Abstract
In the present work, several novel optically active nanostructure poly(amide–imide)s (PAI)s were synthesized via step-growth polymerization reaction of chiral diacids based on pyromellitic dianhydride-derived dicarboxylic acids containing different natural amino acids such as l-alanine, S-valine, l-leucine, l-isoleucine, l-methionine, and l-phenylalanine with 2-(3,5-diaminophenyl)-benzimidazole under green conditions using molten tetrabutylammonium bromide. The new optically active PAIs were achieved in good yields and moderate inherent viscosity up to 0.41 dL/g. The synthesized polymers were characterized with FT-IR, 1H-NMR, X-ray diffraction, field emission scanning electron microscopy (FE-SEM), elemental and thermogravimetric analysis (TGA) techniques. These polymers show high solubility in organic polar solvents due to the presence of amino acid and benzimidazole pendant group at room temperature. FE-SEM results show that, these chiral nanostructured PAIs have spherical shapes and the particle size is around 20–80 nm. On the basis of TGA data, such PAIs are thermally stable and can be classified as self-extinguishing polymers. In addition due to the existence of amino acids in the polymer backbones, these macromolecules are not only optically active but also could be biodegradable and thus may well be classified under environmentally friendly materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymers symbolize an imperative part of our daily life and over several million tons of the synthetic polymers are formed every year. They have many advantages like higher energy efficiency, less weight, higher performance and durability, and more flexibility in design and processing over other conventional materials (Erdmenger et al. 2010; Saito and Hearn 2008). For the synthesis of these important materials, common organic solvents are used expensively, which are volatile and most of them are flammable, toxic, quite hazardous, and harmful (Azapagic et al. 2003). New methods for polymer processing and synthesis could reduce or eliminate the environmental problems associated with polymer manufacturing as well as increase the energy efficiency and decrease the waste generation. In this regard, the most common explored green solvents for polymer production are ionic liquids (IL)s, supercritical carbon dioxide, and water (Kerton 2009; Nelson 2003; Mallakpour 2011).
The exceptional combination of non-volatility and favorable solubility characteristics of ILs has motivated a growing interest as a replacement for organic solvents, and more generally of volatile organic compounds in many industrial applications (Bideau et al. 2011; Parvulescu and Hardacre 2007). These attractive features of ILs made them fashionable candidates in a broad number of applications in various fields including organic synthesis, extraction/separation, electrochemical analysis, catalysis, and chemical sensors (Betz et al. 2011; Chen et al. 2010; Moniruzzaman et al. 2010; Olivier-Bourbigou et al. 2010; Quijano et al. 2011). Actually, negligible vapour pressure allows easy retrieval of the final products by distillation or using biphasic chemical processes without degradation or loss of solvent by evaporation and, consequently, an easy recycling (Earle and Seddon 2000; Hubbard et al. 2011). Also, they have found numerous applications not only as environmentally benign reaction media, but also as catalysts for many types of direct polycondensation due to the high temperatures often employed in this type of reaction. Most of these ILs are expensive and it would be cost effective to use less expansive and safe molten ILs such as tetrabutylammonium bromide (TBAB) (Mallakpour and Mirkarimi 2010). Molten TBAB is low-cost, readily accessible, and has inherent properties like ecological compatibility, operational simplicity, greater selectivity, non-corrosive nature, and no difficulty of reusability (Kantevari et al. 2008).
Among the heat resistant and high performance polymers, polyimides (PI)s have received considerable scientific and technological attention due to their outstanding performance such as dielectrical and mechanical properties as well as thermal and chemical stability (Yu et al. 2010). Several potential applications of PIs have appeared in the literature for advanced technology. Further, interest in their functional construction and application has been increasing to obtain PIs possessing certain specific properties (Chen et al. 2009; Seckin et al. 2010; Yang et al. 2002). However, wholly aromatic PIs do not always provide the optimum properties for many specialty applications owing to deficiencies in processability, solubility, and transparency as well as their relatively high dielectric constant because of their rigid backbones, and strong interactions between chains (Eichstadt et al. 2002; Hsiao and Lin 2005; Wang and Chen 2011). To overcome these problems, much research effort has been focused on the synthesis of soluble and processable PIs in fully imidized form without deterioration of their own excellent properties. Several approaches to fabricate soluble PIs including introduction of flexible linkage and heteroaromatic rings into the polymer backbone or preparation of co-PIs like poly(amide–imide)s (PAI)s have been developed in the past decade (Ding et al. 2002; Mallakpour and Dinari 2011a; Qiu et al. 2007; Shockravi et al. 2009). PAI have better processability than PI and excellent heat resistant properties than polyamide. Among the PAIs, type of chiral is important because the majority of bioorganic molecules are chiral. The optical activity of the polymer can be tuned by choosing a suitable chiral initiator or by starting from a chiral monomer. The introduction of chiral structural units into PAI systems may play an important role in molecular arrangement. It is also interesting to mention that macromolecules containing a high degree of amino acid functionality can lead to the formation of polymers with improved solubility, biodegradability, and biocompatibility (Hsiao et al. 2010; Levesque et al. 2010; Mallakpour et al. 2011; Mallakpour and Dinari 2011b; Sanda and Endo 1999).
The benzimidazole rings with heteroaromatic structure have excellent stabilities derived from its molecular symmetry and aromaticity, so the incorporation of benzimidazole group into PI backbone would improve the solubility without deterioration of their own excellent properties (Ayala et al. 2005; Dubey et al. 2007; Wang and Wu 2003; Wu et al. 2009).
The designing of new nanostructure materials from organic molecules has opened up new fundamental and practical frontiers to improve physical properties of materials significantly for different purposes (Livi et al. 2011). Different nanostructure polymers have been developed for a wide range of advanced uses such as drug delivery devices, conducting wires, and optoelectronic devices (Dai 2004). In addition, amino acid-based systems have been found to show nano-scale ordering into stable hierarchical superstructures administered by the formation of secondary structures in these segments (Klok and Lecommandoux 2001; Mallakpour and Dinari 2011a, b). Based on these above considerations, in this study, we introduced natural amino acid into PAIs backbone which not only classified them as nanostructure materials and moreover induce chirality in the obtained polymers, but also improve the solubility of the resulting macromolecules. To maintain the thermal stability; benzimidazole groups were also introduced into PAIs side chain. These polymers were prepared with direct polycondensation reaction of different amino acids-based diacid monomers and benzimidazole diamine in green medium using molten TBAB. In this way, a good balance of thermal resistance and solubility was obtained. The properties of the obtained polymers, such as specific rotation, viscosity, solubility, thermal stability and morphology are discussed here.
Experimental
Materials
Solvents and chemicals were obtained from the Aldrich Chemical Co. (Milwaukee, WI, USA), Riedel–deHaen AG (Seelze, Germany), and Merck Chemical Co. (Germany). Pyromellitic dianhydride (PMDA) and TBAB (mp = 100–103°C) were purchased from Merck Co. (Darmstadt, Germany) and were used without further purification. N,N′-Dimethylformamide (DMF) were dried over barium oxide and then were distilled under reduced pressure. l-Alanine, S-valine, l-methionine, l-leucine, l-isoleucine and l-phenylalanine were used as obtained without further purification. 3,5-Dinitrobenzoyl chloride, 1,2-phenylenediamine, hydrazine monohydrate, phosphorus pentoxide (P2O5), and methanesulfonic acid (MSA) were obtained from commercial sources and used as received.
Characterization
Proton nuclear magnetic resonance (1H-NMR, 500 MHz) spectra were recorded in dimethylsulfoxide (DMSO-d 6) solution using a Bruker (Germany) Avance 500 instrument. Multiplicities of proton resonance were designated as singlet (s), doublet (d), and multiplet (m). Infrared spectra of the samples were recorded at room temperature in the range of 4,000–400 cm−1, on (Jasco-680, Japan) spectrophotometer. Spectra of solids were obtained using KBr pellets. Vibrational transition frequencies are reported in wavenumber (cm−1). Inherent viscosities (μinh) were measured by a standard procedure using a Cannon–Fenske Routine Viscometer (Germany). Specific rotation ([α] 25D ) was measured with a Jasco (Osaka, Japan) P-1030 polarimeter at the concentration of 0.5 g/dL at 25°C. XRD patterns were recorded using CuKα radiation on a Bruker, D8 Advance, (Germany) diffractometer operating at current of 100 mA and a voltage of 45 kV. The diffractograms were measured for 2θ, in the range of 5º–80º, using CuKα incident beam (λ = 1.51418 Å). Thermal gravimetric analysis (TGA) data were taken on STA503 WinTA instrument in a nitrogen atmosphere at a heating rate of 10°C/min. Surface morphology of polymers were characterized using field emission scanning electron microscopy (FE-SEM) [HITACHI: S-4160].
Monomers synthesis
Synthesis of amino acid containing diacid monomers
Optically active diacid monomers bearing different natural amino acids (4a–4f) were prepared according to our previous works (Mallakpour and Dinari 2011a, b).
Synthesis of dinitro and diamine
The pure dinitro intermediate, 2-(3,5-dinitrophenyl)-benzimidazole 7 was prepared from 3,5-dinitrobenzoyl chloride and 1,2-phenylenediamine using MSA and P2O5 (yield: 70%; m.p. 331–332°C) (Alvarez-Gallego et al. 2008; Ayala et al. 2005).
FT-IR (KBr, cm−1): 3,336 (m), 3,102 (W), 1,593 (w), 1,541 (s), 1,346 (s), 1,075 (m), 808 (m), 731 (s) and 789 (w).
2-(3,5-Diaminophenyl)-benzimidazole 8 was prepared by reduction of dinitro precursor in refluxed ethanol using palladium and hydrazine monohydrate (yield: 77%; m.p. 242–243°C) (Alvarez-Gallego et al. 2008; Ayala et al. 2005).
FT-IR (KBr, cm−1): 3,409 (s, br), 3,063 (w), 1,607 (s), 1,577 (s), 1,484 (w), 1,446 (m), 1,274 (w), 841 (m) and 743 (s). 1H NMR (400 MHz, DMSO-d 6, δ, ppm): 4.91 (s, 4H, NH2), 5.96 (s, 1H, Ar–H), 6.61 (s, 2H, Ar–H), 7.12–7.17 (d, 2H, Ar–H, J = 8.56 Hz), 7.51–7.56 (m, 2H, Ar–H), 12.62 (s, 1H, NH benzimidazole). Elem. Anal. Calcd. for C13H12N4 (224.23): C, 69.62%; H, 5.39%; N, 24.98. Found. C, 69.60%; H, 5.36%; N, 24.88%.
Polymers synthesis
The PAIs were prepared by the following general procedure: as an example for the preparation of PAI9a, a mixture of 0.10 g (2.36 × 10−4 mol) of diacid 4a and 0.30 g (9.42 × 10−4 mol) of TBAB was ground until a powder was formed and then it was transferred into a 25-mL round-bottom flask and 0.06 g (2.36 × 10−4 mol) of diamine 8 was added to the mixture. It was heated until homogeneous solution was formed and then TPP (0.49 mL, 3.70 × 10−3 mol) was added. The solution was stirred for 12 h at 120°C, and the viscous solution was precipitated in 30 mL of methanol. The white solid was filtered off and dried to give 0.13 g (87%) of PAI9a. The other PAIs were prepared by a similar procedure.
PAI9a: μ inh = 0.41 dL/g, [α] 25D = −67.60 (c = 0.5 g/dL, DMP). FT-IR peaks (KBr, cm−1): 3,393 (br), 3,063 (w), 2,946 (m), 1,775 (s), 1,724 (s), 1,684 (m), 1,577 (m), 1,487 (m), 1,455 (w), 1,375 (m), 1,347 (m), 1,222 (m), 1,108 (w), 848 (w), 747 (m), 697 (m), 617 (w) and 558 (m).
PAI9b: White solid, yield: 90%; μinh = 0.43 dL/g, [α] 25D = −80.41 (c = 0.5 g/dL, DMP). FT-IR peaks (KBr, cm−1): 3,436 (br), 2,926 (m), 1,775 (m), 1,724 (s), 1,636 (m), 1,575 (s), 1,416 (m), 1,380 (m), 1,188 (w), 1,114 (m), 926 (m), 877 (m),727 (m), 700 (m) and 562 (w). 1H-NMR (500 MHz, DMSO-d 6, δ, ppm): 0.87–1.04 (m, 12H), 2.77–2.79 (m, 2H), 4.86–4.87 (d, 2H, J = 8.15 Hz), 6.91–6.92 (distorted d, 2H, Ar–H), 7.23–7.25 (distorted d, 2H, Ar–H), 7.63 (s, 1H, Ar–H), 7.83 (s, 2H, Ar–H), 10.58 (s, 2H, NH amide), 12.78–12.86 (br, 1H, NH benzimidazole). Elem. Anal. Calcd. for C33H28N6O6: C, 65.55%; H, 4.67%; N, 13.90. Found C, 65.26%; H, 4.52%; N, 13.78%.
PAI9c: Off white solid, yield: 94%; μinh = 0.51 dL/g, [α] 25D = −77.23 (c = 0.5 g/dL, DMP). FT-IR peaks (KBr, cm−1): 3,367 (br), 3,071 (w), 2,925 (m), 1,775 (m), 1,721 (s), 1,617 (m), 1,586 (m), 1,487 (m), 1,382 (m), 1,355 (m), 1,215 (m), 1,076 (w), 895 (m), 750 (m), 688 (m), 617 (w) and 555 (m). 1H-NMR (500 MHz, DMSO-d 6, δ, ppm): 1.96–2.03 (m, 10H), 2.55–2.57 (m, 4H), 5.11-5.13 (m, 2H), 6.74–6.76 (m, 2H, Ar–H), 7.34–7.36 (m, 2H, Ar–H), 7.78 (s, 1H, Ar–H), 7.98–8.02 (m, 2H, Ar–H), 10.40 (s, 2H, NH amide), 12.64–12.80 (br, 1H, NH benzimidazole). Elem. Anal. Calcd. for C33H28N6O6S2: C, 59.55%; H, 4.67%; N, 12.90; S, 9.59. Found C, 58.89%; H, 4.06%; N, 12.44%; S, 9.47.
PAI9d: White solid, yield: 83%; μinh = 0.46 dL/g, [α] 25D = −84.72 (c = 0.5 g/dL, DMP). FT-IR peaks (KBr, cm−1): 3,434 (br), 3,066 (w), 2,967 (m), 2,934 (m), 1,777 (m), 1,722 (s), 1,617 (m), 1,577 (s), 1,488 (m), 1,418 (w), 1,385 (m), 1,220 (m), 1,164 (w), 1,087 (m), 970 (w), 752 (m), 690 (m), 620 (m) and 558 (m). 1H-NMR (500 MHz, DMSO-d 6, δ, ppm): 0.85–0.92 (m, 12H), 1.52–1.54 (m, 2H), 1.98–2.02 (m, 4H), 4.81–4.83 (m, 2H), 6.92–6.93 (distorted d, 4H, Ar–H), 7.19-7.21 (d, 2H, Ar–H, J = 9.45 Hz), 7.37 (s, 1H, Ar–H), 7.70 (s, 2H, Ar–H), 8.31 (s, 2H, Ar–H), 10.37 (s, 2H, NH amide), 12.80–12.84 (br, 1H, NH benzimidazole). Elem. Anal. Calcd. for C35H32N6O6: C, 66.45%; H, 5.10%; N, 13.28. Found C 66.17%; H, 5.00%; N, 13.08%.
PAI9e: White solid, yield: 89%; μinh = 0.48 dL/g, [α] 25D = –78.45 (c = 0.5 g/dL, DMP). FT-IR peaks (KBr, cm−1): 3,423 (br), 3,074 (w), 2,987 (m), 2,917 (w), 1,774 (m), 1,718 (s), 1,628 (m), 1,576 (w), 1,439 (w), 1,385 (s), 1,364 (m), 1,288 (m), 1,158 (w), 1,108 (m), 1,011 (w), 898 (m), 833 (m), 730 (m), 627 (m) and 562 (w). 1H-NMR (500 MHz, DMSO-d 6, δ, ppm): 0.83–1.03 (m, 12H), 1.48–1.50 (m, 4H), 1.94–1.96 (m, 2H), 4.77–4.79 (m, 2H), 6.85–6.86 (distorted d, 4H, Ar–H), 7.13–7.15 (d, 2H, Ar–H, J = 8.76 Hz), 7.34 (s, 1H, Ar–H), 7.66 (s, 2H, Ar–H), 8.25 (s, 2H, Ar–H), 10.45 (s, 2H, NH amide), 12.86–12.92 (br, 1H, NH benzimidazole). Elem. Anal. Calcd. for C35H32N6O6: C, 66.45%; H, 5.10%; N, 13.28. Found C, 65.89%; H, 5.03%; N, 13.17%.
PAI9f: Off white solid, yield: 94%; μinh = 0.51 dL/g, [α] 25D = −77.23 (c = 0.5 g/dL, DMP). FT: FT-IR peaks (KBr, cm−1): 3,479 (br), 3,165 (w), 3,038 (w), 2,937 (m), 1,771 (s), 1,720 (s), 1,634 (m), 1,602 (w), 1,498 (m), 1,456 (m), 1,385 (s), 1,366 (s), 1,258 (w), 1,226 (m), 1,170 (m), 1,079 (m), 1,045 (w), 941 (s), 918 (m), 881 (m), 828 (m), 733 (s), 700 (s), 631 (m), 568 (s) and 492 (w). 1H-NMR (500 MHz, DMSO-d 6, δ, ppm): 3.37–3.39 (m, 4H), 5.31 (d, 2H, J = 7.65 Hz), 7.14–6.16 (m, 14H, Ar–H), 7.67 (s, 1H, Ar–H), 7.98 (s, 2H, Ar–H), 8.21 (s, 2H, Ar–H), 10.34 (s, 2H, NH amide), 12.67-12.71 (br, 1H, NH benzimidazole). Elem. Anal. Calcd. for C41H30N6O6: C, 70.26%; H, 4.00%; N, 11.99. Found C, 69.94%; H, 3.97%; N, 11.76%.
Result and discussion
Synthesis of chiral diacids
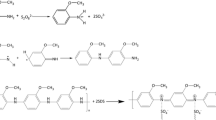
Chiral diacid monomers 4a–4f were prepared by the condensation reaction of one equimolar of dianhydride 1 and one equimolar of different natural amino acids (l-alanine, S-valine, l-methionine, l-leucine, l-isoleucine, and l-phenylalanine) in refluxing acetic acid, as shown in Scheme 1.
Synthesis of diamine
Diamine 8 was prepared with a two-step procedure outlined in Scheme 2. In the first step, the dinitro compound was obtained by the direct condensation of 1,2-phenylenediamine and 3,5-dinitrobenzoyl chloride with Eaton’s reagent (1:10 P2O5/MSA) (Eaton et al. 1973; Leykin et al. 2010) as the condensing agent and solvent. In the second step, nitro groups were reduced to the corresponding amino groups with hydrazine hydrate as the reducing agent and palladium as the catalyst. By this procedure, pure diamine 8 was attained after recrystallization.
Preparation of the organosoluble chiral PAIs
In the current investigation, in order to extend the utilization of ILs in polymers synthesis, molten TBAB was used as solvent and catalyst for the formation of several novel optically active, organo-solube and thermally stable nanostructure PAIs by the direct polymerization reaction of compound 8 with different chiral monomers 4a–4f (Scheme 3). In this method, the polymerization reaction was performed under oil-bath heating in the presence of molten TBAB and TPP. It is interesting to mention that the above polyamidation in the absence of either TPP or TBAB will not occur. Therefore, both TPP and TBAB are required which will act both as catalyst and solvent (Mallakpour and Mirkarimi 2010). The inherent viscosities of the resulting polymers under optimized condensations were in the range of 0.41–0.58 dL/g and yields were 87–94%. The incorporation of a chiral unit into the polymer backbone was obtained by measuring the specific rotations of polymers that represent all PAIs are optically active.
Characterized techniques
FT-IR and 1H-NMR study
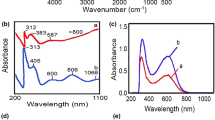
The FT-IR spectrum of dinitro 7 revealed a strong peak at 3,336 cm−1, which was assigned to the NH stretching group, and two absorption bands at 1,541 and 1,346 cm−1, which were characteristic peaks for NO2 asymmetric and symmetric, respectively (Fig. 1). In the FT-IR spectra of synthesized diamine 8, the characteristic peaks for NH2 functions and the absence of the original peaks arising from the NO2 groups in the corresponding dinitro intermediate provided that this compound was successfully prepared (Fig. 1). Absorption of amine NH2 and NH benzimidazole bonds appeared around 3,394 and 3,323 cm−1 and the peak at 1,627 cm−1 confirms the presence of NH deformation. Two absorption bands at 1,577 and 1,484 cm−1 were characteristic peaks for aromatic rings. The benzimidazole groups gave bands at 1,484 cm−1 (in plane deformation of the benzimidazole ring), 1,590 cm−1 (ring vibration of conjugation between fused benzene and imidazole rings) and a shoulder at 1,630 cm−1 (C–N stretching).
In the 1H-NMR of diamine 8, appearances of the N–H protons of benzimidazole group at 12.62 ppm as broad singlet peaks indicate the presence of this group in the diamine side chain. The absorption of aromatic protons appeared in the range of 5.96–7.50 ppm. The proton of the amine groups appeared as broad singlet peaks at 4.91 ppm.
The structures of new PAIs were confirmed by FT-IR and 1H-NMR spectroscopy and elemental analysis techniques. The N–H stretching bands of the amide and benzimidazole groups could be observed by FT-IR spectroscopy for all the polymers around 3,400–3,300 cm−1. FT-IR spectra of all polymers also show the characteristic absorption peaks for the imide ring at 1,775 and 1,714 cm−1 due to the symmetrical and asymmetrical carbonyl stretching vibrations. All of them exhibited medium absorptions at 1384 and 718–720 cm−1 that show the presence of the imide heterocycle ring in these polymers. The N–H bending and C–N stretching bands at 1,550 cm−1 could also be observed. For example, the FT-IR spectrum of PAI9a (Fig. 1) displays characteristic absorption bands for the imide ring at around 1,722 cm−1, which is indicative of the asymmetrical and symmetrical C=O stretching vibration, and at 1,375, 1,064, and 731 cm−1 due to imide ring deformation. The N–H stretching band of the amide and benzimidazole groups observed around 3,320 cm−1, and the C=O stretching band of amide group at 1,669 cm−1. Other PAIs had similar functional groups.
In the 1H-NMR spectrum of these PAIs, appearances of the N–H protons of benzimidazole and amide groups at 12.60–12.80 and 10.35–10.51 ppm indicate the presence of these groups in the polymers side chain, as well as main chain, respectively. The resonance of aromatic protons appeared in the range of 6.73–8.35 ppm. The proton of the chiral center appeared as multiplets in the range of 4.72–5.34 ppm. The resonance of the diastrotopic hydrogens bonded to neighbor carbon of chiral center appeared in the range of 1.97–3.37 ppm as two discrete multiplets peaks. The resonance of the CH3 protons groups of the amino acids appeared as a broad multiplet peak at 0.86–1.37 ppm.
Morphology investigations
Morphology of the PAIs was recorded by X-ray diffraction which is the most commonly used methods to study the structure of nanostructure materials. For PAI9a, PAI9b and PAI9c, it could be observed that except one crystalline peak, there is a lack of any diffraction peak in the range of 2θ angle (Fig. 2). The diffraction patterns reveal the presence of a little percentage of crystalline phases compared to an amorphous one, primarily because of the bulky pendant groups. Furthermore, the amorphous nature of these polymers is also reflected in their good solubility, which is in agreement with the general rule that the solubility decreases with increasing crystallinity.
In this study, it is worth mentioning that the FE-SEM pictures of the synthesized PAIs showed that the obtained macromolecules have nanostructured morphology (Fig. 3). Also, in order to study the effect of ultrasound wave on morphology of these polymers, synthesized PAIs were irradiated for 30 min in cold ethanol mixture under ultrasonic waves. By comparing FE-SEM images before (Fig. 3a–d) and after (Fig. 3a′–d′) ultrasonic irradiation, the results showed that after sonication process, the size of PAI particles was decreased. According to these data, before sonication process, PAIs showed the spherical shapes and the particle size is around 40–80 nm, while after this process, the size of PAIs decreased and showed smaller particle in the range of 20–65 nm.
Solubility of polymers
The solubility of these thermally stable polymers was measured qualitatively (0.01 g of polymeric sample in 2 mL of solvent) in various solvents and the results demonstrate that the synthesized PAIs were well dissolved in aprotic polar solvents such as DMF, N-methyl-2-pyrrolidinone, N,N-dimethylacetamide and dimethylsulfoxide at room temperature. But these polymers were insoluble in general organic solvents such as xylenes, dioxane, CHCl3 and acetone. In THF, only PAI9f based on l-phenylalanine as a chiral moiety diacid monomer was soluble. The enhanced solubility of the resulting PAIs is attributed not only to the bulkiness of the benzimidazole and amino acid pendant groups but also to the amide and imide groups in polymer backbone.
Thermal properties
Thermogravimetric analysis was applied to evaluate the thermal properties of the PAIs at a heating rate of 10°C/min, under a nitrogen atmosphere. The results of TGA given in Fig. 4 show that the PAIs as a class possess outstanding thermal stability. The polymers exhibited a one-step pattern of decomposition with no significant weight loss below 400°C. From these data, it is clear that all of the PAIs are thermally stable owing to existence of various linkages such as imide bond and benzimidazoles groups in polymer backbones. The PAIs with an aliphatic chain as part of the side chain are not as thermally stable as the aromatic polymer. At 800°C, the residue is up to 50% for all PAIs. These values are remarkably high and they vary according to the structure of the polymers.
Char yield can be applied as decisive factor for estimated limiting oxygen index (LOI) of the polymers based on Van Krevelen and Hoftyzer equation (Van Krevelen and Hoftyzer 1976).
where CR = char yield
All polymers have LOI values around 37–40 which were calculated from their char yield at 800°C. On the basis of LOI values, all macromolecules can be classified as self-extinguishing polymers (Table 1). According to Table 1, it is clear that the PAI9f (based on l-phenylalanine) has higher thermal stability than others (Fig. 4). It could be pertained to aromatic, rigid structure of phenylalanine for PAI9f compare to aliphatic, flexible structure of other macromolecules.
Conclusions
From the results achieved in this investigation, it can reach to the following conclusions: the chiral dicarboxylic acids, containing a rigid pyromellitoyl and flexible amino acid groups and diamine with benzimidazoles pendant group were used for the preparation of novel organosoluble optically active aromatic PAIs. From the chemical point of view, the incorporation of benzimidazole diamine into the backbone of diverse polymer systems results in versatile polymers with interesting properties such as thermal stability and good solubility. Furthermore, in this work molten TBAB was used as a solvent and catalyst for the polymerization that makes the polycondensation reactions with safe operation, low pollution and rapid access to products which make it as an environmentally friendly and green method, and also decreases the cost of polymerization reaction. FE-SEM micrographs of these amino acid containing fabricated polymers showed that, all of them have nanostructure that nanosize particle is at range of 20–80 nm. Also, the effect of ultrasonic irradiation on the size and shape of polymer nanoparticles was studied and the results show that the sizes of polymer particles were decreased after sonication process. In addition, due to the existence of amino acids in the macromolecules backbone, these polymers are anticipated to be biodegradable and are therefore could be classified under environmental benign materials. These materials are excellent candidates for the utilization in different systems such as the chiral medium in asymmetric synthesis and chiral stationary phases in high-performance liquid chromatography (HPLC) for resolution of racemic mixtures.
References
Alvarez-Gallego Y, Ruffmann B, Silva V, Silva H, Lozano AE, de la Campa JG, Nunes SP, de Abajo J (2008) Sulfonated polynaphthalimides with benzimidazole pendant groups. Polymer 49:3875–3883
Ayala V, Maya EM, Garcia JM, De La Campa JG, Lozano AE, De Abajo J (2005) Synthesis, characterization, and water sorption properties of new aromatic polyamides containing benzimidazole and ethylene oxide moieties. J Polym Sci Part A Polym Chem 43:112–121
Azapagic A, Emsley A, Hamerton I (2003) Polymers: the environment and sustainable development. Wiley, New York
Betz D, Altmann P, Cokoja M, Herrmann WA, Kuhn FE (2011) Recent advances in oxidation catalysis using ionic liquids as solvents. Coord Chem Rev 255:1518–1540
Bideau JL, Viau L, Vioux A (2011) Ionogels, ionic liquid based hybrid materials. Chem Soc Rev 40:907–925
Chen K, Kazuaki XC, Endo YN, Higa M, Okamoto K (2009) Synthesis and properties of novel sulfonated polyimides bearing sulfophenyl pendant groups for fuel cell application. Polymer 50:510–516
Chen X, Liu J, Wang J (2010) Ionic liquids in the assay of proteins. Anal Methods 2:1222–1226
Dai L (2004) Polymer nanostructures. In: Nalwa HS (ed) Encyclopedia of nanoscience and nanotechnology. American scientific publishers, USA, pp 763–790
Ding Y, Bikson B, Nelson JK (2002) Polyimide membranes derived from poly(amic acid) salt precursor polymers. Synthesis and characterization. Macromolecules 35:905–911
Dubey R, Subbiah N, Moorthy HN (2007) Comparative studies on conventional and microwave assisted synthesis of benzimidazole and their 2-substituted derivative with the effect of salt form of reactant. Chem Pharm Bull 55:115–117
Earle J, Seddon KR (2000) Green solvents for the future. Pure Appl Chem 72:1391–1398
Eaton PE, Carlson GR, Lee JT (1973) Phosphorus pentoxide–methanesulfonic acid. A convenient alternative to polyphosphoric acid. J Org Chem 38:4071–4073
Eichstadt AE, Ward TC, Bagwell MD, Farr IV, Dunson DL, McGrath JE (2002) Synthesis and characterization of amorphous partially aliphatic polyimide copolymers based on bisphenol-A dianhydride. Macromolecules 35:7561–7568
Erdmenger T, Guerrero-Sanchez C, Vitz J, Hoogenboom R, Schubert US (2010) Recent developments in the utilization of green solvents in polymer chemistry. Chem Soc Rev 39:3317–3333
Hsiao SH, Lin KH (2005) Polyimides derived from novel asymmetric ether diamine. J Polym Sci Part A Polym Chem 43:331–341
Hsiao SH, Liou GS, Kung YC, Lee YJ (2010) Synthesis and characterization of electrochromic poly(amide–imide)s based on the diimide-diacid from 4,4′-diamino-4″-methoxytriphenylamine and trimellitic anhydride. Eur Polym J 46:1355–1366
Hubbard CD, Illner P, van Eldik R (2011) Understanding chemical reaction mechanisms in ionic liquids: successes and challenges. Chem Soc Rev 40:272–290
Kantevari S, Chary MV, Das APR, Vuppalapati SVN, Lingaiah N (2008) Catalysis by an ionic liquid: Highly efficient solvent-free synthesis of aryl-14H-dibenzo[a.j]xanthenes by molten tetrabutylammonium bromide under conventional and microwave heating. Catal Commun 9:1575–1581
Kerton FM (2009) Alternative solvents for green chemistry in RSC green chemistry book series. The royal society of chemistry, Cambridge
Klok HA, Lecommandoux S (2001) Supramolecular materials via block copolymer self-assembly. Adv Mater 13:1217–1223
Levesque CL, Moehn S, Pencharz PB, Ball RO (2010) Review of advances in metabolic bioavailability of amino acids. Livest Sci 133:4–9
Leykin AY, Fomenkov AI, Galpern EG, Stankevich A IV, Rusanov L (2010) Some aspects of polybenzimidazoles’ synthesis in P2O5 containing condensation media. Polymer 51:4053–4057
Livi S, Duchet-Rumeau J, Gerard JF (2011) Nanostructuration of ionic liquids in fluorinated matrix: influence on the mechanical properties. Polymer 52:5223–5231
Mallakpour S (2011) Synthesis of soluble poly(amide–ether–imide–urea)s bearing amino acid moieties in the main chain under green media (ionic liquid). Amino acids 40:487–492
Mallakpour S, Dinari M (2011a) Insertion of novel optically active poly(amide–imide) chains containing pyromellitoyl-bis-l-phenylalanine linkages into the nanolayered silicates modified with l-tyrosine through solution intercalation. Polymer 52:2514–2523
Mallakpour S, Dinari M (2011b) Progress in synthetic polymers based on natural amino acids. J Macromol Sci Part A Pure Appl Chem 48:644–679
Mallakpour S, Mirkarimi F (2010) Synthesis and characterization of novel, optically active polyamides derived from S-valine natural amino acid and bulky anthracenic side chain. Amino Acids 39:1255–1263
Mallakpour S, Asadi P, Sabzalian MR (2011) Synthesis of biodegradable chiral poly(ester-imide)s derived from valine-, leucine- and tyrosine-containing monomers. Amino Acid 41:1215–1222
Moniruzzaman M, Nakashima K, Kamiya N, Goto M (2010) Recent advances of enzymatic reactions in ionic liquids. Biochem Eng J 48:295–314
Nelson WM (2003) Green solvents for chemistry: perspectives and practice in Green chemistry series, 1st edn. Oxford University Press, Oxford
Olivier-Bourbigou H, Magna L, Morvan D (2010) Ionic liquids and catalysis: recent progress from knowledge to applications. Appl Catal A 373:1–56
Parvulescu VI, Hardacre C (2007) Catalysis in ionic liquid. Chem Rev 107:2615–2665
Qiu Z, Chen G, Zhang Q, Zhan S (2007) Synthesis and gas transport property of polyimide from 2,2-disubstituted biphenyltetracarboxylic dianhydrides (BPDA). Eur Polym J 43:194–204
Quijano G, Couvert A, Amrane A, Darracq G, Couriol C, Cloirec PL, Paquin L, Carrie D (2011) Potential of ionic liquids for VOC absorption and biodegradation in multiphase systems. Chem Eng Sci 66:2707–2712
Saito K, Hearn M (2008) Novel polymer science in green chemistry. Chem Aust 9:8–12
Sanda F, Endo T (1999) Syntheses and functions of polymers based on amino acids. Macromol Chem Phys 200:2651–2661
Seckin T, Vural S, Koytepe S (2010) Preparation and structural properties of Fe3O4-polyimide hybrid nanocomposites. Polym Bull 64:115–126
Shockravi A, Abouzari-Lotf E, Javadi A, Atabaki F (2009) Preparation and properties of new ortho-linked polyamide-imides bearing ether, sulfur, and trifluoromethyl linkages. Eur Polym J 45:1599–1606
Van Krevelen DW, Hoftyzer PJ (1976) Properties of polymers, 3rd edn. Elsevier scientific publishing, New York
Wang YW, Chen WC (2011) New photosensitive colorless polyimide-silica hybrid optical materials: synthesis, properties and patterning. Mater Chem Phys 126:24–30
Wang HH, Wu SP (2003) Synthesis of thermally stable aromatic poly(imide amide benzimidazole) copolymers. J Appl Polym Sci 90:1435–1444
Wu SY, Yuen SM, Ma CCM, Huang YL (2009) Synthesis and properties of aromatic polyimide, poly(benzoxazole imide), and poly(benzoxazole amide imide). J Appl Polym Sci 113:2301–2312
Yang CP, Hsiao SH, Hsu MF (2002) Organosoluble and light-colored fluorinated polyimides from 4,40-bis(4-amino-2-trifluoromethylphenoxy)biphenyl and aromatic dianhydrides. J Polym Sci Part A Polym Chem 40:524–534
Yu YY, Chien WC, Tsai TW (2010) High transparent soluble polyimide/silica hybrid optical thin films. Polym Testing 29:33–40
Acknowledgments
The partial financial support from the Research Affairs Division, Isfahan University of Technology (IUT), Isfahan is gratefully acknowledged. Also, thankfully acknowledged of Iran Nanotechnology Initiative Council (INIC), National Elite Foundation (NEF), and Center of Excellence in Sensors and Green Chemistry (IUT) for partial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallakpour, S., Dinari, M. Novel nanostructure amino acid-based poly(amide–imide)s enclosing benzimidazole pendant group in green medium: fabrication and characterization. Amino Acids 43, 1605–1613 (2012). https://doi.org/10.1007/s00726-012-1236-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1236-8