approximately the same activity toward chlorpyrifos oxon and phenyl acetate (Davies et al. 1996). High HTase activity is associated with R192 and L55 alleles, whereas low HTase activity is associated with Q192 and M55 alleles. HTase activity is strongly correlated with POase activity, which suggests that the POase is a good surrogate for the biological HTase activity (Jakubowski et al. 2001; Lacinski et al. 2004a, b).
Human allozymes Q192 and R192 PON1 have been recently purified and their activities tested with phenyl acetate, paraoxon, and Hcy-thiolactone. When Hcy-thiolacone was used as a substrate, the R192 PON1 isoform had 2.5- and 2.6-fold higher V max and k cat/K m, respectively, than the Q192 PON1 isoform. K m values of Q192 and R192 PON1 variants for Hcy-thiolactone were 23.5 and 22.6 mM, respectively (Bayrak et al. 2011). These values are in agreement with previously reported K m value for Hcy-thiolactone of 23 mM (Jakubowski 2000a). Rabbit liver PON1 have also been purified and characterized recently. The molecular weight of this protein is close to 40 kDa. The K m of the rabbit enzyme for phenyl acetate and Hcy-thiolactone are 0.55 ± 0.024 and 17.31 ± 1.2 mM, respectively (Bayrak et al. 2010).
The influence of polymorphism at position 192 on PON1 activity is consistent with the fact that the residue at this position is part of the active site wall (in the crystallized PON1 variant there is Lys at position 192) (Harel et al. 2004).
The polymorphism at position 55 does not affect the catalytic efficiency of the enzyme, but it has been shown that the PON1 M55 allele is correlated with decreased mRNA and protein levels (Garin et al. 1997; Leviev et al. 1997; Mackness et al. 1998; Brophy et al. 2000). Isoform M55 is more susceptible for proteolysis than isoform L55 (Leviev et al. 2001). This is consistent with the role of Leu55 in packing the propeller’s central tunnel and of its neighboring residues Glu53 and Asp54, which interact with calcium ions (Harel et al. 2004).
PON1 function from animal studies
Paraoxonase 1 has been studied by toxicologists since the 1960s, but it has been molecular biologists working on animal models, who provided important insights into the physiological roles of PON1. Pon1 −/− mice are more sensitive to atherosclerosis induced by high-fat diet and to organophosphate toxicity than the wild-type (wt) animals. Pon1-deficient mice develop significantly larger atherosclerotic lesions in their aortas compared to their wt counterparts (Shih et al. 1998, 2000). HDL from Pon1 −/− mice does not protect low-density lipoprotein (LDL) from oxidation (Shih et al. 1998). Pon1-deficient mice are also more susceptible to neurotoxicity induced by intraperitoneal injections of Hcy-thiolactone than wt animals (Jakubowski 2011, 2012; Borowczyk et al. 2012a).
Additional data on PON1 anti-atherosclerotic function came from studies with transgenic mice overexpressing human PON1. Human PON1 expressed in mice protects against a high-fat diet-induced atherosclerosis (Tward et al. 2002). An increased protection against oxidative stress induced by copper ions, as indicated by lecithin:cholesterol acyltransferase (LCAT) activity, has also been observed. Excess PON1 inhibits lipid hydroperoxide formation on HDL. Thus, overexpression of PON1 protects HDL integrity and function (Oda et al. 2002). Furthermore, human PON gene cluster transgenic overexpression in mice represses atherogenesis and promotes atherosclerotic plaque stability (She et al. 2009). PON3 is also an HDL-associated enzyme with biological activity similar to PON1 (Reddy et al. 2001). PON3 transgenic mice exhibit decreased atherosclerosis compared to their wt littermates (Shih et al. 2007).
In PON1-transfected macrophages and in Pon1-deficient mice, PON1 directly reduces macrophage and aortic oxidative status: a phenomenon associated with decreased superoxide anion production and increased glutathione content (Rozenberg et al. 2005). A study of the effects of PON1 on macrophage oxidative stress and on streptozotocin-induced diabetes development in mice has shown that PON1 overexpression is associated with decreased diabetes-induced macrophage oxidative stress, decreased diabetes development, and decreased mortality, in comparison to wt and Pon1-deficient mice (Rozenberg et al. 2008).
PON1 and protection of LDL against oxidative damage
Even though experiments in mouse models show that manipulation of PON1 expression affects atherosclerosis, the mechanisms involved are unclear. It is often incorrectly stated in the literature that hydrolytic enzymatic activities of PON1 toward oxidized lipids can account for its anti-atherosclerotic function, e.g., (Bhattacharyya et al. 2008). However, the preponderance of available evidence indicates that PON1 does not have an intrinsic anti-oxidative function (Vos 2008; Perla-Kaján and Jakubowski 2010) and that the protective effect of PON1 is indirect. Although Mackness et al. have shown that PON1 as well as HDL inhibits the Cu2+-induced generation of lipid peroxides in LDL (Mackness et al. 1993), their findings are controversial, as other investigators have shown that PON1 does not have intrinsic redox activity (see below). There is also controversy regarding whether the protective activity of PON1 depends on the polymorphism at position 192 (Aviram et al. 1998) or not (Cao et al. 1999). The degree of protection tends to relate to POase activity (Mackness et al. 1993), but is independent of POase activity (Cao et al. 1999). The protection occurs in a concentration-dependent manner (Mackness et al. 1993; Aviram et al. 1998) and is not sensitive to EDTA (Aviram et al. 1998; Cao et al. 1999), meaning that the putative protective activity does not require calcium ions, while other PON1 activities are calcium dependent (POase, AREase, HTase, diazoxonase). The protection of LDL is not eliminated by heating PON1 protein (56 °C, 10 min) (Cao et al. 1999); however, more intensive heat treatment (60 °C, 15 min) inhibits LDL protection (Aviram et al. 1998). Blocking the PON1’s free sulfhydryl group at position 283 with p-hydroxymercuribenzoate inhibits both AREase activity and protection of LDL from oxidation (Aviram et al. 1998). However, HTase activity is not affected by iodoacetate or iodoacetamide, which indicates that thiol groups are not required for activity (Jakubowski 2000a). Mutation of Cys 283 to Ala or Ser abolishes antioxidative function of PON1 (against LDL oxidation), even though the enzyme retains POase and AREase activities. It has been suggested that PON1’s AREase/POase activities and the protection against LDL oxidation do not involve the active site on the enzyme in exactly the same way (Aviram et al. 1998).
Some investigators reported that PON1 directly protects LDL against oxidation, but subsequent studies have shown that PON1 does not have an intrinsic antioxidant function. For example, Marathe et al. showed that trace amounts of platelet-activating factor (PAF) acetylhydrolase, a serine esterase associated with HDL particles, contaminate PON1 preparations and that PON1 lacking PAF acetylhydrolase does not have phospholipase activity toward PAF or pro-atherogenic oxidized phospholipids (Marathe et al. 2003).
Teiber et al. have studied the antioxidant properties of serum PON1 using in vitro assays with copper or the free radical generator 2,2′-azobis-2-amidinopropane hydrochloride. The antioxidant activity of different purified PON1 preparations did not correlate with their AREase, lactonase, or phospholipase A2 activities and could largely be accounted for the “antioxidant” activity of the detergent present in PON1 preparations (Teiber et al. 2004).
Recombinant PONs do not protect human LDL against Cu2+-induced oxidation in vitro, and no antioxidant activity copurifies with any of the PON1 preparations. The authors stated that antioxidant activity of human serum PON1 was attributable to a low molecular mass contaminant and to the detergent in the preparation (Draganov et al. 2005).
Hcy-thiolactonase activity of PON1 protects against protein N-homocysteinylation
More light on the mechanism of cardioprotective functions of PON1 was shed by the discovery of its natural substrate, Hcy-thiolactone (Jakubowski 2000a). Hcy-thiolactone is a product of Hcy editing reaction catalyzed by methionyl-tRNA synthetase (Jakubowski 2011, 2012). The presence of an HTase activity in human sera was first reported by Dudman et al. (Dudman et al. 1991), but it was not yet known which enzyme was responsible for the HTase activity. Serum HTase has been purified to homogeneity about a decade later and shown to be identical with PON1 (Jakubowski 2000a). Substrate specificity and steady-state enzyme kinetic studies of human serum HTase/PON1 have shown that phenyl acetate and paraoxon are non-competitive inhibitors of HTase, and that Hcy-thiolactone, phenyl acetate, and paraoxon are hydrolyzed at different sites of the HTase/PON1 enzyme (Jakubowski 2000a). HTase activity of PON1 strongly correlates with POase activity in various populations (Jakubowski et al. 2001; Lacinski et al. 2004; Perla-Kaján and Jakubowski 2010).
Hcy-thiolactone is a reactive metabolite that modifies protein lysine residues in a process known as N-homocysteinylation (Jakubowski 1997, 1999). Plasma Hcy-thiolactone and N-linked protein Hcy (N-Hcy-protein) are present in the human body (Jakubowski 2000b; Jakubowski et al. 2000; Chwatko and Jakubowski 2005a, b) and are greatly elevated in genetic or nutritional hyperhomocysteinemia (Glowacki and Jakubowski 2004; Chwatko et al. 2007; Jakubowski et al. 2008; Perla-Kajan et al. 2008). Hcy-thiolactone and N-Hcy-protein are also elevated in hyperhomocysteinemic mouse models (Chwatko et al. 2007; Jakubowski et al. 2009).
N-Homocysteinylation affects protein structure and function, e.g., enzymes lose their activity (Liu et al. 1997; Jakubowski 1999; Ferretti et al. 2003), N-Hcy-fibrinogen acquires prothrombotic properties (Sauls et al. 2006; Undas et al. 2006), and N-Hcy-proteins become autoimmunogenic (Perla et al. 2004; Undas et al. 2004). N-Homocysteinylation of HDL changes the conformation and properties of apolipoprotein. The modification causes significant decrease of POase and lactonase activities of the PON1 protein, which could affect the ability of HDL to protect against oxidative damage and protein N-homocysteinylation and contribute to atherosclerosis in patients with hyperhomocysteinemia (Ferretti et al. 2010). N-Hcy-ApoA1 is present in normal human sera and exhibits great inter-individual variability (Ishimine et al. 2010).
Protein N-homocysteinylation could account for detrimental effects of hyperhomocysteinemia. A hypothesis of Hcy-thiolactone-mediated vascular disease (Jakubowski 1997) explains the toxicity of Hcy by its conversion to Hcy-thiolactone, which then modifies proteins to generate N-Hcy-protein, leading in turn to cell death, inflammation, autoimmune response, and in consequence to atherosclerosis, thrombosis, and neurotoxicity (Jakubowski 2008, 2010, 2011, 2012) (Fig. 1).
The Hcy-thiolactone hypothesis. In humans and animals, Hcy is formed from dietary protein Met as a result of cellular methylation reactions. In this pathway, Met is first activated by ATP to yield S-adenosylmethionine (AdoMet). As a result of the transfer of its methyl group to an acceptor, AdoMet is converted to S-adenosylhomocysteine (AdoHcy). Enzymatic hydrolysis of AdoHcy is the only known source of Hcy in the human body. Levels of Hcy are regulated by remethylation to Met, catalyzed by Met synthase (MS), and transsulfuration to cysteine, the first step of which is catalyzed by cystathionine β-synthase (CBS). The remethylation requires vitamin B12 and 5,10-methyl-tetrahydrofolate (CH3-THF), generated by 5,10-methylene-THF reductase (MTHFR). The transsulfuration requires vitamin B6. Hcy is also metabolized to a thioester, Hcy-thiolactone, by methionyl-tRNA synthetase. Hcy-thiolactone modifies proteins generating N-Hcy-protein. Hydrolysis of Hcy-thiolactone by PON1 protects against its neurotoxicity and protein N-homocysteinylation [adapted from (Chwatko et al. 2007)]
The discovery of HTase activity of PON1 led to a hypothesis that PON1 could be atheroprotective also by its ability to detoxify Hcy-thiolactone and minimize protein N-homocysteinylation (Jakubowski 2000a) (Fig. 1). Support for that hypothesis came from discoveries that PON1 protects against the accumulation of N-Hcy-protein in vitro (Jakubowski et al. 2000, 2001) and in vivo (Perla-Kaján and Jakubowski 2010). Plasma N-Hcy-protein is negatively correlated with serum HTase activity (r = −0.43, P = 0.01) in cystathionine β-synthase-deficient patients. Enzymatic activities of the PON1 protein measured with artificial substrates correlate less strongly (r = −0.36, P = 0.025 for POase activity) or do not correlate at all (phenyl acetate hydrolase and TBLase activities) with plasma N-Hcy protein. Furthermore, the inverse in vivo relationship between N-Hcy-protein and HTase activity was recapitulated in separate in vitro N-homocysteinylation experiments. These findings provide evidence that the HTase activity of PON1 is a major determinant of plasma N-Hcy-protein levels in vivo (Perla-Kaján and Jakubowski 2010), which is also in agreement with earlier in vitro findings that high activity forms of PON1 afford better protection against protein N-homocysteinylation than low activity forms (Jakubowski et al. 2001).
Lowering serum HTase activity of PON1 by leptin administration in rats increases the level of N-linked Hcy in plasma proteins, but has no effect on plasma total Hcy. It has been suggested that the decreased capacity to metabolize Hcy-thiolactone and concomitant increase in protein N-homocysteinylation contribute to the pro-atherogenic effect of chronic hyperleptinemia, which is independent of oxidative stress (Beltowski et al. 2010).
Recent studies on Pon1 −/− mice have shown that PON1 plays a protective role against neurotoxicity of Hcy-thiolactone (Borowczyk 2012a). Pon1 −/− mice, compared to wt animals, have elevated levels of Hcy-thiolactone in the brain, while in other organs the levels of Hcy-thiolacone are not influenced by Pon1 gene deletion. When hyperhomocysteinemia was induced in Pon1 −/− and Pon1 +/+ mice, the levels of plasma tHcy increased 5.6- and 10.4-fold, respectively. Pon1 −/− mice excreted 2.4-fold more Hcy-thiolactone in urine than wt animals, while urinary tHcy levels were similar. A surprising finding of this study was that after Hcy-thiolactone was injected intraperitoneally (i.p.) into the mice, the turnover of plasma Hcy-thiolactone was similar in Pon1 −/− and wt littermates. The half-life of plasma Hcy-thiolactone in vivo was about 5 min, while in vitro in serum from Pon1 +/+ and Pon1 −/− mice it was 73 and >1,000 min, respectively. These values suggest that PON1 contributes at most 10 % to Hcy-thiolactone clearance from the mouse blood in vivo and indicate that there are other more efficient mechanisms of Hcy-thiolactone clearance from the circulation. One of such mechanisms involves kidneys as Pon1 −/− mice excrete more Hcy-thiolactone than wt counterparts. After i.p. injection of Hcy-thiolactone, plasma tHcy increased to higher levels in Pon1 +/+ mice than in knockout animals.
Pon1 −/− mice are more sensitive than wt littermates to i.p. injection of Hy-thiolactone, as manifested by higher incidence of seizures, shorter seizure latency, and higher levels of Hcy-thiolactone and N-Hcy-protein in the brain (Borowczyk 2012a). These findings suggest that PON1 protects against neurotoxicity caused by Hcy-thiolactone.
Association of PON1 genotypes and activity
As mentioned in previous sections, the two most commonly studied PON1 polymorphisms, M55L and Q192R, influence PON1 function. The HTase activity of PON1 is modified by polymorphisms at position 55 and 192 in American and European populations: products of PON1 L55 and R192 alleles have higher HTase activity than PON1 M55 and Q192 alleles (Jakubowski et al. 2001; Lacinski et al. 2004). In a study of a Tunisian population, PON1 192RR, and PON1 55MM genotypes were associated with higher HTase activity (Koubaa et al. 2009).
Other PON1 activities are also modified by gene polymorphism. For example, a large, prospective study of a US population of patients carried out at the Cleveland Clinic (n = 1,399) demonstrated a significant association between PON1 genotype and serum PON1 POase and AREase activities. Genotypes PON1 192QQ and PON1 192RR had lowest and highest activity, respectively. Moreover, participants with the PON1 192QQ genotype showed higher risk of mortality and of major adverse cardiac events than subjects with the PON1 192RR or 192QR genotype. Elevated systemic levels of fatty acids oxidation products that are increased in atherosclerotic plaque and plasma of participants with cardiovascular disease were associated with low PON1 activity and the PON1 192QQ genotype (Bhattacharyya et al. 2008).
The association of PON1 polymorphisms and activity was also investigated in Turkish patients with CAD and healthy controls. Genotypes PON1 55 and PON1 192 were associated with PON1 activity for all subjects. Genotypes RR, QR, and QQ have the highest, intermediate, and lowest activity, respectively. When subjects were divided into the patient and control groups, PON1 55 and PON1 192 genotypes were significantly associated with PON1 activity in patients, but not in controls. PON1 activity in patients with PON1 55LL genotype was significantly elevated compared with individuals with MM genotype. Similarly, in patients carrying RR and QR alleles, PON1 activity levels were significantly increased compared to patients with QQ genotype. In contrast, PON1 55/192 was not associated with the analyzed parameters in controls (Aydin et al. 2009).
Also in another Turkish population of CAD and healthy subjects, POase and HTase activity of PON1 was affected by Q192R polymorphism. Genotype QQ had the lowest activity while QR and RR had intermediate and highest activity, respectively (Bayrak et al. 2011).
The association of PON1 polymorphisms with risk factors for cardiovascular disease has been studied. Fan et al. examined gene polymorphisms in genes associated with Hcy metabolism (among which was PON1) with cardiovascular risk markers such as serum Hcy, CRP, and plasma fibrinogen in a study of adult women in USA (n = 3,409). They did not find any association of polymorphism in PON1 with Hcy levels (Fan et al. 2010). On the other hand, the results of another study that examined polymorphisms Q192R and L55M in the population on the Island of Crete showed significant association of the polymorphisms with blood pressure, fasting blood glucose, triglycerides, apolipoprotein B, serum iron, and Hcy (Zafiropoulos et al. 2010).
PON1 genotype and disease
Observation of the atheroprotective function of PON1 that emerged from animal studies has directed the interest of epidemiologists to the role of PON1 in human disease. As the PON1 allozymes differ in their enzymatic activities, one of the questions to answer is whether there are differences in PON1 genotype frequencies between patients and controls. After almost two decades of studies, the answer to this question is ambiguous. PON1 genotype frequencies differ in human populations; high activity alleles are more prevalent in blacks than in whites, while low activity alleles are more frequent in whites than in blacks (Jakubowski et al. 2001). Some studied populations show no differences in allele frequency between patients and controls, while others indicate an association of a particular genotype with a disease state. For example, the frequency of PON1 192RR genotype did not differ between CAD cases and healthy subjects in a UK (Domagala et al. 2006), Turkish (Bayrak et al. 2011), and Southeastern USA population (Coombes et al. 2011). However, in North-West Indian Punjabis (Gupta et al. 2011) and another Turkish population (Aydin et al. 2009), R allele was significantly elevated in CAD patients compared to controls. QR and RR genotypes showed significant association with type 2 diabetes mellitus in a North-West Indian population (Gupta et al. 2011).
A recent study claims that PON1 participates in the bioactivation of the antithrombotic pro-drug, clopidogrel, to an active thiol, and that PON1 Q192R polymorphism is a major determinant of clopidogrel efficacy in individuals with CAD who underwent stent implantation (Bouman et al. 2011). The bioactivation involves a thiolactone intermediate, which is formed in a cytochrome P450 (P450)-dependent monooxygenation of its thiophene ring leading to 2-oxo-clopidogrel. The opening of the thiolactone ring of 2-oxo-clopidogrel is catalyzed by PON1. Compared with PON1 192RR homozygous individuals, 192QQ subjects showed risk of stent thrombosis, lower PON1 plasma activity, lower plasma concentrations of active metabolite of clopidogrel, and lower platelet inhibition (Bouman et al. 2011). However, other investigators immediately expressed reservations regarding the validity of the Bouman et al. findings (Camps et al. 2011; Dansette et al. 2011) and subsequent studies do not support those findings. For example, Dansette et al. (2012), have shown that there are two metabolic pathways for the opening of the thiolactone ring of 2-oxo-clopidogrel. The major one that was previously described results from a P450-dependent redox bioactivation of 2-oxo-clopidogrel and leads to two thiol diastereomers bearing an exocyclic double bond. The second, minor one results from a hydrolysis of 2-oxo-clopidogrel, which is dependent on PON1, and leads to an isomer, in which the double bond has migrated from an exocyclic to an endocyclic position in the piperidine ring. Furthermore, the major active thiol isomer present in the plasma of clopidogrel-treated subjects is the one that is formed via a cytochrome P450-dependent pathway. In addition, overwhelming evidence from clinical studies involving thousands of patients now indicates that the response to clopidogrel therapy does not depend on PON1 Q192R polymorphism (Kreutz et al. 2012; Lewis and Shuldiner 2012).
The distribution of PON1 Q192R genotypes differed significantly between Han Chinese patients with stroke and controls. Genotype QQ was more frequent in controls, while QR and RR were more frequent in patients with concurrent stenosis (Man et al. 2010) and in another group of ischemic stroke patients (Can Demirdöğen et al. 2008). However, a large study on young survivors of ischemic stroke (n = 501) and matched controls (n = 1,211) did not identify significant association between PON1 polymorphism and premature ischemic stroke (Giusti et al. 2010). Genotype distribution for PON1 SNPs was not significantly different between patients suffering from abdominal aortic aneurysm (AAA) and controls (Giusti et al. 2008). The gene frequency for the PON1 192 polymorphisms (QQ, RR, and QR) was not significantly different between preeclamptic women and healthy pregnant women in a Turkish population. The same observation was drawn for the PON1 55 polymorphisms (LL, MM, and LM) (Isbilen et al. 2009).
The distribution of PON1 55 MM, ML, and LL genotypes between patients and controls also differs depending on the population. LL and LM genotypes and L allele of PON1 55 were more frequent in Turkish patients with CAD, while MM genotype and M allele were more frequent in healthy controls (Aydin et al. 2009). In L55M polymorphism, there was no difference in allele frequency between CAD subjects and controls (Gupta et al. 2011).
A study with a Korean population of ischemic stroke patients and controls did not identify significant differences in PON polymorphisms between the ischemic stroke and control subjects. This study confirmed that there was an association between allele L of PON1 55 and tHcy. Homozygotes for PON1 55L had a higher plasma concentration of tHcy (Shin et al. 2008).
On the other hand in a study with a Turkish population with acute hemispheric ischemic stroke and controls, PON1 genotypes were associated with the risk of stroke. PON1 55LL genotype was associated with a 1.78-fold increase in the risk of ischemic stroke (Demirdöğen et al. 2009).
PON1 activity and disease
Since the gene frequencies for PON1 192R and PON1 192Q vary significantly among different ethnic groups (Richter et al. 2010), it was suggested that all epidemiological studies into the role of PON1 and disease, in addition to the genetic polymorphisms, should include a measurement of the enzyme itself (Mackness et al. 2001). Enzyme activity (POase and HTase) was found to be a better predictor for cardiovascular disease than the PON1 genotype (Brophy et al. 2000; Jakubowski et al. 2001), and PON1 activity but not Q192R polymorphism was related to the extent of atherosclerosis (Bayrak et al. 2011).
In clinical studies, enzymatic activity of PON1 is measured with several substrates: paraoxon (POase), phenyl acetate (AREase), diazoxon (Furlong 2006), 5-thiobutyl butyrolactone (TBLase) (Kosaka et al. 2005), and Hcy-thiolactone (HTase) (Jakubowski 2000a). Out of these, only Hcy-thiolactone is a natural substrate of PON1. Activity measured with artificial substrates may not reflect physiological activity of the enzyme. It has been shown that HTase activity correlates strongly with POase (Jakubowski et al. 2001; Lacinski et al. 2004; Perla-Kaján and Jakubowski 2010; Sztanek et al. 2012) and less strongly with TBLase. However, HTase does not correlate significantly with AREase activity (Perla-Kaján and Jakubowski 2010). Measuring activity with phenyl acetate measures the AREase activity of PON1, which is considered a reliable measurement of enzyme level because rates of phenyl acetate hydrolysis do not differ between PON1 192Q and R alloforms as they do for paraoxon and Hcy-thiolactone hydrolysis. Recently, a new chemiluminescent method employing a derivative of methylacridinium triflate, measuring AREase activity of PON1, was evaluated (Mu et al. 2012).
The majority of studies on healthy subjects and patients have shown that in pathological states such as CAD, renal failure, Alzheimer’s disease, diabetes mellitus, age-related macular degeneration (AMD), and ischemic stroke, the activity of PON1 is diminished, and usually negatively correlated with Hcy level. Serum from hyperhomocysteinemic subjects has an impaired ability to induce cholesterol efflux from lipid-loaded macrophages compared with healthy controls. The activity of POase in serum is significantly reduced in subjects with elevated Hcy concentration (Holven et al. 2008), although acute mild hyperhomocysteinemia caused by L-Met loading does not influence POase activity (Türkeli et al. 2010). As mentioned in previous sections, PON1 circulates in the blood associated with ApoA1 in HDL (Blatter et al. 1993). Hcy reduces liver expression of ApoA1 and decreases its blood level in mouse models (Liao et al. 2006; Mikael et al. 2006). In humans, ApoA1 and HDL-cholesterol are also negatively correlated with plasma Hcy concentrations (Qujeq et al. 2001; Lacinski et al. 2004; Domagala et al. 2006; Liao et al. 2006; Mikael et al. 2006; Guéant-Rodriguez et al. 2011).
A meta-analysis of 43 studies showed significant decrease of POase and AREase activity in CAD patients compared with controls (Zhao et al. 2012). Since HTase activity of PON1 was suggested to contribute to an atheroprotective role of PON1 (Jakubowski 2000a, 2010; Jakubowski et al. 2001; Perla-Kaján and Jakubowski 2010), studies on the relationship between CAD and HTase activity of PON1 should provide useful information and have recently drawn more attention. In a study of a Japanese population, HTase activity was diminished in CAD patients compared with healthy controls and decreased inversely with the number of affected vessels and according to PON1 polymorphism. CAD patients had lower levels of HDL cholesterol and increased levels of tHcy and ox-LDL. HTase activity in patients was negatively associated with tHcy and Hs CRP levels, but positively associated with apoB and triglyceride levels (Koubaa et al. 2009).
Also a study on a Turkish population showed that POase and HTase activities were significantly decreased in CAD patients compared to controls. In the patients group, a negative correlation was observed between POase and HTase activities and the extent of atherosclerosis (expressed as Gensini score). It is worth mentioning that the negative correlation between the Gensini score and HTase activity was higher and more significant than that with POase activity, which could suggest that HTase activity was a better indicator of physiological function of PON1 than activity measured with an artificial substrate. Both PON1 activities positively correlated with HDL levels. HDL concentration was lower and the cholesterol/HDL ratio was higher in the patient group, but neither of them correlated with the severity and extent of CAD (Bayrak et al. 2011). PON1 activity was found to be diminished also in CAD patients from Marocco compared with controls (Amine et al. 2011). Similar results were obtained in a study of North-West Indian Punjabis (350 angiographically proven CAD patients and 300 healthy controls), a distinct ethnic group with high incidence of CAD. The serum POase and AREase activities were significantly lower in CAD patients as compared to the controls. POase activity was modulated by all studied polymorphisms except L55M, while AREase activity was not affected by them (Gupta et al. 2011).
Not in all studies though CAD patients are characterized by lower PON1 activity. In a study of a Manchester and Blackpool UK population, a group of CAD patients had higher mean HTase activity than a control subject group (Domagala et al. 2006). However, mean HTase activity did not differ between CAD subjects, myocardial infarction (MI) subjects, and controls in a study of population from Poznan, Poland. This discrepancy could be due to the small number of subjects analyzed (51 CAD patients, 73 MI patients, and 60 controls) (Lacinski et al. 2004).
Serum POase and HTase activities were also investigated in patients after acute coronary syndrome receiving treatment with aspirin and ticlopidine. The two activities of PON1 were significantly associated with each other and did not correlate with any parameters for platelet aggregation, hypertension, sleep apnea, and diabetes mellitus. In contrast, serum PON1 activities seemed to be involved in cardiac function; brain natriuretic peptide and ejection fraction were significantly correlated with serum HTase (r = −0.2767, P = 0.0214) and POase activity (r = 0.2558, P = 0.0339), respectively. POase activity also demonstrated a significant association with increased levels of ankle-brachial index (Ohmori et al. 2012).
Decreased PON1 activity (AREase and POase) (Itahara et al. 2000; Rajković et al. 2010) and concentration (Suehiro et al. 2002) are found in patients undergoing hemodialysis (HD). Also, subjects with chronic kidney disease (CKD) have lower PON1 activity compared to controls (Atamer et al. 2008). HD and renal transplanted (RT) patients have significantly lower TBLase activities compared to the control subjects (Sztanek et al. 2012). Significantly lower POase activities are found in HD patients compared to the RT group (Kimak et al. 2011; Sztanek et al. 2012). Patients with end-stage renal disease (ESRD) have decreased lactonase activity compared to control subjects, which increased after HD to levels similar to those of control subjects. One mechanism for lower lactonase activity in ESRD patients may be inhibition by uremic toxins and oxidative stress (Gugliucci et al. 2011). Another possible mechanism of diminished PON1 activity in renal disease patients involves Hcy and/or its metabolite. High Hcy levels are commonly present in plasma of CKD patients (Perna et al. 2004). As a consequence, hemodialyzed patients with ESRD have increased levels of S- and N-homocysteinylated proteins (Perna et al. 2006).
Paraoxonase 1 activity is negatively correlated with Hcy in HD, RT (Varga et al. 2009), and CKD patients (Atamer et al. 2008; Varga et al. 2009). PON1 activity is also negatively correlated with cystatin C (Varga et al. 2009), malondialdehyde (MDA), lipoprotein (a), apoA1 (Atamer et al. 2008), and asymmetric dimethylarginine (AMDA) levels (Sztanek et al. 2012). Decreased PON1 activity in CKD patients could be one of the reasons for the higher chance of developing atherosclerosis in those subjects. In fact, serum PON1 activity predicts the cardiovascular-related and all-cause mortality of HD patients (Ikeda et al. 2007) and is a new biomarker of HDL dysfunction.
Demirdöğen et al. found POase and AREase activities and PON1 activity ratio (POase/AREase) to be lower in ischemic stroke patients than in controls (Can Demirdöğen et al. 2008). The same authors did not find differences in POase, AREase, and diazoxonase activities between stroke and controls subjects from a Turkish population (Demirdöğen et al. 2009).
Paraoxonase activity is decreased (Wehr et al. 2009; Zengi et al. 2011) and negatively correlated with Hcy levels (Wehr et al. 2009) in Alzheimer’s disease (AD) patients. Furthermore, HTase activity is diminished in the brains of subjects with AD, suggesting that this dysfunctionality could contribute to the pathology of AD (Suszynska et al. 2010; Borowczyk 2012b).
Decrease of serum PON1 activities is associated with type 2 diabetes mellitus in several populations (Amine et al. 2011; Gupta et al. 2011). In these patients, POase activity is significantly correlated with the plasma HDL level, age, and myocardial flow reserve (Dunet et al. 2011). There is a tendency for a negative association between TBLase activity and the thickness of the carotid intima media. Coronary heart disease is the number one cause of mortality in type 2 diabetes mellitus. Reduced PON1 activity could lead to accelerated hardening of arteries, and TBLase activity may be an indicator of atherosclerosis severity in patients suffering from type 2 diabetes mellitus (Kosaka et al. 2005).
A growing body of evidence suggests that Hcy is implicated in many diseases of the ocular system, including retinal atherosclerosis (Ghorbanihaghjo et al. 2008), central retinal vein occlusion (CRVO) (Sodi et al. 2008), and exudative AMD (Javadzadeh et al. 2010). Intravitreal Hcy-thiolactone injection in mice causes degeneration of multiple retinal cells and homocysteinylation of retinal proteins (Chang et al. 2011). A negative correlation between serum PON1 and plasma Hcy was detected in CRVO patients that show a significantly lower serum PON1 AREase activity and higher plasma Hcy level compared with controls (Angayarkanni et al. 2008).
Subjects with AMD also have significantly lower serum PON1 activity and higher serum levels of Hcy than controls. In those patients, there is a negative correlation between PON1 AREase activity and Hcy level as well as between the activity and MDA, a lipid peroxidation product, used as a marker of oxidative stress (Ates et al. 2009). Disequilibrium between oxidative stress and antioxidant levels has been proposed to be one of the mechanisms of exudative AMD. Javadzadeh et al. investigated Hcy level, PON1 activity (using paraoxon and phenyl acetate as substrates), and ox-LDL levels in patients with exudative AMD and healthy controls. The distribution of PON1 phenotypes was significantly different between patients with exudative AMD and control subjects, i.e., the phenotype with the lowest activity (AA) was significantly more frequent in exudative AMD patients compared with healthy subjects, while phenotypes with intermediate (AB) and high (BB) activity were more frequent in controls. Except in BB phenotype, patients with AA and AB phenotypes had higher plasma Hcy levels in comparison to those of controls. The mean ox-LDL levels were significantly higher in the patients than controls. However, no significant differences in the comparison of Hcy and ox-LDL levels between the three PON1 phenotypes in both control and patients were found (Javadzadeh et al. 2012).
Barathi et al. reported that Hcy-thiolactone is positively correlated with HTase activity of PON1 in the eye’s vitreous in patients with proliferative diabetic retinopathy (n = 13) and macular hole (n = 8) as well as in ex vivo cultured bovine retinal capillary endothelial cells. Furthermore, vitreous Hcy-thiolactone levels and HTase activity were significantly elevated in patients with proliferative diabetic retinopathy, compared with macular hole patients, and thus can be used as markers of diabetic retinopathy (Barathi et al. 2010).
Conclusions
Paraoxonase 1, carried on HDL in the circulation, is an enzyme with several activities enabled by different conformations of the active site. It can contribute to atheroprotective functions of HDL due to its ability both to reduce oxidative stress and to minimize protein damage by N-homocysteinylation. Links between PON1 polymorphisms 192 and 55 and disease were investigated. Some studied populations show differences in allele frequency between patients and controls, while others do not.
The discovery of an atheroprotective function of PON1 has led to intensive studies of PON1 association with other risk factors for atherosclerosis and CAD, including homocysteine. Because of its essential role in protecting against toxic pesticides and cardiovascular disease, much attention has been devoted to pharmacological and dietary modulators of PON1 activity (Costa et al. 2005, 2011). Enzymatic activities of PON1 are diminished in pathological states, thus it would seem worthy to evaluate whether elevation of PON1 levels improves outcomes in patients. As shown in many studies, plasma Hcy is negatively correlated with HTase and POase activities of PON1. Thus, lowering Hcy levels should be beneficial by elevating PON1 activities. Indeed, short-term oral folic acid (5 mg/day) supplementation with or without methylcobalamin is an effective approach to decrease Hcy levels and increase HTase/PON activity in patients with type 2 diabetes. This could be a promising novel approach to protect against vascular complications in patients with diabetes (Weijun et al. 2008). Antioxidants also have been shown to up-regulate PON1. One of them is quercetin, a plant flavonoid present in red wine, with a number of bioactivities (Gong et al. 2009). Moderate alcohol up-regulates liver PON1 gene expression and serum activity, whereas heavy alcohol consumption has the opposite effects in animal models and humans (Lakshman 2009). To paraphrase a popular proverb: a glass of wine a day keeps your PON1 awake.
In spite of intensive studies and impressive advances especially concerning the structure, in the PON1 research, there are still questions awaiting examination. Results from computational docking and experiments collected for lipophilic lactones, phenyl acetate, and paraoxon provide an explanation of PON1’s promiscuity. As the data show that HTase activity of PON1 could contribute to its athero- and neuroprotective function, it would be worth performing computational docking for Hcy-thiolactone as well as structure–function studies of the HTase activity of PON1. Other questions to answer are: Why does the deletion of Pon1 gene not influence Hcy-thiolactone levels in mouse organs other than the brain? What are the mechanisms of Hcy-thiolactone detoxification in those organs?
References
Adkins S, Gan KN et al (1993) Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: glutamine or arginine at position 191, for the respective A or B allozymes. Am J Hum Genet 52(3):598–608
Amine K, Atouk A et al (2011) Paraoxonase-1 (PON1) activity in patients with coronary artery diseases and in diabetic patients. Ann Biol Clin (Paris) 69(6):671–677
Anderson J, Muhlestein J et al (2000) Plasma homocysteine predicts mortality independently of traditional risk factors and C-reactive protein in patients with angiographically defined coronary artery disease. Circulation 102(11):1227–1232
Angayarkanni N, Barathi S et al (2008) Serum PON1 arylesterase activity in relation to hyperhomocysteinaemia and oxidative stress in young adult central retinal venous occlusion patients. Eye (Lond) 22(7):969–974
Atamer A, Kocyigit Y et al (2008) Effect of oxidative stress on antioxidant enzyme activities, homocysteine and lipoproteins in chronic kidney disease. J Nephrol 21(6):924–930
Ates O, Azizi S et al (2009) Decreased serum paraoxonase 1 activity and increased serum homocysteine and malondialdehyde levels in age-related macular degeneration. Tohoku J Exp Med 217(1):17–22
Aviram M, Billecke S et al (1998) Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R. Arterioscler Thromb Vasc Biol 18(10):1617–1624
Aydin M, Gokkusu C et al (2009) Association of genetic variants in Methylenetetrahydrofolate Reductase and Paraoxonase-1 genes with homocysteine, folate and vitamin B12 in coronary artery disease. Mol Cell Biochem 325(1–2):199–208
Barathi S, Angayarkanni N et al (2010) Homocysteinethiolactone and paraoxonase: novel markers of diabetic retinopathy. Diabetes Care 33(9):2031–2037
Bayrak A, Bayrak T et al (2011) Serum PON-1 activity but not Q192R polymorphism is related to the extent of atherosclerosis. J Atheroscler Thromb 19(4):376–384
Bayrak T, Bayrak A et al (2010) Purification and kinetic properties of rabbit liver paraoxonase 1. J Chromatogr B Analyt Technol Biomed Life Sci 878(21):1791–1795
Bayrak A, Bayrak T et al (2011) Differential hydrolysis of homocysteine thiolactone by purified human serum (192)Q and (192)R PON1 isoenzymes. J Chromatogr B Analyt Technol Biomed Life Sci 879(1):49–55
Beltowski J, Wojcicka G et al (2010) Modulation of paraoxonase 1 and protein N-homocysteinylation by leptin and the synthetic liver X receptor agonist T0901317 in the rat. J Endocrinol 204(2):191–198
Ben-David M, Elias M et al (2012) Catalytic versatility and backups in enzyme active sites: the case of serum paraoxonase 1. J Mol Biol 418(3–4):181–196
Bhattacharyya T, Nicholls SJ et al (2008) Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 299(11):1265–1276
Blatter MC, James RW et al (1993) Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur J Biochem 211(3):871–879
Borowczyk K, Shih DM et al (2012a) Metabolism and neurotoxicity of homocysteine thiolactone in mice: evidence for a protective role of paraoxonase 1. J Alzheimers Dis 30(2):225–231
Borowczyk K, Tisończyk J et al (2012b) Metabolism and neurotoxicity of homocysteine thiolactone in mice: protective role of bleomycin hydrolase. Amino Acids. doi:10.1007/s00726-011-1207-5
Bouman HJ, Schömig E et al (2011) Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med 17(1):110–116
Brophy VH, Jarvik GP et al (2000) Analysis of paraoxonase (PON1) L55M status requires both genotype and phenotype. Pharmacogenetics 10(5):453–460
Camps J, Joven J et al (2011) Paraoxonase-1 and clopidogrel efficacy. Nat Med 17(9):1041–1042
Can Demirdöğen B, Türkanoğlu A et al (2008) Paraoxonase/arylesterase ratio, PON1 192Q/R polymorphism and PON1 status are associated with increased risk of ischemic stroke. Clin Biochem 41(1–2):1–9
Cao H, Girard-Globa A et al (1999) Paraoxonase protection of LDL against peroxidation is independent of its esterase activity towards paraoxon and is unaffected by the Q– > R genetic polymorphism. J Lipid Res 40(1):133–139
Chang HH, Lin DP et al (2011) Intravitreal homocysteine-thiolactone injection leads to the degeneration of multiple retinal cells, including photoreceptors. Mol Vis 17:1946–1956
Chwatko G, Jakubowski H (2005a) The determination of homocysteine-thiolactone in human plasma. Anal Biochem 337(2):271–277
Chwatko G, Jakubowski H (2005b) Urinary excretion of homocysteine-thiolactone in humans. Clin Chem 51(2):408–415
Chwatko G, Boers GHJ et al (2007) Mutations in methylenetetrahydrofolate reductase or cystathionine beta-syntase gene, or a high-methionine diet, increase homocysteine thiolactone levels in humans and mice. Faseb J 21(8):1707–1713
Coombes RH, Crow JA et al (2011) Relationship of human paraoxonase-1 serum activity and genotype with atherosclerosis in individuals from the Deep South. Pharmacogenet Genomics 21(12):867–875
Costa LG, Vitalone A et al (2005) Modulation of paraoxonase (PON1) activity. Biochem Pharmacol 69(4):541–550
Costa LG, Giordano G et al (2011) Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: the hunt goes on. Biochem Pharmacol 81(3):337–344
Dansette PM, Rosi J et al (2011) Paraoxonase-1 and clopidogrel efficacy. Nat Med 17(9):1040–1041
Dansette PM, Rosi J et al (2012) Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem Res Toxicol 25(2):348–356
Davies HG, Richter RJ et al (1996) The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet 14(3):334–336
Demirdöğen BC, Demirkaya S et al (2009) Analysis of paraoxonase 1 (PON1) genetic polymorphisms and activities as risk factors for ischemic stroke in Turkish population. Cell Biochem Funct 27(8):558–567
Domagala TB, Lacinski M et al (2006) The correlation of homocysteine-thiolactonase activity of the paraoxonase (PON1) protein with coronary heart disease status. Cell Mol Biol 52(5):4–10
Draganov DI, Teiber JF et al (2005) Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res 46(6):1239–1247
Dudman NP, Hicks C et al (1991) Homocysteine thiolactone disposal by human arterial endothelial cells and serum in vitro. Arterioscler Thromb 11(3):663–670
Dunet V, Ruiz J et al (2011) Effects of paraoxonase activity and gene polymorphism on coronary vasomotion. EJNMMI Res 1(1):27
Fan AZ, Yesupriya A et al (2010) Gene polymorphisms in association with emerging cardiovascular risk markers in adult women. BMC Med Genet 11:6
Ferretti G, Bacchetti T et al (2003) Effect of homocysteinylation on human high-density lipoproteins: a correlation with paraoxonase activity. Metabolism 52(2):146–151
Ferretti G, Bacchetti T et al (2010) Effect of homocysteinylation on high density lipoprotein physico-chemical properties. Chem Phys Lipids 163(2):228–235
Furlong CE, Holland N et al (2006) PON1 status of farm worker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenet Genomics 16(3):183–190
Gan KN, Smolen A et al (1991) Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos 19(1):100–106
Garin MC, James RW et al (1997) Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J Clin Invest 99(1):62–66
Ghorbanihaghjo A, Javadzadeh A et al (2008) Lipoprotein(a), homocysteine, and retinal arteriosclerosis. Mol Vis 14:1692–1697
Giusti B, Saracini C et al (2008) Genetic analysis of 56 polymorphisms in 17 genes involved in methionine metabolism in patients with abdominal aortic aneurysm. J Med Genet 45(11):721–730
Giusti B, Saracini C et al (2010) Early-onset ischaemic stroke: analysis of 58 polymorphisms in 17 genes involved in methionine metabolism. Thromb Haemost 104(2):231–242
Glowacki R, Jakubowski H (2004) Cross-talk between Cys(34) and lysine residues in human serum albumin revealed by N-homocysteinylation. J Biol Chem 279(12):10864–10871
Gong M, Garige M et al (2009) Quercetin up-regulates paraoxonase 1 gene expression with concomitant protection against LDL oxidation. Biochem Biophys Res Commun 379(4):1001–1004
Guéant-Rodriguez RM, Spada R et al (2011) Homocysteine is a determinant of ApoA-I and both are associated with ankle brachial index, in an ambulatory elderly population. Atherosclerosis 214(2):480–485
Gugliucci A, Kinugasa E et al (2011) Serum paraoxonase 1 (PON1) lactonase activity is lower in end-stage renal disease patients than in healthy control subjects and increases after hemodialysis. Clin Chem Lab Med 49(1):61–67
Gupta N, Binukumar BK et al (2011a) Serum paraoxonase-1 (PON1) activities (PONase/AREase) and polymorphisms in patients with type 2 diabetes mellitus in a North-West Indian population. Gene 487(1):88–95
Gupta N, Singh S et al (2011b) Paraoxonase 1 (PON1) polymorphisms, haplotypes and activity in predicting cad risk in North-West Indian Punjabis. PLoS ONE 6(5):e17805
Harel M, Aharoni A et al (2004) Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol 11(5):412–419
Hassett C, Richter RJ et al (1991) Characterization of cDNA clones encoding rabbit and human serum paraoxonase: the mature protein retains its signal sequence. Biochemistry 30(42):10141–10149
Holven KB, Aukrust P et al (2008) The antiatherogenic function of HDL is impaired in hyperhomocysteinemic subjects. J Nutr 138(11):2070–2075
Humbert R, Adler DA et al (1993) The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet 3(1):73–76
Ikeda Y, Suehiro T et al (2007) Human serum paraoxonase concentration predicts cardiovascular mortality in hemodialysis patients. Clin Nephrol 67(6):358–365
Isbilen E, Yilmaz H et al (2009) Association of paraoxonase 55 and 192 gene polymorphisms on serum homocysteine concentrations in preeclampsia. Folia Biol (Praha) 55(2):35–40
Ishimine N, Usami Y et al (2010) Identification of N-homocysteinylated apolipoprotein AI in normal human serum. Ann Clin Biochem 47(Pt 5):453–459
Itahara T, Suehiro T et al (2000) Serum paraoxonase and arylesterase activities in hemodialysis patients. J Atheroscler Thromb 7(3):152–158
Jakubowski H (1997) Metabolism of homocysteine thiolactone in human cell cultures—possible mechanism for pathological consequences of elevated homocysteine levels. J Biol Chem 272(3):1935–1942
Jakubowski H (1999) Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. Faseb J 13(15):2277–2283
Jakubowski H (2000a) Calcium-dependent human serum homocysteine thiolactone hydrolase—a protective mechanism against protein s-homocysteinylation. J Biol Chem 275(6):3957–3962
Jakubowski H (2000b) Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. J Nutr 130(2):377S–381S
Jakubowski, H. (2008). Paraoxonase 1 (PON1), a junction between the metabolism of homocysteine and lipids. Paraoxonases Their Role Dis Dev Xenobiotic Metabol 6:87–102
Jakubowski H (2010) The Role of Paraoxonase 1 in the Detoxification of Homocysteine Thiolactone. Paraoxonases Inflamm Infection Toxicol 660:113–127
Jakubowski H (2011) Quality control in tRNA charging—editing of homocysteine. Acta Biochim Pol 58(2):149–163
Jakubowski H (2012) Quality control in tRNA charging. Wiley Interdiscip Rev RNA 3(3):295–310
Jakubowski H, Zhang L et al (2000) Homocysteine thiolactone and protein homocysteinylation in human endothelial cells—implications for atherosclerosis. Circ Res 87(1):45–51
Jakubowski H, Ambrosius WT et al (2001) Genetic determinants of homocysteine thiolactonase activity in humans: implications for atherosclerosis. FEBS Lett 491(1–2):35–39
Jakubowski H, Boers GHJ et al (2008) Mutations in cystathionine beta-synthase or methylenetetrahydrofolate reductase gene increase N-homocysteinylated protein levels in humans. Faseb J 22(12):4071–4076
Jakubowski H, Perla-Kajan J et al (2009) Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. Faseb J 23(6):1721–1727
Javadzadeh A, Ghorbanihaghjo A et al (2010) Plasma oxidized LDL and thiol-containing molecules in patients with exudative age-related macular degeneration. Mol Vis 16:2578–2584
Javadzadeh A, Ghorbanihaghjo A et al (2012) Serum paraoxonase phenotype distribution in exudative age-related macular degeneration and its relationship to homocysteine and oxidized low-density lipoprotein. Retina 32(4):658–666
Kelso GJ, Stuart WD et al (1994) Apolipoprotein J is associated with paraoxonase in human plasma. Biochemistry 33(3):832–839
Khersonsky O, Tawfik DS (2005) Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry 44(16):6371–6382
Kimak E, Hałabiś M et al (2011) Association between moderately oxidized low-density lipoprotein and high-density lipoprotein particle subclass distribution in hemodialyzed and post-renal transplant patients. J Zhejiang Univ Sci B 12(5):365–371
Kosaka T, Yamaguchi M et al (2005) Investigation of the relationship between atherosclerosis and paraoxonase or homocysteine thiolactonase activity in patients with type 2 diabetes mellitus using a commercially available assay. Clin Chim Acta 359(1–2):156–162
Koubaa N, Nakbi A et al (2009) Association of homocysteine thiolactonase activity and PON1 polymorphisms with the severity of acute coronary syndrome. Clin Biochem 42(9):771–776
Kreutz RP, Nystrom P et al (2012) Influence of paraoxonase-1 Q192R and cytochrome P450 2C19 polymorphisms on clopidogrel response. Clin Pharmacol 4:13–20
Lacinski M, Skorupski W et al (2004) Determinants of homocysteine-thiolactonase activity of the paraoxonase-1 (PON1) protein in humans. Cell Mol Biol (Noisy-le-grand) 50(8):885–893
Lacinski M, Skorupski W et al (2004) Determinants of homocysteine-thiolactonase activity of the paraoxonase-1 (PON1) protein in humans. Cell Mol Biol 50(8):885–893
Lakshman R, Garige M et al (2009) Is alcohol beneficial or harmful for cardioprotection? Genes Nutr 5(2):111–120
Leviev I, Negro F et al (1997) Two alleles of the human paraoxonase gene produce different amounts of mRNA. An explanation for differences in serum concentrations of paraoxonase associated with the (Leu-Met54) polymorphism. Arterioscler Thromb Vasc Biol 17(11):2935–2939
Leviev I, Deakin S et al (2001) Decreased stability of the M54 isoform of paraoxonase as a contributory factor to variations in human serum paraoxonase concentrations. J Lipid Res 42(4):528–535
Lewis JP, Shuldiner AR (2012) Paraoxonase 1 Q192R variant and clopidogrel efficacy: fact or fiction? Circ Cardiovasc Genet 5(2):153–155
Liao D, Tan H et al (2006) Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I Protein synthesis and enhancing HDL cholesterol clearance. Circ Res 99(6):598–606
Liu G, Nellaiappan K et al (1997) Irreversible inhibition of lysyl oxidase by homocysteine thiolactone and its selenium and oxygen analogues. Implications for homocystinuria. J Biol Chem 272(51):32370–32377
Mackness MI, Arrol S et al (1993) Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis 104(1–2):129–135
Mackness B, Mackness MI et al (1998) Serum paraoxonase (PON1) 55 and 192 polymorphism and paraoxonase activity and concentration in non-insulin dependent diabetes mellitus. Atherosclerosis 139(2):341–349
Mackness B, Davies GK et al (2001) Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol 21(9):1451–1457
Man BL, Baum L et al (2010) Genetic polymorphisms of Chinese patients with ischemic stroke and concurrent stenoses of extracranial and intracranial vessels. J Clin Neurosci 17(10):1244–1247
Marathe GK, Zimmerman GA et al (2003) Platelet-activating factor acetylhydrolase, and not paraoxonase-1, is the oxidized phospholipid hydrolase of high density lipoprotein particles. J Biol Chem 278(6):3937–3947
Marsillach J, Mackness B et al (2008) Immunohistochemical analysis of paraoxonases-1, 2, and 3 expression in normal mouse tissues. Free Radic Biol Med 45(2):146–157
Mikael LG, Genest J et al (2006) Elevated homocysteine reduces apolipoprotein A-I expression in hyperhomocysteinemic mice and in males with coronary artery disease. Circ Res 98(4):564–571
Mu X, Yu N et al (2012) Evaluation of a new substrate for measurement of serum PON arylesterase activity. Talanta 88:711–716
Oda MN, Bielicki JK et al (2002) Paraoxonase 1 overexpression in mice and its effect on high-density lipoproteins. Biochem Biophys Res Commun 290(3):921–927
Ohmori T, Yano Y et al (2012) Lack of association between serum paraoxonase-1 activity and residual platelet aggregation during dual anti-platelet therapy. Thromb Res 129(4):e36–e40
Perla J, Undas A et al (2004) Purification of antibodies against N-homocysteinylated proteins by affinity chromatography on N omega-homocysteinyl-aminohexyl-Agarose. J Chromatograph B-Anal Technol Biomed Life Sci 807(2):257–261
Perla-Kaján J, Jakubowski H (2010) Paraoxonase 1 protects against protein N-homocysteinylation in humans. FASEB J 24(3):931–936
Perla-Kajan J, Stanger O et al (2008) Immunohistochernical detection of N-homocysteinylated proteins in humans and mice. Biomed Pharmacother 62(7):473–479
Perna AF, Ingrosso D et al (2004) Homocysteine metabolism in renal failure. Curr Opin Clin Nutr Metab Care 7(1):53–57
Perna AF, Satta E et al (2006) Increased plasma protein homocysteinylation in hemodialysis patients. Kidney Int 69(5):869–876
Qujeq D, Omran TS et al (2001) Correlation between total homocysteine, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol in the serum of patients with myocardial infarction. Clin Biochem 34(2):97–101
Rajković MG, Rumora L et al (2010) Effect of non-genetic factors on paraoxonase 1 activity in patients undergoing hemodialysis. Clin Biochem 43(18):1375–1380
Reddy ST, Wadleigh DJ et al (2001) Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol 21(4):542–547
Richter RJ, Jarvik GP et al (2010) Paraoxonase 1 status as a risk factor for disease or exposure. Adv Exp Med Biol 660:29–35
Rozenberg O, Shih DM et al (2005) Paraoxonase 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis 181(1):9–18
Rozenberg O, Shiner M et al (2008) Paraoxonase 1 (PON1) attenuates diabetes development in mice through its antioxidative properties. Free Radic Biol Med 44(11):1951–1959
Sauls DL, Lockhart E et al (2006) Modification of fibrinogen by homocysteine thiolactone increases resistance to fibrinolysis: a potential mechanism of the thrombotic tendency in hyperhomocysteinemia. Biochemistry 45(8):2480–2487
She ZG, Zheng W et al (2009) Human paraoxonase gene cluster transgenic overexpression represses atherogenesis and promotes atherosclerotic plaque stability in ApoE-null mice. Circ Res 104(10):1160–1168
Shih DM, Gu L et al (1998) Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 394(6690):284–287
Shih DM, Xia YR et al (2000) Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem 275(23):17527–17535
Shih DM, Xia YR et al (2007) Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ Res 100(8):1200–1207
Shin BS, Oh SY et al (2008) The paraoxonase gene polymorphism in stroke patients and lipid profile. Acta Neurol Scand 117(4):237–243
Sodi A, Giambene B et al (2008) Atherosclerotic and thrombophilic risk factors in patients with recurrent central retinal vein occlusion. Eur J Ophthalmol 18(2):233–238
Suehiro T, Ikeda Y et al (2002) Serum paraoxonase (PON1) concentration in patients undergoing hemodialysis. J Atheroscler Thromb 9(3):133–138
Suszynska J, Tisonczyk J et al (2010) Reduced homocysteine-thiolactonase activity in Alzheimer’s disease. J Alzheimers Dis 19(4):1177–1183
Sztanek F, Seres I et al (2012) Decreased paraoxonase 1 (PON1) lactonase activity in hemodialyzed and renal transplanted patients. A novel cardiovascular biomarker in end-stage renal disease. Nephrol Dial Transplant. doi:10.1093/ndt/gfr753
Teiber JF, Draganov DI et al (2004) Purified human serum PON1 does not protect LDL against oxidation in the in vitro assays initiated with copper or AAPH. J Lipid Res 45(12):2260–2268
Türkeli H, Caycı T et al (2010) Paraoxonase-1 activity determination via paraoxon substrate yields no significant difference in mild hyperhomocysteinemia. Int J Cardiol 145(1):42–43
Tward A, Xia YR et al (2002) Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 106(4):484–490
Undas A, Perla J et al (2004) Autoantibodies against N-homocysteinylated proteins in humans—Implications for atherosclerosis. Stroke 35(6):1299–1304
Undas A, Stepien E et al (2006) Folic acid administration and antibodies against homocysteinylated proteins in subjects with hyperhomocysteinemia. Thromb Haemost 96(3):342–347
Varga E, Seres I et al (2009) Serum cystatin C is a determinant of paraoxonase activity in hemodialyzed and renal transplanted patients. Dis Markers 26(3):141–148
Vos E (2008) Homocysteine levels, paraoxonase 1 (PON1) activity, and cardiovascular risk. JAMA 300(2):168–169 (author reply 169)
Wehr H, Bednarska-Makaruk M et al (2009) Paraoxonase activity and dementia. J Neurol Sci 283(1–2):107–108
Weijun G, Juming L et al (2008) Effects of plasma homocysteine levels on serum HTase/PON activity in patients with type 2 diabetes. Adv Ther 25(9):884–893
Zafiropoulos A, Linardakis M et al (2010) Paraoxonase 1 R/Q alleles are associated with differential accumulation of saturated versus 20:5n3 fatty acid in human adipose tissue. J Lipid Res 51(7):1991–2000
Zengi O, Karakas A et al (2011) Urinary 8-hydroxy-2′-deoxyguanosine level and plasma paraoxonase 1 activity with Alzheimer’s disease. Clin Chem Lab Med 50(3):529–534
Zhao Y, Ma Y et al (2012) Association between PON1 activity and coronary heart disease risk: a meta-analysis based on 43 studies. Mol Genet Metab 105(1):141–148
Acknowledgments
Supported in part by a grant from the National Science Center, Poland (MAY-2011/02/1/NZ1/00010).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Perła-Kaján, J., Jakubowski, H. Paraoxonase 1 and homocysteine metabolism. Amino Acids 43, 1405–1417 (2012). https://doi.org/10.1007/s00726-012-1321-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1321-z