Abstract
Hyperammonemia is considered to be the main cause of decreased levels of the branched-chain amino acids (BCAA), valine, leucine, and isoleucine, in liver cirrhosis. In this study we investigated whether the decrease in BCAA is caused by the direct effect of ammonia on BCAA metabolism and the effect of ammonia on BCAA and protein metabolism in different types of skeletal muscle. M. soleus (SOL, slow-twitch, red muscle) and m. extensor digitorum longus (EDL, fast-twitch, white muscle) of white rat were isolated and incubated in a medium with or without 500 μM ammonia. We measured the exchange of amino acids between the muscle and the medium, amino acid concentrations in the muscle, release of branched-chain keto acids (BCKA), leucine oxidation, total and myofibrillar proteolysis, and protein synthesis. Hyperammonemia inhibited the BCAA release (81% in SOL and 60% in EDL vs. controls), increased the release of BCKA (133% in SOL and 161% in EDL vs. controls) and glutamine (138% in SOL and 145% in EDL vs. controls), and increased the leucine oxidation in EDL (174% of controls). Ammonia also induced a significant increase in glutamine concentration in skeletal muscle. The effect of ammonia on intracellular BCAA concentration, protein synthesis and on total and myofibrillar proteolysis was insignificant. The data indicates that hyperammonemia directly affects the BCAA metabolism in skeletal muscle which results in decreased levels of BCAA in the extracellular fluid. The effect is associated with activated synthesis of glutamine, increased BCAA oxidation, decreased release of BCAA, and enhanced release of BCKA. These metabolic changes are not directly associated with marked changes in protein turnover. The effect of ammonia is more pronounced in muscles with high content of white fibres.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The branched-chain amino acids (BCAA), valine, leucine, and isoleucine, are essential amino acids which exert a specific regulatory effect on the rates of protein synthesis and degradation (Tischler et al. 1982; Nair and Short 2005). The decrease in plasma levels of these BCAA is a characteristic abnormality of patients with liver cirrhosis, and the decreased BCAA levels are considered an important pathogenic factor in hepatic encephalopathy and protein-energy malnutrition development (Iber et al. 1957; Fischer and Baldessarini 1971; Blonde-Cynober et al. 1994; Holecek et al. 1996a, b). These alterations in BCAA concentrations and their metabolic properties serve as the rationale for the recommendation to use the BCAA-supplemented diets in treatment of different forms of liver disease (Fischer et al. 1976; Marchesini et al. 2003; Nakaya et al. 2007; Urata et al. 2007).
The pathogenesis of BCAA deficiency in patients with chronic liver disease is not completely clear. Several factors have been proposed as the cause of decreased plasma BCAA levels, including hyperinsulinemia, hyperglucagonemia, catecholamines, and starvation (Soeters and de Boer 1984; Marchesini et al. 1979; Eriksson et al. 1982; Hayashi et al. 1981; Yamato et al. 1995). Now it appears that ammonia has a crucial role. Several studies have shown an inverse relationship between the plasma ammonia and BCAA concentrations and/or that ammonia infusion decreases BCAA levels both in blood and in tissues (Hayashi et al. 1981; Leweling et al. 1996; Yamato et al. 1995; Holecek et al. 2000b). It was suggested that skeletal muscle takes up ammonia from the blood and detoxifies it via the synthesis of glutamine from glutamate. BCAA deficiency may result from the intensified synthesis of glutamate from α-ketoglutarate and BCAA (Leweling et al. 1996). However, the direct evidence of the supposed effect of ammonia on BCAA metabolism in skeletal muscle is absent.
Considering the physiological functions and pharmacological properties of BCAA, the relationship between ammonia and the development of protein-energy malnutrition, characterized by loss of energy stores and by wasting of skeletal muscle, also seems important. The prevalence of cachexia in patients with liver cirrhosis is about 50%, its pathogenesis is not fully understood, and effective therapeutic possibilities are not known (Peng et al. 2007).
The main objective of this study was to test the hypothesis whether the reduction of BCAA levels in the extracellular fluid of cirrhotic patients is caused by a direct effect of hyperammonemia on the BCAA metabolism in muscle, excluding the effect of neurohumoral changes associated with hyperammonemia, such as alterations in glucose and insulin concentrations and changes in pH (Fernandez et al. 1988). Therefore, we studied the effect of ammonia on BCAA metabolism using the in vitro technique. We examined the effect of ammonia addition into the incubation medium on amino acid concentrations in skeletal muscle, on amino acid exchange between the muscle and the extracellular fluid, on BCAA oxidation, and on release of branched-chain keto acids (BCKA) from the muscle. To solve the question of possible effects of hyperammonemia on protein metabolism, the changes both in protein synthesis and proteolysis were examined. As demonstrated in several studies, muscles mostly composed of white (fast-twitch) fibres have different metabolic features compared to muscles mostly composed of red (slow-twitch) fibres (Hasselgren et al. 1986; Muthny et al. 2008). Concerning possible differences in BCAA metabolism between red and white skeletal muscle regarding ammonia, the higher BCAA aminotransferase activity may be of importance in the red muscle (Yang and Birkhahn 1997) and higher glutamine synthetase activity in the white one (Hickson et al. 1996). Therefore, our studies were performed in both types of skeletal muscle, in m. soleus (SOL, slow-twitch, red muscle) and in m. extensor digitorum longus (EDL, fast-twitch, red muscle).
Materials and methods
Animals
Male Wistar rats (body weight 40–60 g) obtained from BioTest, Konarovice, CZ, were used in this study. The rats were housed under controlled conditions (12-h light–dark cycle, 22°C, 55–65% relative humidity) with free access to standard laboratory chow and water. All procedures involving animal manipulation were performed in accordance with guidelines set by the Institutional Animal Use and Care Committee of Charles University, Prague, CZ.
Materials
l-[1-14C]leucine was purchased from GE Healthcare Life Sciences (Buckinghamshire, UK); amino acids, cycloheximide, Folin–Ciocalteu’s phenol reagent, N-succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (Suc-LLVY-MCA), and albumin were purchased from Sigma Chemical (St Louis, MO, US); Aminoplasmal 15 from B. Braun Medicals (Melsungen, DE). Hyamine hydroxide was obtained from Packard Instrument (Meriden, CT). The remaining chemicals were obtained from Sigma Chemicals (St Louis, MO, US), Waters (Milford, MA, US), and Lachema (Brno, CZ).
Experimental design
The animals were killed in pentobarbital narcosis (6 mg/100 g body weight, intraperitoneally) by exsanguination via abdominal aorta. Soleus (SOL) and extensor digitorum longus (EDL) muscles of both legs were dissected according to Maizels et al. (1977) and fixed via the tendons to stainless steel clips to provide slight tension (at approximately resting length), and immediately transferred into 2.5 ml of modified Krebs–Heinseleit bicarbonate buffer with 6 mM glucose and 2 mU/ml insulin (pH 7.4, 37°C). The medium was saturated with O2/CO2 (19:1).
The muscles were preincubated for 30 min in a thermostatically controlled bath (37°C) with a shaking device (70 cycles/min) to ensure stable intramuscular concentrations of components present in the medium. After the preincubation, the muscles were quickly rinsed in 0.9% NaCl, blotted and transferred to a second set of vials containing fresh incubation media enriched with an ammonium acetate/bicarbonate buffer to reach 500 μM ammonia in the medium (ammonia group) or sodium acetate/bicarbonate buffer. Muscles of the left leg were used for determination of the effect of ammonia, and muscles of the right leg served as controls. Other components present in the medium were dependent on the parameter measured as described below. The viability of the incubated muscles was previously confirmed in our laboratory (Safranek et al. 2003) as well as by other authors (Fang et al. 2005). Three separate studies were performed.
Study 1: the effect of hyperammonemia on amino acid exchange between the muscle and the medium, the release of BCKA, and amino acid concentration in muscle (18 animals)
The muscles were incubated for 2 h in the medium enriched with amino acids in approximately physiological concentrations. At the end of incubation, the muscles were quickly removed, rinsed and homogenized in 0.6 ml of 2% (v/v) perchloric acid and then centrifuged for 5 min at 12,000g. The supernatant was used for determination of free amino acid concentration in muscle. Results were expressed in μmol/g wet muscle.
The amino acid exchange and the BCKA release were calculated by the formula:
where C t120 and C t0 are amino acid concentrations in the medium at the end and at the beginning of the incubation, V is the volume of incubation medium, and t is the duration of incubation.
Amino acid concentrations were determined with liquid chromatograph (Waters, Milford, MA) after precolumn derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, BCKA with liquid chromatograph (Shimadzu, Kyoto, Japan) after precolumn derivatization with o-phenylenediamine (Kandar et al. 2009).
Study 2: the effect of hyperammonemia on proteolysis and chymotrypsin-like activity (15 animals)
The total and myofibrillar protein breakdown was estimated after a 2-h incubation of the muscle in a medium enriched with cycloheximide (0.5 mM), which prevented the reincorporation of the released amino acids into the proteins. Since tyrosine is neither synthesized nor degraded in skeletal muscle (Fulks et al. 1975), its release into the medium reflects total proteolysis. The release of 3-methylhistidine, a characteristic product of the myofibrillar breakdown, served for the myofibrillar proteolysis calculation. The calculation of the rates of amino acid release was based on their respective concentrations in the medium, the weight of the muscle, and time of the incubation. Tyrosine and 3-methylhistidine were determined via liquid chromatography after a precolumn derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (Cohen and Michaud 1993) or fluorescamine (Wassner et al. 1980).
The chymotrypsin-like activity of proteasome was determined using the fluorogenic substrate Suc-LLVY-MCA (Gomes-Marcondes and Tisdale 2002) as follows. The muscles were homogenized in 0.4 ml of ice-cold 20 mM Tris buffer, pH 7.5, containing 2 mM ATP, 5 mM MgCl2 and 1 mM dithiothreitol. The homogenates were centrifuged for 10 min at 18,000g at 4°C. The cellular supernatant (0.1 ml) was incubated with 0.1 ml of substrate Suc-LLVY-MCA (0.1 mM), with and without inhibitor MG132 (0.02 mM), for 1 h on ice. Adding 0.4 ml of 100 mM sodium acetate buffer of pH 4.3, stopped the reaction. The fluorescence of the samples was immediately determined at the excitation wavelength of 340 nm and the emission wavelength of 440 nm (Tecan InfiniteTM 200). The standard curve was established for 7-amino-4-methylcoumarin (AMC), which permitted the expression of CTLA as nmol of AMC/g protein per hour. The activity was adjusted for the protein concentration of the supernatant (Lowry et al. 1951). The differences after the subtraction of inhibited from non-inhibited activities were used for the calculations.
Study 3: the effect of hyperammonemia on leucine oxidation and protein synthesis (9 animals)
Leucine oxidation and protein synthesis rates were measured after 1-h incubation of the muscle in 2.5 ml of medium enriched with amino acids in approximately physiological concentrations and l-[1-14C]leucine (0.6 μCi/ml) (Carbó et al. 2000; Safranek et al. 2003).
Protein synthesis
The muscles were removed from the incubation flasks, quickly rinsed in cold 6% (v/v) perchloric acid, blotted, and homogenized in 0.6 ml of 6% (v/v) perchloric acid. The homogenate was centrifuged for 5 min at 12,000g. The supernatant was used for measurement of l-[1-14C]leucine radioactivity and leucine concentration to calculate muscle free leucine specific activity (SALeu). The precipitated pellet was washed three times by 2% (v/v) perchloric acid and then hydrolyzed in 1 M NaOH. Aliquots were taken for measurement of protein content (Lowry et al. 1951) and l-[1-14C]leucine radioactivity in muscle protein. SALeu and protein synthesis rates were calculated by the following formulas:
Leucine oxidation
At the end of the incubation period, 0.4 ml of hyamine hydroxide was added into the well hanging above the incubation medium, the reaction was stopped by the addition of 35% (v/v) perchloric acid solution (0.2 ml) into the incubation medium, and the flasks were shaken for 1 h to ensure complete absorption of 14CO2 into the hyamine hydroxide. The hanging wells containing hyamine were placed into counting vials containing 10 ml of scintillation mixture and counted in the liquid scintillation radioactivity counter LS 6000 (Beckman Instruments, CA, USA). The efficiency of 14CO2 recovery estimated by adding sodium[14C]bicarbonate to the medium was 96%. Therefore the recovery factor (FR) 0.96 was used for calculation of leucine oxidation by the formula:
Statistical analysis
The results are expressed as the mean ± SEM. F test followed by paired t test (to estimate the effect of ammonia on specific type of the muscle) and unpaired t test (to estimate the difference in sensitivity to ammonia between SOL and EDL) have been used for the analysis of the data. Differences were considered significant at P < 0.05. Statistical software NCSS 2001 (Kaysville, UT, US) was applied.
Results
Exchange of amino acids between the muscle and the medium
The enhanced release of glutamine and decreased release of BCAA, glutamate and alanine was observed from the muscles incubated under hyperammonemic conditions. The effect of ammonia on BCAA release was more pronounced in EDL (60% of controls) than in SOL (81% of controls) (Table 1).
Release of BCKA
Addition of ammonia into the incubation medium significantly enhanced the release of all three BCKA. The effect was more pronounced in EDL (161% of controls) than in SOL (133% of controls) (Table 2).
Leucine oxidation
Ammonia significantly stimulated the leucine oxidation in EDL (174% of controls). The effect in SOL was insignificant (Fig. 1).
Protein synthesis and proteolysis
Insignificant changes in leucine incorporation in muscle protein, in tyrosine and 3-MH release, and in CHTLA activity indicate that the incubation of skeletal muscle in the medium with high concentration of ammonia had no effect on protein synthesis and proteolysis. The higher values of SALeu in EDL are related to lower BCAA concentrations in EDL than in SOL. The effect of ammonia on SALeu was insignificant (Table 3).
Amino acid concentrations in muscle
The most impressive effect of incubation of isolated muscles in the medium with ammonia was a significant increase in glutamine concentration. Changes in the BCAA concentration were insignificant (Table 4).
Discussion
There are differences in relative distribution of BCAA aminotransferase and BCKA dehydrogenase, and in their activities in human and rat tissues (Khatra et al. 1977; Sweatt et al. 2004; Suryawan et al. 1998), and therefore the results should be interpreted cautiously. In a noteworthy study, Suryawan et al. (1998) demonstrated that some BCAA aminotransferase activity is present in human liver while almost absent in rat liver, and that human muscle contains approximately 54% of total BCKA dehydrogenase activity with only 13% in the liver, while in rats, 3% is found in the muscle and 83% in the liver. Aside from skeletal muscle and the liver, other organs, particularly the brain and kidneys, contribute significantly to whole-body BCAA oxidation.
The results obtained in this study clearly demonstrate that hyperammonemia induces marked alterations in the BCAA metabolism in skeletal muscle, resulting in the decrease in BCAA concentration in the extracellular fluid. The results strongly support the hypothesis that the mechanism by which hyperammonemia decreases the BCAA level is based on a simple biochemical regulation principle of enzymatic reactions, by alterations in reactant concentrations. The main changes are summarized in Fig. 2 and discussed below.
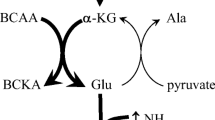
Hypothetical scheme of the effect of hyperammonemia on BCAA metabolism in skeletal muscle resulting in the decrease of BCAA concentration in extracellular fluid. Ammonia is in skeletal muscle detoxified by synthesis of glutamine from glutamate. The preferential source of nitrogen for synthesis of glutamate from α-ketoglutarate are the BCAA. The BCKA formed from BCAA in aminotransferase reaction are oxidized in skeletal muscle and/or released to the blood. The suggested mechanism of decreased net release of BCAA (or increased uptake from ECF) is their enhanced exchange with glutamine via L-transporter system. ECF the extracellular fluid, BCAA branched-chain amino acids, BCKA branched-chain keto acids, BCA-CoA branched-chain acyl-CoA, α-KG alpha-ketoglutarate, Glu glutamate, Gln glutamine, Ala alanine. 1 branched-chain aminotransferase, 2 branched-chain α-ketoacid dehydrogenase, 3 glutamine synthetase
Hyperammonemia enhances the conversion of BCAA to BCKA in muscle
The initial step in the BCAA catabolism, which occurs mostly in skeletal muscle due to a high activity of BCAA aminotransferase, involves the transfer of the amino group with α-ketoglutarate to form glutamate and BCKA. Glutamate can be converted to glutamine (in the presence of ammonia) or it can pass its amino group to pyruvate to form alanine. Since these reactions are reversible and near equilibrium, the principal regulatory mechanism of the BCAA aminotransferase are changes in the concentration of substrates and products of these reactions (Harper et al. 1984).
When hyperammonemia develops, the skeletal muscle takes up ammonia from the blood and detoxifies it by the synthesis of glutamine from glutamate in the reaction catalysed by glutamine synthetase. As BCAA are the main source of nitrogen for synthesis of glutamate from α-ketoglutarate, the consequence of activated synthesis of glutamine is the enhanced consumption of BCAA and the production of BCKA in BCAA aminotransferase reaction. The increase in BCAA aminotransferase activity in rat muscle by hyperammonemia was demonstrated by Hod et al. (1982).
As BCAA are known also as essential donors of nitrogen for synthesis of alanine from pyruvate, the increased demands for glutamate caused by hyperammonemia should decrease the alanine synthesis and release from the muscle. Therefore, the finding of inverse alteration in the concentrations of glutamine (increase) and alanine (decrease) under hyperammonemic conditions indicates, together with decreased release of BCAA and increased release of BCKA, the direct relationship among hyperammonemia, glutamine synthesis, the rate of BCAA aminotransferase reaction, and the BCAA consumption.
The higher increase in glutamine concentration in muscles incubated in medium with ammonia (around 2,000 nmol/g per hour) than can be accounted for BCAA losses (around 500 nmol/g per hour) demonstrates that other sources of glutamate participate in synthesis of glutamine. An important source is undoubtedly extracellular fluid, as indicated in the present study by decreased net release of glutamate in both muscles. We also assume the involvement of other sources of amino nitrogen in synthesis of glutamate and/or the shift from glutamate utilization in other reactions, such as synthesis of glutathione, to glutamine synthesis. Glutathione depletion has been observed both in hyperammonemia (Essa and Subramanian 2006) and other conditions of activated glutamine synthesis (Hammarqvist et al. 1997).
Hyperammonemia enhances both the BCKA release to the extracellular fluid and BCAA oxidation in muscle
The enhanced flux of BCAA through the BCAA aminotransferase reaction results in an increased production of BCKA, which can be oxidized in skeletal muscle or released to the extracellular fluid. In our study, both these pathways were found to have been activated, as demonstrated by the enhanced release of BCKA into the incubation medium, and by the stimulation of leucine oxidation.
The main cause of release of BCKA from skeletal muscle to the blood is the low activity of the BCKA dehydrogenase in muscle. These BCKA are then taken up and oxidized by tissues with high BCKA dehydrogenase activity, such as adipose tissue, liver and the heart (Harper et al. 1984), or reaminated to BCAA, particularly in the liver (Walser et al. 1973). The latter may occur in a condition of enhanced supply of glutamate or BCKA and/or in case of a lack of BCAA or alpha-ketoglutarate. The enhanced production of glutamate and activated resynthesis of BCAA may be associated with glutaminase reaction in the liver to provide ammonia for urea synthesis. This suggestion can be supported by finding a higher synthesis of BCAA from BCKA in the liver perfused by a medium with glutamine than by a glutamine-deficient medium (Holecek et al. 2003). We suppose that in case of severe hepatic injury, the conversion of BCKA to BCAA induced by the enhanced availability of glutamine, may be impaired and may be the additional mechanism decreasing the BCAA levels in cirrhotic patients.
The way in which hyperammonemia activates the BCAA oxidation might be explained by changes in the BCKA dehydrogenase reaction, which is the first irreversible and the rate-limiting step in the BCAA oxidation in skeletal muscle, and therefore commits the BCAA to degradation (Harper et al. 1984). The BCKAD is a multienzyme complex located on the inner surface of the inner mitochondrial membrane which is highly regulated by a phosphorylation (inhibition)–dephosphorylation (activation) mechanism. The BCKA dehydrogenase kinase, which is considered to be the key regulator of BCKA dehydrogenase activity (Harris et al. 2004), is allosterically inhibited by BCKA, particularly by α-ketoisocaproate (Schauder 1988; Brosnan and Brosnan 2006). Therefore the activation of BCKA dehydrogenase by the enhanced supply of BCKA (produced by activated aminotransferase reaction as described above), seems to be the main mechanism by which hyperammonemia enhances the BCAA oxidation.
Our measurements show that the ammonia-induced fall of BCAA release from muscle (around 500 nmol/g per hour) was higher than the increase in their oxidation and the release of BCKA from muscle. Considering insignificant changes in intracellular BCAA levels, the possible cause should be the increase in BCKA concentration in intracellular space and/or enhanced BCAA utilization in protein synthesis, although the increase in protein synthesis observed both in red and white muscle was insignificant.
Hyperammonemia affects transport of BCAA across the cell membrane
The decrease in BCAA levels in incubation medium and the enhanced release of BCKA and leucine oxidation in muscles incubated in hyperammonemic conditions were not associated with the decrease in BCAA levels in skeletal muscle. Considering unaffected rates of protein synthesis and proteolysis, the explanation should be sought in terms of alterations in amino acid transport across the cell membrane.
There are several transport systems with overlapping substrate specificity which may be involved. An example may be a System L, that serves for neutral amino acids. System L operates as an obligatory amino acid exchanger which can couple the uptake of BCAA with the efflux of cytoplasmic amino acids such as glutamine (Meier et al. 2002). Therefore, the enhanced efflux of glutamine could prevent intracellular depletion of BCAA via increase in the influx of BCAA from the extracellular fluid, in spite of their enhanced catabolism induced by hyperammonemia.
Effect of hyperammonemia on protein synthesis and proteolysis in muscle
In our experimental conditions we did not succeed in demonstrating that hyperammonemia affects protein synthesis, proteolysis, or BCAA concentration in muscle. We assume that this finding indicates that the fall of BCAA in extracellular fluid and enhanced concentrations of glutamine both in intracellular and extracellular space preceded the fall in intracellular BCAA levels. This suggestion is supported by observations of Leweling et al. (1996), who infused ammonium salts in rats for 2 or 6 h. Administration of ammonium brought about a decline in BCAA plasma concentrations after 2 h and in muscle after 6 h; although the increase of glutamine associated with the fall of glutamate and alanine was observed both in plasma and muscle already after 2 h.
Therefore, alterations in protein metabolism may develop later, and may be activated by humoral changes induced by ammonia, especially by alterations in leucine, BCKA, and glutamine concentrations. Leucine has a potent stimulatory effect on protein synthesis, probably via activation of translation initiation factors (Nair and Short 2005), BCKA have an inhibitory effect on proteolysis (Tischler et al. 1982), and glutamine probably has both a stimulatory effect on protein synthesis (Iresjo et al. 2005) and an inhibitory effect on proteolysis (Holecek et al. 2000a). These findings indicate that changes induced by hyperammonemia may in fact result in a decrease in muscle protein turnover rather than in an increase, and that the cause of any potential muscle wasting induced by ammonia should be a greater decrease in protein synthesis compared to the respective decrease in proteolysis.
Effect of hyperammonemia on the BCAA metabolism in muscle depends on the type of fibre
The results indicate that EDL (muscle composed of white, fast-twitch fibres, which derives energy mostly from anaerobic glycolysis and is adapted to short duration contraction movements) is more susceptible to the effect of hyperammonemia than SOL (muscle composed of red, slow-twitch fibres, with a greater capacity for aerobic metabolism and adapted to maintaining a relatively sustained contraction). This is clearly demonstrated not only by lower release of BCAA into medium, but also by higher release of BCKA, and higher increase in leucine oxidation in EDL than in SOL. We assume that the higher increase in BCAA utilization in white muscle may be due to higher glutamine synthetase activity, which, under hyperammonemic conditions enables more rapid consumption of glutamate and thus activates the flux of amino nitrogen through BCAA aminotransferase and BCKA dehydrogenase reactions. The physiological importance of this phenomenon may be related to higher capacity of fast-twitch muscle to produce ammonia during contraction (Terjung et al. 1985). When considering that red fibres are the major components of respiratory muscles (Polla et al. 2004), the finding that hyperammonemia affects groups of muscles with a higher proportion of white fibres may be also of clinical importance.
Conclusion
In conclusion, our study provides clear evidence that hyperammonemia activates the BCAA catabolism in skeletal muscle, which is associated with enhanced release of BCKA to the extracellular fluid, with increased BCAA oxidation in muscle, and with decreased BCAA concentration in extracellular fluid. These metabolic changes are not associated with marked changes in protein turnover. The effect of ammonia is more pronounced in muscles with high amount of white fibres.
Abbreviations
- BCAA:
-
Branched-chain amino acids
- BCKA:
-
Branched-chain keto acids
- BCA-CoA:
-
Branched-chain acyl-CoA
- α-KG:
-
Alpha-ketoglutarate
- Glu:
-
Glutamate
- Gln:
-
Glutamine
- Ala:
-
Alanine
- ECF:
-
Extracellular fluid
- SALeu :
-
Leucine-specific activity
References
Blonde-Cynober F, Plassart F, Rey C, Coudray-Lucas C et al (1994) Assessment of the carbon tetrachloride-induced cirrhosis model for studies of nitrogen metabolism in chronic liver disease. Ann Nutr Metab 38:238–248
Brosnan JT, Brosnan ME (2006) Branched-chain amino acids: enzyme and substrate regulation. J Nutr 136:207S–211S
Carbó N, Ribas V, Busquets S et al (2000) Short-term effects of leptin on skeletal muscle protein metabolism in the rat. J Nutr Biochem 11:431–435
Cohen SA, Michaud DP (1993) Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem 211:279–287
Eriksson LS, Hagenfeldt L, Wahren J (1982) Intravenous infusion of alpha-oxoisocaproate: influence on amino acid and nitrogen metabolism in patients with liver cirrhosis. Clin Sci (Lond) 62:285–293
Essa MM, Subramanian P (2006) Pongamia pinnata modulates the oxidant–antioxidant imbalance in ammonium chloride-induced hyperammonemic rats. Fundam Clin Pharmacol. 20:299–303
Fang CH, Li BG, James JH et al (2005) Protein breakdown in muscle from burned rats is blocked by insulin-like growth factor and glycogen synthase kinase-3beta inhibitors. Endocrinology 146:3141–3149
Fernandez JM, Croom WJ Jr, Johnson AD et al (1988) Subclinical ammonia toxicity in steers: effects on blood metabolite and regulatory hormone concentrations. J Anim Sci 66:3259–3266
Fischer JE, Baldessarini RJ (1971) False neurotransmitters and hepatic failure. Lancet 2:75–80
Fischer JE, Rosen HM, Ebeid AM et al (1976) The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 80:77–91
Fulks RM, Li JB, Goldberg AL (1975) Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem 250:290–298
Gomes-Marcondes MCC, Tisdale MJ (2002) Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett 180:69–74
Hammarqvist F, Luo JL, Cotgreave IA et al (1997) Skeletal muscle glutathione is depleted in critically ill patients. Crit Care Med 25:78–84
Harper AE, Miller RH, Block KP (1984) Branched-chain amino acid metabolism. Annu Rev Nutr 4:409–454
Harris RA, Joshi M, Jeoung NH (2004) Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun 313:391–396
Hasselgren PO, Talamini M, James JH et al (1986) Protein metabolism in different types of skeletal muscle during early and late sepsis in rats. Arch Surg 121:918–923
Hayashi M, Ohnishi H, Kawade Y et al (1981) Augmented utilization of branched-chain amino acids by skeletal muscle in decompensated liver cirrhosis in special relation to ammonia detoxication. Gastroenterol Jap 16:64–70
Hickson RC, Wegrzyn LE, Osborne DF, Karl IE (1996) Glutamine interferes with glucocorticoid-induced expression of glutamine synthetase in skeletal muscle. Am J Physiol 270:E912–E917
Hod G, Chaouat M, Haskel Y et al (1982) Ammonia uptake by skeletal muscle in the hyperammonaemic rat. Eur J Clin Invest 12:445–450
Holecek M, Mraz J, Tilser I (1996a) Plasma amino acids in four models of experimental liver injury in rats. Amino Acids 10:229–241
Holecek M, Tilser I, Skopec F et al (1996b) Leucine metabolism in cirrhotic rats. J Hepatol 24:209–216
Holecek M, Skopec F, Skalska H et al (2000a) Effect of alanyl-glutamine on leucine and protein metabolism in endotoxemic rats. JPEN J Parenter Enteral Nutr 24:215–222
Holecek M, Sprongl L, Tichy M et al (2000b) Effect of hyperammonemia on leucine and protein metabolism in rats. Metabolism 49:1330–1334
Holecek M, Rysava R, Safranek R et al (2003) Acute effects of decreased glutamine supply on protein and amino acid metabolism in hepatic tissue. A study using isolated perfused rat liver. Metabolism 52:1062–1067
Iber FL, Rosen H, Levenson SM et al (1957) The plasma amino acids in patients with liver failure. J Lab Clin Med 50:417–425
Iresjo BM, Svanberg E, Lundholm K (2005) Reevaluation of amino acid stimulation of protein synthesis in murine- and human-derived skeletal muscle cells assessed by independent techniques. Am J Physiol Endocrinol Metab 288:E1028–E1037
Kandar R, Zakova P, Jirosova J et al (2009) Determination of branched chain amino acids, methionine, phenylalanine, tyrosine and α-keto acids in plasma and dried blood samples using HPLC with fluorescence detection. Clin Chem Lab Med 47:565–572
Khatra BS, Rajender KC, Sewell CW, Rudman D (1977) Distribution of branched-chain α-keto acid dehydrogenases in primate tissues. J Clin Invest 59:558–564
Leweling H, Breitkreutz R, Behne F et al (1996) Hyperammonemia-induced depletion of glutamate and branched-chain amino acids in muscle and plasma. J Hepatol 25:756–762
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maizels EZ, Ruderman NB, Goodman MN et al (1977) Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem J 162:557–568
Marchesini G, Forlani G, Zoli M et al (1979) Insulin and glucagon levels in liver cirrhosis. Relationship with plasma amino acid imbalance of chronic hepatic encephalopathy. Dig Dis Sci 24:594–601
Marchesini G, Bianchi G, Merli M et al (2003) Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology 124:1792–1801
Meier C, Ristic Z, Klauser S, Verrey F (2002) Activation of system l heterodimeric amino acid exchangers by intracellular substrates. EMBO J 21:580–589
Muthny T, Kovarik M, Sispera L et al (2008) Protein metabolism in slow- and fast-twitch muscle during turpentine-induced inflammation. Int J Exp Pathol 89:64–71
Nair KS, Short KR (2005) Hormonal and signaling role of branched-chain amino acids. J Nutr 135:1547S–1552S
Nakaya Y, Okita K, Suzuki K, Hepatic Nutritional Therapy (HNT) Study Group et al (2007) BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition 23:113–120
Peng S, Plank LD, McCall JL et al (2007) Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr 85:1257–1266
Polla B, D’Antona G, Bottinelli R, Reggiani C (2004) Respiratory muscle fibres: specialisation and plasticity. Thorax 59:808–817
Safranek R, Holecek M, Kadlcikova J et al (2003) Effect of acute acidosis on protein and amino acid metabolism in rats. Clin Nutr 22:437–443
Schauder P (1988) Do branched chain keto amino acids regulate their own metabolism? Hepatology 8:1716–1718
Soeters PB, de Boer J (1984) Why are plasma branched chain amino acid levels diminished in patients with liver cirrhosis. In: Adibi SA, Fekl W, Langenbeck U, Schauder P (eds) Branched chain amino and keto acids in health and disease. Karger, Basel, pp 483–496
Suryawan A, Hawes JW, Harris RA et al (1998) A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr 68:72–81
Sweatt AJ, Wood M, Suryawan A et al (2004) Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab 286:E64–E76
Terjung RL, Dudley GA, Meyer RA (1985) Metabolic and circulatory limitations to muscular performance at the organ level. J Exp Biol 115:307–318
Tischler ME, Desautels M, Goldberg AL (1982) Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem 257:1613–1621
Urata Y, Okita K, Korenaga K et al (2007) The effect of supplementation with branched-chain amino acids in patients with liver cirrhosis. Hepatol Res 37:510–516
Walser M, Lund P, Ruderman NB, Coulter AW (1973) Synthesis of essential amino acids from their α-keto analogue by perfused rat liver and muscle. J Clin Invest 52:2865–2877
Wassner SJ, Schlitzer L, Li JB (1980) A rapid, sensitive method for the determination of 3-methylhistidine levels in urine and plasma using high-pressure liquid chromatography. Anal Biochem 104:284–289
Yamato M, Muto Y, Yoshida T (1995) Clearance rate of plasma branched-chain amino acids correlates significantly with blood ammonia level in patients with liver cirrhosis. Int Hepatol Commun 3:91–96
Yang Q, Birkhahn RH (1997) Branched-chain transaminase and keto acid dehydrogenase activities in burned rats: evidence for a differential adaptation according to sex. Nutrition 13:640–645
Acknowledgments
This study was supported by Research Project MSM 0021620820. We are grateful for the technical assistance of H. Buzkova and R. Rysava. Many thanks to Nishant Sunil Joshi from London, UK, for his assistance with revision of English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holecek, M., Kandar, R., Sispera, L. et al. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids 40, 575–584 (2011). https://doi.org/10.1007/s00726-010-0679-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0679-z