Abstract
Carnosine is present in high concentrations in skeletal muscle where it contributes to acid buffering and functions also as a natural protector against oxidative and carbonyl stress. Animal studies have shown an anti-diabetic effect of carnosine supplementation. High carnosinase activity, the carnosine degrading enzyme in serum, is a risk factor for diabetic complications in humans. The aim of the present study was to compare the muscle carnosine concentration in diabetic subjects to the level in non-diabetics. Type 1 and 2 diabetic patients and matched healthy controls (total n = 58) were included in the study. Muscle carnosine content was evaluated by proton magnetic resonance spectroscopy (3 Tesla) in soleus and gastrocnemius. Significantly lower carnosine content (−45%) in gastrocnemius muscle, but not in soleus, was shown in type 2 diabetic patients compared with controls. No differences were observed in type 1 diabetic patients. Type II diabetic patients display a reduced muscular carnosine content. A reduction in muscle carnosine concentration may be partially associated with defective mechanisms against oxidative, glycative and carbonyl stress in muscle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperglycemia results in exacerbated oxidative stress and glycation, both responsible for the harmful lipid and protein glycoxidation products such as 4-hydroxynonenal (HNE), advanced glycation end products (AGE’s) and protein carbonyls. Carnosine (β-alanine-l-histidine) is a dipeptide predominantly and abundantly found in skeletal muscle where it contributes to pH regulation over the physiological pH-transit range (Artioli et al. 2010; Derave et al. 2010; Sale et al. 2010). In vitro studies indicate that carnosine also has the potential to act as a scavenger of reactive oxygen species, reactive aldehydes and ketones, protein carbonyls, and inflammatory species (Boldyrev 2006). The consequence of this is a lowering in the carnosine concentration in plasma and in retinal, renal and diaphragm tissue, as it was demonstrated in diabetic rodent models (Buse et al. 1980; Pfister et al. 2011; Riedl et al. 2011; Sauerhofer et al. 2007).
The observation of carnosine deficiency in animal models of diabetes has directed research towards the therapeutical strategy of oral carnosine administration against the development of diabetes and its complications (Aldini et al. 2011; Lee et al. 2005; Pfister et al. 2011; Riedl et al. 2011; Sauerhofer et al. 2007). There is evidence showing in db/db mice (i.e., a type 2 diabetes model) supplemented with l-carnosine that fasting plasma glucose as well as HbA1c levels rise significantly later and remain lower. It has been demonstrated that l-carnosine administration also attenuates the development of insulin resistance in obese Zucker rats (Aldini et al. 2011; Sauerhofer et al. 2007).
Recently, carnosinase (CN-1) expression (i.e., the carnosine-degrading enzyme) has been implicated in the progression of diabetes and the etiology of its complications. Overexpression of CNDP1, the gene encoding CN-1, leads to early onset of diabetes in db/db mice. In humans, a polymorphism in the gene CNDP1 resulting in increased CN-1 activity has been associated with diabetic complications in type 2 diabetes patients. However, controversy exists about the role of carnosine/carnosinase in type 1 diabetic patients (Janssen et al. 2005; Mooyaart et al. 2010).
In humans, carnosine is almost undetectable in serum due to the presence of a highly active serum carnosinase (Harris et al. 2006), and over 99% of the body store is found in skeletal muscle (Boldyrev 2006). As a result it is possible that the muscle carnosine store is an important element in the role of carnosine in the development of diabetes and its complications. However, there are no data available on the changes in skeletal muscle carnosine content in diabetic patients.
Material and methods
This is a prospective, case–control, two-center study. 15 patients with type 1 diabetes and 15 matched control subjects were studied in Ghent (Belgium). 14 patients with type 2 diabetes and 14 matched controls were studied in Sao Paulo (Brazil). None of the subjects were vegetarians. Muscle carnosine content was evaluated in type I fibre- and type II fibre-predominant lower leg muscles (i.e., soleus and gastrocnemius, respectively) using a 3 Tesla non-invasive proton magnetic resonance spectroscopy (1H-MRS) technique. Carnosine data were calculated based on the C2-peak of carnosine and expressed relative to the water signal, as described previously by our group (Baguet et al. 2009). Type 2 diabetic patients (n = 14; gender: 8 females, 6 males; age: 60 ± 6 years; disease duration: 7 ± 3 years; HbA1c: 7.5 ± 0.3%; BMI: 32 ± 1 kg/m2) were compared with control subjects (n = 14) matched for age, BMI, gender and diet. Type 1 diabetic patients (n = 15; gender: male; age: 35 ± 7 years; disease duration; 18 ± 8 years, HbA1c: 7.4 ± 0.9%; BMI: 24 ± 2 kg/m2) were compared with age-, BMI- and gender-matched healthy controls (n = 15). Data from each center were evaluated by a Student’s t-test and expressed as mean ± SD. The significance level was set at P < 0.05. The study protocols were approved by the Ethical Committees of the Universities of Sao Paulo and Ghent.
Results
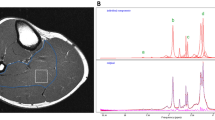
Gastrocnemius and soleus carnosine contents were not different between type 1 diabetes patients and controls (Fig. 1). In contrast, diabetic type 2 patients presented significantly lower carnosine content in gastrocnemius (−45%, P = 0.004; diabetics: 0.054 ± 0.015 vs. controls: 0.098 ± 0.039 a.u.), but not in soleus (diabetics: 0.056 ± 0.030 vs. controls: 0.050 vs. 0.026 a.u.; P = 0.63), when compared with the controls (Fig. 2).
Discussion
The current study showed for the first time that there is a reduced muscle carnosine content in diabetic patients, which seems to be confined to type-2 diabetes and fast-twitch muscle. As skeletal muscle is one of the largest organs and has by far the largest carnosine concentration in the body then it is likely that the whole body carnosine reserve is reduced in type-2 diabetes.
A possible explanation for the observation in the present study of a reduced muscle carnosine content was that this was already present in these individuals before the development of diabetes and therefore possibly facilitated the development of insulin resistance and type 2 diabetes, as has been suggested in animal studies (Aldini et al. 2011; Sauerhofer et al. 2007). However, in contrast to these findings, Kim (2009) shown that older (mean age 60.7 years) male Korean patients with impaired glucose tolerance, which is a status preceding diabetes, had the same muscle carnosine content in the vastus lateralis as younger trained and healthy Korean subjects. Therefore, a more likely explanation is that muscle carnosine was decreased as a result of the diabetic disease characteristics, such as the insulin resistance, hyperglycemia and oxidative and carbonyl stress. In vitro studies have indicated that carnosine acts as a sacrificial peptide and it is able to inhibit the generation of and to quench cytotoxic products, such as HNE, protein carbonyls and AGEs (Boldyrev 2006). In order to affect the muscle carnosine level, such effects would have to be accumulative over time and would need to exceed the capacity of the muscle fibres to resynthesize carnosine from beta-alanine synthesized in the liver or obtained through the diet.
Given that the carnosine content is much higher in type II compared to type I fibres, then it is possible that some of the disparity found in gastrocnemius muscle between type 2 diabetics and controls could be secondary to a shift towards a higher type I fibre composition. However, this seems unlikely as studies in vastus lateralis point to an increase, rather than a decrease, in type II fibres in type 2 diabetes (Oberbach et al. 2006). A decline in the availability in type 2 diabetics of beta-alanine from the diet or from endogenous synthesis in the liver from uracil, which is limiting to carnosine synthesis in muscle, could be a further factor but in this case should have affected values in both muscle groups.
The reduction in carnosine in type 2 diabetic patients was observed only in gastrocnemius, of which the majority of fibers are fast-twitch type II fibers. A decline in the levels of carnosine in muscle of elderly people, which was also restricted to type II fibres, has previously been reported by Tallon et al. in patients diagnosed with osteoarthritis of the knee (Tallon et al. 2007).
Our data suggest that the muscle carnosine storage is a limited pool of anti-cytotoxic protectors, which can decline under specific pathological conditions where oxidative stress and glycation are exacerbated. Supposedly, any such decrease in muscle carnosine may place the patient at a greater risk for the further development and progression of diabetic complications (or even diabetes per se). In these cases where reduced muscle carnosine may imply a greater risk for diabetes progression and/or complications, simple changes in the diet [i.e., increasing the intake of foods that are rich in beta-alanine, the rate-limiting factor for carnosine synthesis, or the introduction of an oral supplementation of beta-alanine (Harris et al. 2006)], could provide a therapeutic benefit in type 2 diabetes by restoring the carnosine reserve. This hypothesis, however, needs to be further confirmed.
A striking observation in the current study is that the decrease in muscle carnosine occurred only in type 2, but not type 1 diabetes. Oxidative stress-induced lipid and protein oxidation, as well as chronic low-grade inflammation are detrimental for the insulin signaling pathway in skeletal muscle, leading to peripheral insulin resistance, which is a hallmark of type 2, but not type 1 diabetes. The transsarcolemmal uptake of beta-alanine, the rate-limiting precursor of carnosine synthesis, is a sodium-dependent process (Bakardjiev and Bauer 1994). Insulin is known to stimulate the sarcolemmal Na+-K+-ATPase pump activity, and secondary to this the activity of sodium-dependent transporters. Thus, although no evidence is available in muscle at present, beta-alanine uptake and the resulting carnosine synthesis is potentially regulated by insulin action, as is the case with other amino acid transport systems in skeletal muscle (Stephens et al. 2006), which could be an alternative explanation why carnosine content is reduced in the insulin-resistant muscles of type 2 diabetic patients.
It is also likely that carnosine metabolism is different between the two disease types. Supporting this assumption, a reduced risk for diabetic nephropathy associated with the 5-allele of the CNDP1 gene, which results in lower serum carnosinase activity, has mainly been confirmed in type 2 but not in type 1 diabetes (Mooyaart et al. 2010).
In conclusion, we have shown that type 2 diabetic patients present markedly reduced carnosine levels in a type II fibre-predominant muscle. These data strengthen the emerging role of carnosine in the etiology of diabetes-associated complications and comorbidities and suggests that further investigations should focus on the origin of the decline in carnosine, the impact on carnosine metabolism in other tissues, and the possible therapeutic role of carnosine restoration in type 2 diabetes.
Abbreviations
- CN-1:
-
Carnosinase-1
- CNDP-1:
-
Carnosine dipeptidase-1 gene
- HNE:
-
4-Hydroxynonenal
- MRS:
-
Magnetic resonance spectroscopy
References
Aldini G, Orioli M, Rossoni G et al (2011) The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J Cell Mol Med 15:1339–1354
Artioli GG, Gualano B, Smith A, Stout J, Lancha AH Jr (2010) Role of beta-alanine supplementation on muscle carnosine and exercise performance. Med Sci Sports Exerc 42:1162–1173
Baguet A, Reyngoudt H, Pottier A et al (2009) Carnosine loading and washout in human skeletal muscles. J Appl Physiol 106:837–842
Bakardjiev A, Bauer K (1994) Transport of beta-alanine and biosynthesis of carnosine by skeletal muscle cells in primary culture. Eur J Biochem 225:617–623
Boldyrev AA (2006) Carnosine and oxidative stress in cells and tissues. Nova Science Publishers, New York
Buse MG, Weigand DA, Peeler D, Hedden MP (1980) The effect of diabetes and the redox potential on amino acid content and release by isolated rat hemidiaphragms. Metabolism 29:605–616
Derave W, Everaert I, Beeckman S, Baguet A (2010) Muscle carnosine metabolism and beta-alanine supplementation in relation to exercise and training. Sports Med 40:247–263
Harris RC, Tallon MJ, Dunnett M et al (2006) The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30:279–289
Janssen B, Hohenadel D, Brinkkoetter P et al (2005) Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 54:2320–2327
Kim HJ (2009) Comparison of the carnosine and taurine contents of vastus lateralis of elderly Korean males, with impaired glucose tolerance, and young elite Korean swimmers. Amino Acids 36:359–363
Lee YT, Hsu CC, Lin MH, Liu KS, Yin MC (2005) Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur J Pharmacol 513:145–150
Mooyaart AL, Valk EJ, van Es LA et al (2010) Genetic associations in diabetic nephropathy: a meta-analysis. Diabetologia 54:544–553
Oberbach A, Bossenz Y, Lehmann S et al (2006) Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29:895–900
Pfister F, Riedl E, Wang Q et al (2011) Oral carnosine supplementation prevents vascular damage in experimental diabetic retinopathy. Cell Physiol Biochem 28:125–136
Riedl E, Pfister F, Braunagel M et al (2011) Carnosine prevents apoptosis of glomerular cells and podocyte loss in STZ diabetic rats. Cell Physiol Biochem 28:279–288
Sale C, Saunders B, Harris RC (2010) Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino Acids 39:321–333
Sauerhofer S, Yuan G, Braun GS et al (2007) L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 56:2425–2432
Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL (2006) Insulin stimulates l-carnitine accumulation in human skeletal muscle. FASEB J 20:377–379
Tallon MJ, Harris RC, Maffulli N, Tarnopolsky MA (2007) Carnosine, taurine and enzyme activities of human skeletal muscle fibres from elderly subjects with osteoarthritis and young moderately active subjects. Biogerontology 8:129–137
Acknowledgments
This study was financially supported by grants from the Research Foundation—Flanders (FWO 1.5.149.08 and G.0046.09) and from Fundação de Amparo à Pesquisa do Estado de São Paulo (Grant numbers 2005/56464-9 and 2010/11221-0). The contribution of Johannes Ruige, Andries Pottier, Bert Celie, Melodie Arts, Koen De Meulenaer, Pieter Metsu, Vitor Painelli, and Rebeca Lugaresi is greatly acknowledged.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gualano, B., Everaert, I., Stegen, S. et al. Reduced muscle carnosine content in type 2, but not in type 1 diabetic patients. Amino Acids 43, 21–24 (2012). https://doi.org/10.1007/s00726-011-1165-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1165-y