Abstract
The therapeutic benefits of l-arginine (ARG) supplementation in humans, often clearly observed in short-term studies, are not evident after long-term use. The mechanisms for the development of ARG tolerance are not known and cannot be readily examined in humans. We have developed a sensitive in vitro model using a low glucose/low arginine culture medium to study the mechanisms of ARG action and tolerance using two different human endothelial cells, i.e., Ea.hy926 and human umbilical venous endothelial cells. Cultured cells were incubated with different concentrations of ARG and other agents to monitor their effects on endothelial nitric oxide synthase (eNOS) expression and function, as well as glucose and superoxide (O ·−2 ) accumulation. Short-term (2 h) exposure to at least 50 μM ARG moderately increased eNOS activity and intracellular glucose (p < 0.05), with no change in eNOS mRNA or protein expression. In contrast, 7-day continuous ARG exposure suppressed eNOS expression and activity. This was accompanied by increase in glucose and O ·−2 accumulation. Co-incubation with 100 μM ascorbic acid, 300 U/ml polyethylene glycol-superoxide dismutase (PEG-SOD), 100 μM l-lysine or 30 μM 5-chloro-2-(N-2,5-dichlorobenenesulfonamido)-benzoxazole (a fructose-1,6-bisphosphatase inhibitor) prevented the occurrence of cellular ARG tolerance. Short-term co-incubation of ARG with PEG-SOD improved cellular nitrite accumulation without altering cellular ARG uptake. These studies suggest that ARG-induced oxidative stress may be a primary causative factor for the development of cellular ARG tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Arginine (ARG), a conditionally essential amino acid, is involved in regulating multiple physiological processes (Schulman et al. 2006). It serves as a substrate for various isoforms of nitric oxide synthase (NOS) to produce nitric oxide (NO), an important signaling molecule for various organs and tissues, including those in the cardiovascular, neurological and immune systems. In addition, ARG plays an important role in removing ammonia from the body through the urea cycle (Ha and Milner 1979), and serves as a substrate for the endogenous synthesis of creatinine and proline (Wu and Morris 1998).
Because of these various physiological functions, ARG, in dietary supplementation (ARG-SUPP), has been shown to produce beneficial therapeutic effects in a variety of disease states. In the NIH website MedlinePlus (http://www.nlm.nih.gov/medlineplus/druginfo/natural/875.html, 2011), the use of ARG in as many as 24 diseases was discussed. Indications graded as “possibly effective” (n = 10) include congestive heart failure, bladder inflammation, intermittent claudication, erectile dysfunction, etc. Those rated as “possibly ineffective” (n = 2) are heart attack and pre-eclampsia, while those rated as “insufficient evidence to rate effectiveness for” (n = 12) include migraine headache, senile dementia, female sexual problems, sickle cell anemia, etc. As a precursor to proline formation, ARG may promote wound healing and the development of muscle mass (Wu and Morris 1998). Thus, ARG is being used by body builders and athletes, mostly without medical supervision.
Many reports have substantiated the short-term benefits of ARG-SUPP in diverse diseases. For example, in patients with stable angina pectoris, 6 g ARG/day for 3 days increased their exercise tolerance (Bednarz et al. 2005), and supplement with two food bars enriched with ARG per day for 2 weeks improved vascular function, exercise capacity and aspects of quality of life (Maxwell et al. 2002). In patients with congestive heart failure, 9 g ARG/day for 7 days prolonged exercise duration (Bednarz 2004). In addition, ARG-SUPP has been found to improve immunity (Popovic et al. 2007), in patients under critical care (Zhou and Martindale 2007) and in sickle cell disease (Romero et al. 2002; Vichinsky 2002).
However, the long-term effects of ARG-SUPP have not been examined extensively. Only two well-conducted clinical studies are available, and both revealed that the short-term therapeutic benefits of ARG are not evident after long-term use. Wilson et al. (2007) showed, in the nitric oxide in peripheral arterial insufficiency (NO-PAIN) trial that ARG-SUPP (3 g/day) for 6 months, in 133 subjects, “did not increase nitric oxide synthesis or improve vascular reactivity and the expected placebo effect observed in studies of functional capacity was attenuated in the ARG-treated group”. These authors characterized these findings as indicating the existence of “l-arginine tolerance” because beneficial effects were observed after 1 month of dosing (Oka et al. 2005). In the vascular interaction with age in myocardial infarction (VINTAGE MI) trial (Schulman et al. 2006), a total of 153 patients after MI was randomly assigned ARG (goal dose of 3 g tid) or matching placebos for 6 months. The results showed no improvement in vascular stiffness measurements or ejection fraction. Strikingly, six patients in the ARG group died during the study period versus none in the placebo group. The authors therefore concluded that ARG “may be associated with high post-infarct mortality”, and stated that ARG “should not be recommended following acute myocardial infarction”, contrary to the beneficial effects shown by the same regiment after 1 month (Bednarz et al. 2005).
The mechanisms responsible for the loss of effect (i.e., tolerance development) and the occurrence of potential toxicity after long-term ARG administration are not known presently. Here, we explored these mechanisms in vitro using two types of human endothelial cells, viz., human umbilical vascular endothelial cells (HUVEC) and Ea.hy926. Our results indicate that repeated exposure of these cells toward ARG induces oxidative stress via superoxide (O ·−2 ) formation, down-regulation of eNOS and apparent glucose accumulation.
Materials and methods
Supplies and reagents
HUVEC were purchased from American Type Culture Collection (Manassas, VA, USA), whereas Ea.hy926, an immortalized cell line derived from human umbilical vein endothelial cells, was obtained as a gift from the University of North Carolina. Culture reagents were obtained from Invitrogen (Carlsbad, CA, USA), and other supplies and chemicals were from Laboratory Product Sales (Rochester, NY, USA) and Sigma-Aldrich (St. Louis, MO, USA). Human eNOS immunoassay kit was purchased from R&D systems (Minneapolis, MN, USA). Quantichrom d-Glucose assay kit utilizing the o-toluidine method was purchased from Bioassay Systems (Hayward, CA, USA). RT-qPCR primer of SYBR Green Human NOS3 was purchased from SABiosciences (Foster City, CA, USA). Deionized water (18 MΩ) was used in all experiments. 5-chloro-2-(N-2,5-dichlorobenzenesulfonamido)-benzoxazole (abbreviated as 5-CDB), an inhibitor of fructose-1,6-bisphosphatase in the gluconeogenesis pathway (IC50 = 6.6 μM) was obtained from EMD Chemicals, Inc. (Gibbstown, NJ, USA).
Cell culture
HUVEC were cultured in physiological F-12K medium containing 100 μM ARG and 5 mM glucose, supplemented with 20% horse serum. Ea.hy926 cells were cultured in two types of Dubecco’s modified eagle medium (DMEM) containing either 100 μM ARG, 5 mM d-glucose (LA/LG), or 400 μM ARG, 25 mM d-Glucose (HA/HG), both supplemented with 10% fetal bovine serum. All culture media contained 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were maintained in a humidified chamber at 37°C with 5% CO2, and passages between 6 and 16 (mean passage number = 9 ± 3) were used in all experiments.
In vitro exposure to ARG
For acute studies, HUVEC and Ea.hy926 cells grown to confluence in six well dishes (well area of 9.6 cm2) were incubated in Locke’s buffer (154 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 2.3 mM CaCl2, 5.6 mM d-glucose, 5 mM HEPES, pH 7.4) for 2 h containing either 0–500 μM ARG, or combinations of 100 μM ARG with or without 30 μM of the gluconeogenesis inhibitor 5-CDB, 100 μM ascorbic acid, 30 μM l-nitroarginine methyl ester (l-NAME), 300 U/ml of superoxide dismutase (SOD) or its cell-permeant poly-ethylene glycol form (PEG-SOD). Continuous effect was assessed by incubating cultured cells with 100 μM ARG in daily refreshed medium consecutively for 7 days, after which cells were washed, and challenged to 50 or 100 μM ARG in the presence or absence of other reagents to determine eNOS action.
After ARG exposure, the cells were washed twice with 1 ml phosphate-buffered saline (PBS) and incubated with trypsin EDTA (0.5 ml) for <3 min before adding equal amount of F-12K medium (0.5 ml). The cells were centrifuged at 300×g for 5 min, washed twice with 1 ml of PBS and lysed using 0.5 ml lysis buffer. The lysed mixtures were centrifuged at 13,000×g for 5 min, and the supernatant (approximately 150–300 μl) was collected and used to analyze protein concentration by the Lowry method (Lowry et al. 1951), using bovine serum albumin as standard. In separate experiments after cells were subjected to acute and chronic exposure to 100 μM ARG, cellular arginase activity was measured with the Quantichrom arginase assay kit (Bioassay Systems, Hayward, CA, USA), according to the manufacturer’s instructions.
Inorganic nitrite and total nitrite/nitrate determination
Cell lysate samples or freshly prepared nitrite standard were first brought to volume of 100 μl with double-deionized water. Samples were protected from light, and 10 μl of freshly prepared diaminonaphthalene (DAN, 0.05 mg/ml in 1 M HCl) was added and mixed immediately. After 10 min incubation at room temperature, the reaction was terminated with 5 μl of 2.8 N NaOH. The intensity of the fluorescent signal produced was measured using a plate reader with excitation at 360 nm and emission read at 420 nm, with a gain setting at 100%.
In order to measure total nitrite/nitrate, nitrate was converted to nitrite by the action of nitrate reductase from Aspergillus niger. Briefly, the samples were incubated with 40 μM NADH and 14 mU of enzyme in a final volume of 50 μl of 20 mM Tris, pH 7.6, followed by 30 min incubation with 10 μl of DAN at room temperature. The reaction was terminated after 30 min with 20 μl of NaOH. Nitrite contents in the samples were then calculated by first subtracting the value of the enzyme blank containing NADH. The values were further normalized using total protein concentration, which were measured according to the Lowry method (1951).
RT-PCR analysis of eNOS mRNA
Total RNA was isolated from Ea.hy.926 cells in LA/LG DMEM medium supplemented with 100 or 300 μM ARG for 7 days using SV total RNA isolation system (Promega, Madison, WI, USA). eNOS mRNA level was analyzed by reactions with RNA PCR kit (one step RNA PCR kit, SABiosciences, USA) using Alien RNA for data normalization. The programmed cycles for eNOS RT-PCR were as follows: 1 cycle of 95°C × 10 min; 40 cycles of 95°C × 15 s, 60°C × 1 min; followed by a melting curve program of 95°C × 1 min, 65°C × 2 min (optics off); 65–95°C/min (optics on). The primer sequence used was as follows: sense 5′-CGA GAT ATC TTC AGT CCC AAG C-3′, antisense 5′-GTG GAT TTG CTG CTC TCT AGG-3′.
Superoxide measurement
O ·−2 production was assessed by dihydroethidium (DHE) fluorescence (Zhao et al. 2003). At the end of the incubations, cells were washed and incubated in Locke’s buffer at a final DHE concentration of 10 nM for 20 min. The resulting mixtures were harvested in acetonitrile (0.2 ml/well), sonicated (10 s), and centrifuged (13,000×g for 5 min at 4°C). The supernatant fraction was air-dried, reconstituted in PBS and the fluorescence intensity (in arbitrary units, AU) was determined, in duplicate, using a micro-plate reader at excitation and emission wavelengths of 490 and 570 nm, respectively.
Effect of PEG-SOD on cellular eNOS activity and ARG uptake
HUVEC grown to confluence in six-well plates for 7 days in modified Ham’s F-12K medium, were washed twice with sterile PBS and preincubated for 5 min in Locke’s buffer, pH 7.4. Cells were then incubated in Locke’s buffer containing 0–300 μM of 15N4-ARG with or without 300 U/ml of PEG-SOD at 37°C for 2 h. The cells were then washed twice with PBS to remove the treatment solutions and detached from six-well plates by incubation with 0.25% trypsin-EDTA at room temperature for 1–2 min, and transferred to a 15 ml tube and spun at 300×g at 4°C for 10 min. The supernatant was removed and the cells were resuspended and washed twice with PBS. Finally, the cells were lysed with 0.5 ml lysis solution (50% cell lysis buffer and 50% PBS) and transferred to a 1.5 ml microcentrifuge tube. After vortexing, the lysate solution was centrifuged at 13,000 rpm at 4°C for 5 min. The supernatants were collected and stored as cell lysate samples for the experimental measurements. An LC-MS/MS assay developed in our laboratory (Shin et al. 2011a, b) was used to monitor the rate of 15N4-ARG uptake into the cultured cells.
Comparison of cellular fluxes of ARG and related amino acids after acute or chronic exposure to 15N4-ARG
We examined whether ARG-tolerant cells would behave similarly in terms of transcellular fluxes of ARG and its related amino acids when compared to non-tolerant cells. In one experiment, EA.hy926 cells were grown to confluence as described, and after washing, the cells were exposed to 100 μM 15N4-ARG for 2 h (15N4-ARG stimulation–acute exposure). In a separate experiment, cells were first exposed to media enriched with 100 μM ARG for 7 days, and after washing, they were exposed to 100 μM 15N4-ARG for 2 h (15N4-ARG stimulation–chronic exposure). The concentrations of ARG and its related amino acids were then measured in cell lysates and in the incubation medium, using an LC-MS/MS method that we reported recently (Shin et al. 2011a, b).
Statistical analysis
Data are presented as mean ± standard deviation (n = 6 replicates) unless otherwise stated. Statistical comparisons among groups were performed using one-way analysis of variance (ANOVA), followed by Fisher’s and Tukey’s post-hoc test procedure (version 15.x; Minitab). Statistical significance was concluded when p < 0.05.
Results
Effect of culture media on eNOS response from acute and continuous exposure to 50 μM ARG
Preliminary studies showed that the traditional incubation medium used to culture Ea.hy926 endothelial cells, i.e., DMEM, which contained 400 μM ARG and 25 mM glucose (Kakoki et al. 2006), produced marginal eNOS response when the cells were challenged with exogenous ARG. Therefore, an alternate medium containing low arginine/low glucose (LA/LG, 100 mM ARG and 5 mM glucose) was employed and compared to the effects of DMEM (called HA/HG here to signify high arginine/high glucose). We compared the effects of the two culture media on Ea.hy926 cells under three ARG-exposure conditions. All cells were cultured for 7 days with the indicated medium, and washed with Locke’s solution before an experimentation. “Acute” treatment denoted exposure to additional 50 μM ARG in Locke’s solution for 2 h while “control” treatment denoted cells that were exposed to Locke’s solution alone for 2 h. “Continuous” treatment denoted cells that have been exposed to an additional 50 μM ARG for 7 days in the two media, washed, and then exposed to 50 μM ARG in Locke’s solution for 2 h.
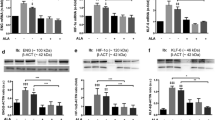
Figure 1a shows that when HA/HG medium was used, acute ARG exposure did not produce a significant increase in nitrite accumulation versus control cells, while cells cultured in LA/LG medium elicited a significant response. Continuous ARG exposure reduced nitrite accumulation from these cells when challenged with 50 μM ARG. Similar results was obtained when combined nitrite and nitrate accumulations were measured (Fig. 1b). The expression of eNOS protein was significantly suppressed by the HA/HG medium when compared to the LA/LG medium under the three ARG treatment conditions and continuous ARG exposure for 7 days led to significant decrease in eNOS expression with both culture media (Fig. 1c). The number of copies of eNOS mRNA, however, was not altered (Fig. 1d). Increased glucose accumulation in the Locke’s solution was observed after both acute and continuous ARG exposure in both culture media, when compared to control treatment (Fig. 1e). This increase appeared to be gradual and continuous over the 7 days of continuous ARG exposure (Fig. 1f).
Comparison of the effects of two incubation media LA/LG versus HA/HG on various responses of Ea.hy.926 cells after challenge by 50 μM ARG in Locke’s solution for 2 h (“acute” and “continuous”) versus by vehicle control. “Acute” denoted cells that were not pre-exposed to ARG while “continuous” denoted cells that were pre-exposed to 100 μM ARG for 7 days followed by washing of cells. Responses were presented as follows: a nitrite accumulation, b nitrite and nitrate accumulation, c eNOS protein expression, d eNOS mRNA expression, e glucose accumulation. f The time-dependent glucose accumulation during the 7-day exposure period during “continuous” exposure. N = 6 replicates. *p < 0.05 versus control; # p < 0.05 between LA/LG versus HA/HG. Similar comparable results were also obtained with HUVEC cells (data not shown)
Superoxide production after continuous exposure to ARG
The role of oxidative stress in mediating the effects of continuous ARG exposure was examined. Exploratory studies showed that Ea.hy926 cells continually exposed to 100 μM ARG for 7 days exhibited increase DHE fluorescence in cell lysates (control 610 ± 142 AU versus ARG exposed 1,621 ± 261 AU, p < 0.05). This elevated O ·−2 accumulation was reproducible in HUVEC (Fig. 2, control), with much higher sensitivity (control 508 ± 131 AU versus ARG exposed 1,764 ± 166 AU, p < 0.05). This increase was abolished by 100 μM ascorbic acid, 30 μM l-NAME, 300 U/ml PEG-SOD, 100 μM l-lysine, and 30 μM of the gluconeogenesis inhibitor 5-CDB; but not with 300 U/ml SOD.
Effects of continuous ARG exposure on eNOS expression and function, and glucose accumulation
Further studies were conducted to examine how some of these agents, such as l-NAME, ascorbic acid, PEG-SOD and 5-CDB might interfere with the ARG-induced changes in eNOS expression and function, as well as glucose accumulation in both Ea.hy926 and HUVEC cells. Table 1 shows that after cells were exposed to 100 μM ARG for 7 days, eNOS protein expression was significantly reduced in both cell lines (treatments A and E). Addition of 30 μM l-NAME (treatment B) did not reverse eNOS protein down-regulation exerted by continuous ARG exposure, while 100 μM ascorbic acid was able to do so (treatment C). Co-incubation with 30 μM 5-CDB appeared to up-regulate eNOS, and this effect was observed with both endothelial cell lines (treatments D and F). Addition of 300 U/ml of PEG-SOD (treatment G) improved eNOS activity and regulated glucose accumulation with no alteration in eNOS protein expression.
eNOS function was determined by combined nitrite/nitrate production upon challenge with 100 μM ARG for 2 h. Continuous ARG exposure for 7 days rendered cells “tolerant” to fresh exposure to ARG in Ea.hy926 cells (treatment A) and HUVEC (treatment E). Co-incubation with l-NAME (treatment B) expectedly reduced the accumulation of cellular nitrite/nitrate to below detection. Co-incubation with 100 μM ascorbic acid (treatment C) enhanced eNOS function, as did co-incubation with 5-CDB in both cell lines (treatments D and F).
Elevated glucose accumulation was observed after 7-day exposure to ARG in both Ea.hy926 cells and HUVEC (treatments A and E). l-NAME (treatment B) was unable to reverse this effect, while ascorbic acid (treatment C) was able to do so. Co-incubation with the gluconeogenesis inhibitor 5-CDB (treatments D and F) did not alter glucose accumulation in control cells, but decreased glucose generation in response to 100 μM ARG exposure for 2 h.
Effect of superoxide scavenging on eNOS activity and ARG uptake
To confirm that ARG-associated superoxide production results in diminished eNOS cellular activity, we co-incubated the cell-permeant PEG-SOD (300 U/ml) with 15N4-ARG for 2 h in HUVEC cells. PEG-SOD was used because preliminary data showed that the cell-inpermeant SOD itself did not affect cellular nitrite accumulation (data not shown). Figure 3a shows that the presence of PEG-SOD significantly enhanced cellular nitrite production at 15N4-ARG substrate concentrations above 50 μM. However, PEG-SOD did not increase 15N4-ARG uptake (Fig. 3b), and no difference was observed in the cellular contents of ARG, l-citrulline, asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) (data not shown). This observation indicated that the increased nitrite accumulation in the presence of PEG-SOD was not due to increased ARG bioavailability. In this short-term experiment, 15N4-ARG did not significantly affect eNOS expression (data not shown).
Arginase activity after acute or chronic ARG exposure
We measured arginase activity after acute and chronic 100 μM ARG exposure, and found that they were decreased versus control (acute treatment 1.07 ± 0.11 units/L, chronic 1.49 ± 0.18 units/L, each different from the control value of 2.61 ± 0.32 units/L, p < 0.002). Thus, increase in arginase activity cannot be a mechanism for the observed ARG tolerance.
Comparison of cellular fluxes of ARG and its related amino acids after either acute or chronic ARG exposure
Under acute exposure conditions, cells stimulated by 100 μM 15N4-ARG showed an expected influx of 15N4-ARG into the cell when compared to control (i.e., Locke’s solution only), as demonstrated by increased cell lysate 15N4-ARG (Table 2a). This influx caused a displacement and efflux of endogenous (unlabelled) ARG into the incubation medium through trans-stimulation. A decrease in cellular ADMA concentration was also noted when cells were stimulated by 15N4-ARG, presumably also due to trans-stimulated efflux. On the other hand, the concentrations of SDMA were not altered. Cellular unlabelled citrulline concentrations appeared to decrease after 15N4-ARG stimulation versus control. However, preliminary studies suggested that this decrease might have been compensated by increased 15N3-citrulline presence (data not shown).
Under chronic ARG-exposure conditions (Table 2b), cells stimulated by 100 μM 15N4-ARG showed patterns of flux behavior of ARG and its related amino acids that were similar to those observed under acute exposure conditions (Table 2a). These results indicate that cellular ARG-tolerance development was not due to decreased ARG influx or increased ADMA presence.
Discussion
Two recent long-term studies have shown that 6-month ARG supplementation produced a loss of its beneficial effects in peripheral artery disease (Wilson et al. 2007) and after acute myocardial infarction (Schulman et al. 2006). However, the mechanisms underlying these findings have not been identified. Because long-term human studies require substantial efforts and resources to carry out, it is of interest to develop in vitro cellular models to enable exploration of the possible mechanisms that may contribute to this phenomenon.
In the present study, we have identified the usefulness of a LA/LG medium in enhancing the sensitivity of two human endothelial cell lines to respond to added ARG in an in vitro system. Using these conditions, we showed that cellular tolerance toward the NO-generating effects of ARG could be demonstrated within one week of exposure to ARG at 100 μM, the higher end of its physiological range. The availability of this cellular model has permitted initial mechanistic studies to be carried out to identify possible contributing factors to provide guidance for future long-term human studies. Based on these studies, we showed that ARG supplementation in the cellular model led to oxidative stress, which was accompanied by cellular eNOS down-regulation and glucose accumulation. These findings therefore provide possible hints for future studies regarding the mechanisms that underlie the phenomenon of ARG tolerance in humans.
Both HUVEC and Ea.hy926 cells are of human origin, commercially available and widely used in experimentation. Expression of eNOS and ARG transport system exist in both cell lines (Sala et al. 2002). Literature studies of ARG effects using cell cultures have not been highly instructive, because the NO response to extracellular ARG was poor, even at high ARG concentrations (Kakoki et al. 2006). We showed (Fig. 1) that the traditional cell culture medium used (i.e., DMEM), contains high concentrations of both ARG (400 μM) and d-glucose (25 mM) which reduced the sensitivity of these cells toward in vitro ARG exposure. Employment of a LA/LG medium (100 μM ARG and 5 mM glucose) increased the sensitivity of the two human endothelial lines to produce NO (using the surrogate measure of nitrite and total nitrite/nitrate) upon in vitro ARG exposure (Fig. 1a, b).
Under these conditions, acute ARG exposure at 50 μM for 2 h produced a trend of decreased eNOS expression (Fig. 1c) but statistical significance was not observed. However, continuous exposure of cultured cells to 50–100 μM ARG for 7 days significantly suppressed the expression of eNOS, with no change in the number of copies of eNOS mRNA (Fig. 1c, d). This result suggests that, in this in vitro model, continuous ARG exposure induced post-translation modification of eNOS rather than its genetic expression. The relevance of this finding to the in vivo system is presently unknown, but is interesting to speculate that the eNOS-NO generating process may be regulated by its own substrate, when excess ARG is available and when its oxidative effects are not removed or overcome.
We showed that in vitro ARG exposure to endothelial cells resulted in glucose accumulation, under both acute and continuous situations, and in both culture media (Fig. 1e). This increased glucose accumulation appeared to be continuous in nature and a steady increase was observed over the study period (Fig. 1f). The induction of glucose accumulation by ARG has been shown in several in vivo studies. For example, Mehta et al. (1995) showed that a 30-min ARG infusion at 500 mg/kg in patients with pulmonary hypertension increased blood glucose from 7.1 to 9.3 mmol/L (p < 0.01). Hasselblatt et al. (2006) showed that an ARG challenge dose at 30 g, infused over 30 min, induced transient increases in glucose in capillary blood, but this increase was blunted in abstinent alcoholics. Trabelsi et al. (1995) demonstrated that intraperitoneal dosing of ARG (at 1 g/kg) increased plasma glucose concentration transiently in hepatic vagotomized and sham-treated rats.
We observed that ARG-induced eNOS down-regulation and glucose accumulation in this in vitro system were accompanied by excess O ·−2 generation (Fig. 2) after continuous ARG exposure. This effect was dependent on the interaction of ARG with eNOS because it was abolished by the classical eNOS inhibitor l-NAME and by the ARG cellular transport competitor l-lysine. Presence of antioxidants such as ascorbic acid and PEG-SOD (but not SOD) prevented ARG-induced O ·−2 accumulation. The phenomena of increased accumulation of glucose and O ·−2 appeared to be related to each other, since the co-incubation of the gluconeogenesis inhibitor 5-CDB abolished glucose accumulation (Table 1) as well as O ·−2 generation (Fig. 2).
Literature evidence indicates that extracellular, not intracellular, ARG is primarily responsible for activating eNOS to produce NO (Hallemeesch et al. 2002; Hardy and May 2002; Luiking et al. 2010; Rajapakse and Mattson 2009; Zani and Bohlen 2005; Shin et al. 2011b). Our results indicate while short-term co-incubation of PEG-SOD with ARG enhanced nitrite accumulation (Fig. 3a), but it had little effect on 15N4-ARG cellular influx (Fig. 3b). Additional data obtained in our laboratory indicated that continuous cellular exposure to ARG for 7 days also did not affect ARG uptake and ADMA displacement (Table 2). These findings further support the view that in vitro cellular ARG tolerance did not result from a modulation of ARG cellular uptake and availability.
The inter-relationship between cellular eNOS expression, nitrite/nitrate production and glucose accumulation was further examined by co-incubation with l-NAME, ascorbic acid and 5-CDB in cells that have been continuously exposed to ARG for 7 days (Table 1). Cellular ARG tolerance (toward a challenge dose of 100 μM ARG for 2 h) was demonstrated by reduced nitrite/nitrate accumulation in both human endothelial cell lines (treatments A and E), which exhibited both eNOS down-regulation and glucose accumulation. The antioxidant ascorbic acid (at 100 μM) reversed all of these effects, consistent with the view that cellular ARG tolerance is mediated by oxidative stress. Interestingly, co-incubation with l-NAME abolished the NO response (as expected), but was unable to prevent the effects of continuous ARG on eNOS down-regulation and increased glucose accumulation. This result suggests that these effects were not entirely dependent on the presence of generated NO. Intriguingly, incubation with the FBPase inhibitor 5-CDB over 7 days not only reduced glucose accumulation in both cells, but it was able to increase cellular eNOS expression and activity (i.e., nitrite/nitrate accumulation). Thus, cellular glucose concentrations appear to influence eNOS function intimately.
The observed glucose accumulation from continuous ARG exposure could alter the intracellular redox-state of cultured cells in several ways (1) by increasing pro-oxidant enzyme activity, thus increasing O ·−2 generation (Srinivasan et al. 2004), (2) by propagating free-radical production (Srinivasan et al. 2004), and (3) by forming mitochondrial derived reactive oxygen species (Mabile et al. 1997; Nishikawa et al. 2000). Hence, when produced under high glucose accumulation (Liu et al. 1997), O ·−2 could modulate the activity of eNOS. Excessive vascular O ·−2 production has been observed in disease states associated with endothelial dysfunction, including hypercholestrmia (Ohara et al. 1993) and diabetes (Tesfamariam and Cohen 1992). Additionally, hyperglycemia involvement in controlling eNOS post-translational modification has also been documented (Du et al. 2001).
As a probe to modify cellular glucose metabolism, we selected the FBPase inhibitor 5-CDB not only because FBPase is a rate-controlling enzyme within the gluconeogenesis pathway, but also because it functions only within the gluconeogenesis pathway, unlike the other two rate limiting enzymes, phosphoenolpyruvate carboxykinase and glucose 6-phosphatase (Granner and Pilkis 1990; Pilkis and Granner 1992). Moreover, adults who are genetically deficient in FBPase activity exhibit relatively normal clinical profiles provided they control their diet and avoid prolonged fasting (Gitzelmann 1995; Gitzelmann and Bosshard 1995). This literature finding could attribute a plausible reason as to why our control cells (i.e., incubation with 5-CDB only) did not show a significant decrease in glucose accumulation.
We believe that the methods used in the present study have established an in vitro cellular model to examine the phenomenon of ARG tolerance. We showed that cells exposed to physiologically relevant concentrations of ARG in vitro manifest oxidative stress, which may be a causative factor for its loss of therapeutic effects upon long-term supplementation in humans (Schulman et al. 2006; Wilson et al. 2007). Further studies to examine the relevance of the current findings in vivo appear warranted.
References
Bednarz K (2004) Am Ende der Welt: eine Reise durch Feuerland und Patagonien. 1. Aufl. edn. Rowohlt, Berlin
Bednarz B, Jaxa-Chamiec T, Maciejewski P, Szpajer M, Janik K, Gniot J, Kawka-Urbanek T, Drozdowska D, Gessek J, Laskowski H (2005) Efficacy and safety of oral l-arginine in acute myocardial infarction. Results of the multicenter, randomized, double-blind, placebo-controlled ARAMI pilot trial. Kardiol Pol 62(5):421–427
Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M (2001) Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 108(9):1341–1348
Gitzelmann R (1995) Galactose-1-phosphate in the pathophysiology of galactosemia. Eur J Pediatr 154(7 Suppl 2):S45–S49
Gitzelmann R, Bosshard NU (1995) Partial deficiency of galactose-1-phosphate uridyltransferase. Eur J Pediatr 154(7 Suppl 2):S40–S44
Granner D, Pilkis S (1990) The genes of hepatic glucose metabolism. J Biol Chem 265(18):10173–10176
Ha YH, Milner JA (1979) Urea cycle operation in the arginine deficient rat. Biochem Med 22(2):149–155
Hallemeesch MM, Lamers WH, Deutz NE (2002) Reduced arginine availability and nitric oxide production. Clin Nutr 21(4):273–279
Hardy TA, May JM (2002) Coordinate regulation of l-arginine uptake and nitric oxide synthase activity in cultured endothelial cells. Free Radic Biol Med 32(2):122–131
Hasselblatt M, Krampe H, Jacobs S, Sindram H, Armstrong VW, Hecker M, Ehrenreich H (2006) Arginine challenge unravels persistent disturbances of urea cycle and gluconeogenesis in abstinent alcoholics. Alcohol Alcohol 41(4):372–378
Kakoki M, Kim HS, Edgell CJ, Maeda N, Smithies O, DL M (2006) Amino acids as modulators of endothelium-derived nitric oxide. Am J Physiol Renal Physiol 291:297–304
Liu P, Hock CE, Nagele R, Wong PY (1997) Formation of nitric oxide, superoxide, and peroxynitrite in myocardial ischemia-reperfusion injury in rats. Am J Physiol 272(5 Pt 2):H2327–H2336
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Luiking YC, Engelen MP, Deutz NE (2010) Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care 13(1):97–104
Mabile L, Meilhac O, Escargueil-Blanc I, Troly M, Pieraggi MT, Salvayre R, Negre-Salvayre A (1997) Mitochondrial function is involved in LDL oxidation mediated by human cultured endothelial cells. Arterioscler Thromb Vasc Biol 17(8):1575–1582
Maxwell AJ, Zapien MP, Pearce GL, MacCallum G, Stone PH (2002) Randomized trial of a medical food for the dietary management of chronic, stable angina. J Am Coll Cardiol 39(1):37–45
Mehta S, Stewart DJ, Langleben D, Levy RD (1995) Short-term pulmonary vasodilation with l-arginine in pulmonary hypertension. Circulation 92(6):1539–1545
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404(6779):787–790
Ohara Y, Peterson TE, Harrison DG (1993) Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91(6):2546–2551
Oka RK, Szuba A, Giacomini JC, Cooke JP (2005) A pilot study of l-arginine supplementation on functional capacity in peripheral arterial disease. Vasc Med 10(4):265–274
Pilkis SJ, Granner DK (1992) Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol 54:885–909
Popovic PJ, Zeh HJ 3rd, Ochoa JB (2007) Arginine and immunity. J Nutr 137(6 Suppl 2):1681S–1686S
Rajapakse NW, Mattson DL (2009) Role of l-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol 36(3):249–255
Romero JR, Suzuka SM, Nagel RL, Fabry ME (2002) Arginine supplementation of sickle transgenic mice reduces red cell density and Gardos channel activity. Blood 99(4):1103–1108
Sala R, Rotoli BM, Colla E, Visigalli R, Parolari A, Bussolati O, Gazzola GC, Dall’Asta V (2002) Two-way arginine transport in human endothelial cells: TNF-alpha stimulation is restricted to system y(+). Am J Physiol Cell Physiol 282(1):C134–C143
Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, Hare JM, Gerstenblith G (2006) l-arginine therapy in acute myocardial infarction: the vascular interaction with age in myocardial infarction (VINTAGE MI) randomized clinical trial. JAMA 295(1):58–64
Shin S, Fung SM, Mohan S, Fung HL (2011a) Simultaneous bioanalysis of l-arginine, l-citrulline, and dimethylarginines by LC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci 879(7–8):467–474
Shin S, Mohan S, Fung HL (2011b) Intracellular l-arginine concentration does not determine NO production in endothelial cells: implications on the Arginine Paradox. Biochem Biophys Res Commun (in press). doi:10.1016/j.bbrc.2011.09.112
Srinivasan S, Hatley ME, Bolick DT, Palmer LA, Edelstein D, Brownlee M, Hedrick CC (2004) Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia 47(10):1727–1734
Tesfamariam B, Cohen RA (1992) Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol 263(2 Pt 2):H321–H326
Trabelsi F, Helie R, Bergeron R, Lavoie JM (1995) Effect of inhibition of gluconeogenesis on arginine-induced insulin secretion. Physiol Behav 57(4):797–802
Vichinsky E (2002) New therapies in sickle cell disease. Lancet 360(9333):629–631. doi:10.1016/S0140-6736(02)09776-3
Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP (2007) l-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation 116(2):188–195
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336(Pt 1):1–17
Zani BG, Bohlen HG (2005) Transport of extracellular l-arginine via cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am J Physiol Heart Circ Physiol 289(4):H1381–H1390
Zhao HT, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B (2003) Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34(11):1359–1368
Zhou M, Martindale RG (2007) Arginine in the critical care setting. J Nutr 137(6 Suppl 2):1687S–1692S
Acknowledgments
We thank Daniel Brazeau and Kathleen Boje for access to their genomic and cell culture facility, respectively. This work was supported in part by NIH grant HL081580.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohan, S., Wu, CC., Shin, S. et al. Continuous exposure to l-arginine induces oxidative stress and physiological tolerance in cultured human endothelial cells. Amino Acids 43, 1179–1188 (2012). https://doi.org/10.1007/s00726-011-1173-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1173-y