Abstract
The novel synthetic derivative of carnosine, (S)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carbonyl-β-alanyl-l-histidine (S-Trolox™-Carnosine, STC) increases the resistance of rats to experimental acute hypobaric hypoxia (AHH) thus protecting brain from the oxidative damage. This effect is accompanied by better preservation of the acquired skills in Morris water maze possibly by increasing efficiency of the brain antioxidant system. In addition, STC caused an increase in life span of both male and female fruit fly Drosophila melanogaster whereas carnosine increased life span only in male fruit flies. The results indicate that development of the drug based on STC could be beneficial in neurology and gerontology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropeptide carnosine is known as the potent protector of brain neurons from the oxidative stress both in vitro and in vivo (Boldyrev 2006). This effect reflects the antioxidant activity of carnosine, which prevents the accumulation of free radicals representing one of the features of oxidative stress (Boldyrev et al. 1987, 1999; Kohen et al. 1988; Aruoma et al. 1989; Boldyrev 1993, 2003). Other mechanisms of carnosine-induced brain protection, however, should not be excluded. These mechanisms may involve in increase in cell viability associated with prevention of protein and nucleic acids oxidation (Boldyrev 2005; Kang 2010), neutralization of toxic aldehydes (Aldini et al. 2011), retaining native properties of DNA (Leinsoo et al. 2006) or modulation of activity of histamine receptors (Miller and O’Dowd 2000; Tanida et al. 2005). All these properties turn special attention to carnosine as useful nutrient and bioactive compound (Hipkiss 2009).
Administration of carnosine to experimental animals in several modes of brain injury (Stvolinsky et al. 1999; Gallant et al. 2000; Dobrota et al. 2010) reduced mortality and improved the behavioral responses of the animals. This feature of carnosine suggests its potential medical application. Although some clinical results demonstrating effects of carnosine during treatment of neurodegenerative diseases in human beings have already been published (Boldyrev et al. 2010), widespread medical use of carnosine is limited by a high rate of its degradation in serum and tissues by an enzyme carnosinase, which dictates the use of high doses and repeated administration of carnosine. One possible way of solving this problem is the development of carnosine derivatives resistant to the enzymatic hydrolysis while preserving its biological activity. We have recently reported a novel carnosine derivative, (S)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carbonyl-β-alanyl-l-histidine (S-Trolox™-Carnosine, STC) which was synthesized by condensing the β-amino group of l-carnosine and the carboxyl group of a lipophilic antioxidant (S)-Trolox™ (Fig. 1).
In our preliminary experiments in rats (Stvolinsky et al. 2010a) and fruit flies (Stvolinsky et al. 2010b) we have compared biological activity of STC and its isomer ®-Trolox™-Carnosine (RTC) with that of carnosine. We found that STC possesses a broader spectrum of the biological activity. With this in mind we continued to study STC (but not RTC) as a more potent candidate. Under in vitro conditions, STC has shown to possess the best ability to control the level of free radicals in neurons as compared with carnosine, Trolox™, and RTC (Stvolinsky et al. 2010a). STC demonstrated similar efficiency as carnosine at two times lower concentration and did not reveal negative adverse side effects such as cell death observed after Trolox™ administration (Stvolinsky et al. 2010a). Moreover, STC was found to be more resistant against serum carnosinase than carnosine suggesting improvement its pharmacokinetic properties and thus higher potential as a drug. Therefore we conducted a comparative study of the efficiency of STC, Trolox™ and l-carnosine as protectors from oxidative stress in the experiments in vivo.
Materials and methods
Acute hypobaric hypoxia
Thirty-five Wistar male rats (350–400 g weight) were randomly divided into five groups: intact animals (n = 7); animals subjected to acute hypobaric hypoxia (AHH) and received saline injections (“AHH + sal”, n = 8); AHH rats treated with carnosine (“AHH + carnosine”, n = 6), Trolox™ (“AHH + Trolox”, n = 6), or STC (“AHH + STC”, n = 8). All compounds and saline were i.p. administered 60 min before AHH in a volume of 1 ml/kg. The doses used were 100, 10, and 50 mg/kg for carnosine, Trolox™ and STC, respectively. These doses were chosen on the bases of previously acquired results where the compounds were compared (Stvolinsky et al. 2010a).
Acute hypobaric hypoxia was created in a hermetic chamber by reducing the pressure for 1 min to 0.165 atmospheres (11,000 m above the sea level). Loss of posture time was recorded under these conditions. Rats were kept in the chamber under these conditions until they stopped breathing. Time between loss of posture and breathing loss was recorded as time to stop breathing. Overall time under hypoxic conditions was recorded as lifetime under hypoxia (Boldyrev et al. 1997). After breathing loss, the pressure was restored, animals were removed from the chamber and restitution time, which is necessary to restore the posture (ability to stand on all the paws) was recorded.
The Morris water maze test was used for assessing the cognitive abilities of animals (Morris 1984). Training was carried out 24 h after AHH using the modification of protocol published by Feldman et al. (2010). During training session, each animal had five 60 s training trials in search the platform in the water pool. The memory test was carried out 24 h after training. During test session, animals were subjected to three 60 s trials of search the platform remained in the pool. The latency to platform (s) was calculated for each animal on each training or testing trial using video recording and custom-made tracking software.
The rats were decapitated 1 h after the end of Morris water maze testing session, brain samples were frozen in liquid nitrogen and stored at −80°C. In order to investigate the total antioxidant capacity of brain tissue the homogenates were prepared using 10% solution in 0.15 M KCl. The resulting homogenates were centrifuged (12,000g, 20 min) and the supernatant was used to assess the total antioxidant capacity of the brain. Test system with a stable radical 1,1-diphenyl-2-picryl hydrazyl (DPPH) was used (Schlesier et al. 2002). Test for reduction of DPPH radical is the fast way to quantify the efficiency of tissue antioxidant defense system without identifying the nature of antioxidant compounds (Koren et al. 2010). Estimation of the antioxidant state of brain using DPPH-test was made at the 3rd day after hypoxic effect—at the period of subsequent reperfusion. Obviously this step is characterized by a clear deficit of intracellular antioxidant defense. During the test, decrease in absorption of a sample containing water–alcohol solution of DPPH (50% ethanol/50% water, final concentration 10 μmol) and brain supernatant was measured at wavelength of 517 nm in a kinetic mode. The rate of DPPH reduction was measured using the molar extinction coefficient equal to 9,690/M/cm and the amount of reduced radicals at 30 min of incubation was calculated and expressed in μmol/g brain tissue.
Statistical analysis of the data was performed using STATISTICA 9 (StatSoft) software. Mann–Whitney test was used to assess the differences between experimental groups.
Results
The quantitative comparison of protective action of the compounds studied in the rats under experimental AHH is presented in Table 1. Carnosine, Trolox™ and STC 2–2.5 times prolonged loss of posture time and lifetime under hypoxia (p < 0.01 compared to “AHH + sal” group) as well as time to stop breathing (carnosine and Trolox™—with twofold efficiency, p < 0.05; STC—with threefold efficiency, p < 0.01). Trolox™ was the most effective in shortening of restitution time and STC—in delaying in time to stop breathing.
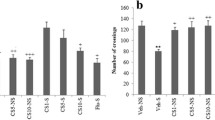
The Morris water maze test showed no difference between the groups at the first three training trials (in Fig. 2a only the first trial is shown) but at trial 4, animals treated with STC, Trolox™ or carnosine showed shorter latencies to the platform than intact and saline-treated AHH rats (statistically significant for Trolox and STC, p < 0.05). In trial 5, however, only “AHH + Trolox” rats showed statistically significant difference from the “AHH + sal” rats (p < 0.05), that for “AHH + carnosine” and “AHH + STC” rats was statistically insignificant from “AHH + sal” group.
Comparison of acquisition (trials 1, 4 and 5, a) and retention (trials 1–3, b) of Morris water maze task for intact animals and animals under AHH treated with saline or the compounds tested. “+”, statistically significant difference comparing with intact animals, p < 0.01; “*”, statistically significant difference comparing with “AHH + sal” group, p < 0.05 (Mann–Whitney U test)
In the course of testing (Fig. 2b, trials 1–3) saline-treated AHH rats showed significantly higher latency than intact group (p < 0.01). The animals treated with any compound tested demonstrated lower latencies compared with the control group of rats and rats exposed to hypoxia; in trial 3 the difference was statistically significant for each of the compounds tested (p < 0.05). Actually this parameter for all three groups of the rats was close to that typical of intact rats. Thus in the concentrations chosen carnosine, Trolox and STC demonstrated equal efficiency.
In order to study possible mechanism of action of these compounds on the brain under conditions used, we evaluated the antioxidant status of the brain extracts of the animals tested using DPPH test (Fig. 3).
Reduction of DPPH radical at 30 min incubation with brain samples of the intact animals and animals subjected to AHH pretreated with either saline or the compounds tested. “+”, statistically significant difference comparing with intact animals, p < 0.01; “*”, statistically significant difference comparing with “AHH + sal” group, p < 0.01; (Mann–Whitney U test)
As it is seen in Fig. 3, AHH results in significant decrease in antioxidant capacity of the brain as indicated by the decrease in reduction of DPPH while the protective effect of the compounds is accompanied with an increased efficiency of DPPH radical reduction by corresponding brain samples. All three compounds studied demonstrated statistically significant increase in antioxidant defense compared to “AHH + sal” rats. In addition, STC statistically significant increased the DPPH radical reduction compared to intact rats.
Discussion
Comparison of carnosine, Trolox™ and STC in AHH rat model showed that under concentrations used these compounds were able to protect rats against oxidative injury. STC showed the same efficiency as carnosine in terms of increase in lifetime under hypoxia, while it assured the longest time to stop breathing. Subsequently, rats treated with STC showed the longest restitution time (Table 1).
Acute hypobaric hypoxia increased latency to find the platform in the Morris water maze test both under training and in testing. We did not find any significant difference between experimental groups at the beginning of training (Fig. 2a, trial 1), which indicates that AHH has no effect on short-term memory. Pronounced differences between the groups were found, however at a transition of information from short-term into the long-term memory (comparing training and test experiments in Fig. 2): for saline treated rats exposed to AHH, significantly higher latency to find the platform was observed during test session. This observation suggests certain violations in the formation, storage and/or function of a long-term memory in this group of rats. The finding that the protectors eliminate elongation of the platform search caused by AHH and improve spatial memory indicates that the compounds tested modulate the long-term memory and contribute to its preservation.
As one can see from Fig. 2b (trial 3) there was no statistically significant difference between carnosine, Trolox™ and STC treated groups at the end of test session. Thus at the concentrations chosen these compounds demonstrated equal protecting activity on brain learning function. Taking into account the molecular weights of carnosine, Trolox and STC (226, 250 and 458, respectively) it may be concluded that STC is only 2.5 times less effective than Trolox™ and 4.5 times more effective than carnosine.
An important question is the mechanism of the protective action of the compounds tested in the AHH model. A variety of scientific publications discuss carnosine and Trolox™ as both direct and indirect antioxidants (Boldyrev 1993, 2006; Gupta and Sharma 2006; Sharma and Kaundal 2007; Kosolapov et al. 2009). Earlier we have demonstrated antioxidant properties of STC on brain neurons under normoxic conditions used primary culture of cerebellum granule cells and flow cytometry (Stvolinsky et al. 2010a). It was found that all the compounds tests decrease the steady-state levels of intracellular free radicals and decelerate neuronal death under normoxia conditions. Thus the data described here using DPPH-test obtained after in vivo hypoxia are in a good correlation with in vitro experiments. However we were unable to find direct correlation between antioxidant ability of these compounds (measured by DPPH test) and their effect on cognitive brain function. This discrepancy led us to a conclusion that physiological activity of these compounds was based not only on their antioxidant properties but also on some other still unknown mechanisms.
It was shown recently that carnosine itself or via formation of histidine and next histamine may modulate the activity of histamine H1 and H2 receptors (Miller and O’Dowd 2000; Tanida et al. 2005). Such indirect effect may provide an additional input in the protecting effect of carnosine and possibly its derivative. The fact that STC is more resistant against carnosinase might produce a favorable biological effect in the body, which makes STC worthwhile to test as a drug candidate against diseases associated with oxidative stress, although precise pharmacokinetic studies of STC will be necessary in the future. Carnosine is known to be actively transported into the body tissues by PepT1 and PepT2 transporters (Biegel et al. 2006). Ability of STC to use this transporting system needs to be studied as well.
Other possibilities also could not be excluded. For instance, if carnosine is incorporated into the metabolic pathway of the animals, its metabolites may influence the biological effects. In the experiments with fruit flies, it was found that carnosine was not detected in the flies’ bodies when they were kept on the standard foodstuff, but histidine and, unexpectedly, homocarnosine (5.67 ± 0.52 and 2.76 ± 0.31 ng/mg tissue, respectively) were observed in tissue homogenates of the flies. When the flies were fed on a carnosine-containing nutriment, carnosine has been detected in tissue homogenates, and at the same time histidine and homocarnosine concentrations were clearly increased (Yuneva et al. 2002). These observations suggest that carnosine is possibly incorporated into the de novo metabolic processes in fruit flies but it is not anticipated how carnosine contributes to the mechanism of the geroprotective effect—by itself or by increasing the contents of the metabolic products, histidine and/or homocarnosine? Evident efficiency of STC as geroprotector, which is expressed in ability to increase the life span of both male and female fruit flies cannot be explained only by gender driven differences in the metabolic processes in the fruit flies (Lints et al. 1983; Iliadi et al. 2009). It also should be addressed to specific properties of STC.
The data presented show that the new carnosine derivative, STC possesses the same antioxidant capacity as carnosine under in vitro (Stvolinsky et al. 2010a) and in vivo (Fig. 3) conditions but has an additional ability to improve the long-term memory (Fig. 2). Moreover, STC, unlike carnosine may increase the life span of both male and female fruit flies. At the same time, STC is more stable against human serum carnosinase attack and may circulate longer in the blood stream (Stvolinsky et al. 2010a). In combination with its more hydrophobic nature one can expect that STC possibly can more easily penetrate the membrane barrier and to be more potent as a membrane protector and anti-stressing agent compared to carnosine. All these data make it necessary to continuation of pharmacokinetic study of STC, its possible accumulation in the cells using PEPT2 system and intrinsic mechanisms of its antiradical activity.
References
Aldini G, Orioli M, Rossoni G, Savi F, Braidotti P, Vistoli G, Yeum KJ, Negrisoli G, Carini M (2011) The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J Cell Mol Med 15(6):1339–1354
Aruoma O, Laughton M, Halliwell B (1989) Carnosine, homocarnosine and anserine: could they act as antioxidants in vivo? Biochem J 264:863–869
Biegel A, Knütter I, Hartrodt B, Gebauer S, Theis S, Luckner P, Kottra G, Rastetter M, Zebisch K, Thondorf I, Daniel H, Neubert K, Brandsch M (2006) The renal type H+/peptide symporter PEPT2: structure-affinity relationships. Amino Acids 31(2):137–156
Boldyrev AA (1993) Does carnosine possess direct antioxidant activity? Int J Biochem 25:1101–1107
Boldyrev AA (2003) Significance of reactive oxygen species for neuronal function. In: Tomasi A et al (eds) Free radicals, NO, and Inflammation: molecular, biochemical and clinical aspects. IOS Press, The Netherlands, pp 153–169
Boldyrev A (2005) Protection of proteins from oxidative stress: a new illusion or a novel strategy? Ann N Y Acad Sci 1057:193–205
Boldyrev A (2006) Carnosine and protection of cells and tissues against oxidative stress, NovaPubl., NY
Boldyrev AA, Dupin AM, Bunin AYa, Babizhaev MA, Severin SE (1987) The antioxidative properties of carnosine, a natural histidine containing dipeptide. Biochem Int 15:1105–1113
Boldyrev AA, Stvolinsky SL, Tyulina OV, Koshelev VB, Hori N, Carpenter D (1997) Biochemical and physiological evidence that carnosine is an endogenous neuroprotector against free radicals. Cell Mol Neurobiol 17(2):259–271
Boldyrev A, Song R, Lawrence D, Carpenter DO (1999) Carnosine protects against excitotoxic cell death independently of effects on reactive oxygen species. Neuroscience 94:571–577
Boldyrev A, Stvolinsky S, Fedorova T, Suslina Z (2010) Carnosine as a natural antioxidant and geroprotector: from molecular mechanisms to clinical trials. Rejuv Res 13(2–3):156–158
Dobrota D, Fedorova T, Stepanova M, Babusikova E, Statelova D, Tatarkova Z, Stvolinsky S, Boldyrev A (2010) Oxidative stress, induced in rat brain by a combination of 3-nitropropionic acid and global ischemia. J Clin Exp Med 3:144–152
Feldman LA, Shapiro ML, Nalbantoglu J (2010) A novel, rapidly acquired and persistent spatial memory task that induces immediate early gene expression. Behav Brain Funct 6:35–46
Gallant S, Kukley M, Stvolinsky S, Bulygina E, Boldyrev A (2000) Effect of carnosine on rats under experimental brain ischemia. Tohoku J Exp Med 191:85–99
Gupta S, Sharma SS (2006) Neuroprotective effects of Trolox™ in global cerebral ischemia in gerbils. Biol Pharm Bull 29:957–961
Hipkiss A (2009) Carnosine and its possible roles in nutrition and health. Adv Food Nutr Res 57:87–154
Iliadi KG, Iliadi NN, Boulianne GL (2009) Regulation of Drosophila life-span: effect of genetic background, sex, mating and social status. Exp Gerontol 44:546–553
Kang JH (2010) Protective effects of carnosine and homocarnosine on ferritin and hydrogen peroxide-mediated DNA damage. BMB Rep 43(10):683–687
Kohen R, Yamamoto Y, Cundy K, Ames BN (1988) Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci USA 85:3175–3179
Koren E, Kohen R, Ginsburg I (2010) Polyphenols enhance total oxidant-scavenging capacities of human blood by binding to red blood cells. Exp Biol Med 235:689–699
Kosolapov VA, Spasov AA, Tibirjkov EV (2009) Antioxidant and membrane protector properties of Trolox™. Exptl Clin Pharmacol 2:47–50
Leinsoo T, Abe H, Boldyrev A (2006) Carnosine and related compounds protect double-helices DNA against oxidative injury. J Evol Biochem Physiol 42:453–456
Lints FA, Bourgois M, Delalieux A, Stoll J, Lints CV (1983) Does the female life span exceed that of the male? A study in Drosophila melanogaster. Gerontology 29:336–352
Miller DJ, O’Dowd A (2000) Vascular smooth muscle actions of carnosine as its zinc complex are mediated by histamine H(1) and H(2) receptors. Biochemistry (Mosc) 65(7):798–806
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Schlesier K, Harwat M, Bőhm V, Bitsch R (2002) Assessment of antioxidant activity by using different in vitro methods. Free Rad Res 36:177–187
Sharma SS, Kaundal RK (2007) Neuroprotective effects of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox™), an antioxidant in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Neurol Res 29(3):304–309
Stvolinsky SL, Kukley ML, Dobrota D, Matejovicova M, Tkac I, Boldyrev AA (1999) Carnosine: an endogeneous neuroprotector in ischemic brain. Cell Molec Neurobiol 19:45–56
Stvolinsky SL, Bulygina ER, Fedorova TN, Meguro K, Sato T, Tyulina OV, Abe H, Boldyrev AA (2010a) Biological activity of novel synthetic derivatives of carnosine. Cell Mol Neurobiol 30(3):395–404
Stvolinsky S, Antipin M, Meguro K, Sato T, Abe H, Boldyrev A (2010b) Effect of carnosine and its Trolox™-modified derivatives on lifespan of Drosophila melanogaster. Rejuv Res 13(4):453–457
Tanida M, Niijima A, Fukuda Y, Sawai H, Tsuruoka N, Shen J, Yamada S, Kiso Y, Nagai K (2005) Dose-dependent effects of l-carnosine on the renal sympathetic nerve and blood pressure in urethane-anesthetized rats. Am J Physiol Regul Integr Comp Physiol 288(2):R447–R455
Yuneva AO, Kramarenko GG, Vetreshchak TV, Gallant S, Boldyrev AA (2002) Effect of carnosine on Drosophila melanogaster life span. Bull Exp Biol Med 133(6):559–661
Acknowledgments
Supported by RFBR Grants ## 09-04-00505, 10-04-01461, and 11-04-00906.
Author information
Authors and Affiliations
Corresponding author
Additional information
To the memory of Steven Gallant, enthusiast in carnosine biochemistry
Rights and permissions
About this article
Cite this article
Stvolinsky, S., Toropova, K., Gordeeva, M. et al. Carnosine and its (S)-Trolox™ derivative protect animals against oxidative stress. Amino Acids 43, 165–170 (2012). https://doi.org/10.1007/s00726-012-1256-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1256-4