Abstract
The aim was to determine the effects of enhanced availability of branched-chain amino acids (BCAAs; leucine, isoleucine, and valine) on ammonia detoxification to glutamine (GLN) and protein metabolism in two types of skeletal muscle under hyperammonemic conditions. Isolated soleus (SOL, slow-twitch) and extensor digitorum longus (EDL, fast-twitch) muscles from the left leg of white rats were incubated in a medium with 1 mM ammonia (NH3 group), BCAAs at four times the concentration of the controls (BCAA group) or high levels of both ammonia and BCAA (NH3 + BCAA group). The muscles from the right leg were incubated in basal medium and served as paired controls. L-[1-14C]leucine was used to estimate protein synthesis and leucine oxidation, and 3-methylhistidine release was used to evaluate myofibrillar protein breakdown. We observed decreased protein synthesis and glutamate and α-ketoglutarate (α-KG) levels and increased leucine oxidation, GLN levels, and GLN release into medium in muscles in NH3 group. Increased leucine oxidation, release of branched-chain keto acids and GLN into incubation medium, and protein synthesis in EDL were observed in muscles in the BCAA group. The addition of BCAAs to medium eliminated the adverse effects of ammonia on protein synthesis and adjusted the decrease in α-KG found in the NH3 group. We conclude that (i) high levels of ammonia impair protein synthesis, activate BCAA catabolism, enhance GLN synthesis, and decrease glutamate and α-KG levels and (ii) increased BCAA availability enhances GLN release from muscles and attenuates the adverse effects of ammonia on protein synthesis and decrease in α-KG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is a scientific consensus that enhanced ammonia concentrations in the blood play a pivotal role in the pathogenesis of hepatic encephalopathy in patients with liver cirrhosis. Recent studies have indicated that ammonia plays an important role in the pathogenesis of muscle wasting, which is a common complication in cirrhosis [3]. The concentration of ammonia in muscles of healthy subjects is ~ 0.4 mM; this increases significantly (~ 5 mM) during liver cirrhosis [4]. A marked increase in ammonia production also occurs in muscles during heavy exercise and promotes both central and peripheral fatigue [8].
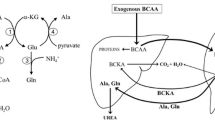
Branched-chain amino acids (BCAAs), i.e., leucine (LEU), isoleucine (ILE), and valine (VAL), are essential substrates and regulators in the synthesis and breakdown of muscle proteins, substrates for energy production, and the main donor of nitrogen to α-KG to form glutamate (GLU), which acts as the direct substrate for ammonia detoxification to glutamine (GLN) in skeletal muscle (Fig. 1). Several studies have shown an inverse relationship between plasma ammonia and BCAA concentrations in cirrhotics and that ammonia increases BCAA catabolism in muscles and decreases BCAA levels in the blood [9, 13, 14, 18]. Due to enhanced catabolism and the potential role of decreased BCAA levels in the pathogenesis of hepatic encephalopathy, fatigue, and muscle wasting, BCAA-enriched supplements are recommended as a therapy for liver disease [11, 22, 23, 27]. However, the results from clinical trials do not provide strong evidence of their beneficial effects [1, 7, 11], and some adverse effects of BCAA supplementation, which may compete with their benefits, have also been suggested [11].
The aims of the present study were to (1) determine how increased ammonia concentrations affect BCAA and protein metabolism and (2) examine the effects of enhanced BCAA availability in muscle on ammonia detoxification to GLN and protein metabolism under hyperammonemic conditions. We examined two types of skeletal muscle with different metabolic properties—soleus (SOL, slow-twitch, red muscle) and extensor digitorum longus (EDL, fast-twitch, white muscle). Because ammonia detoxification to GLN may enhance consumption of α-KG, which may disturb the function of the tricarboxylic acid (TCA) cycle, we also examined the effects of ammonia and BCAA on the concentrations of TCA cycle intermediates. Figure 1 shows the possible effects of ammonia and BCAAs on the TCA cycle.
Materials and methods
Animals and ethics statement
Wistar rats (body weight of 40–60 g) obtained from BioTest (Konarovice, CZ) were used in this study. The rats were housed under controlled conditions (12-h light-dark cycle, 22 °C, and 55–65% relative humidity) with free access to standard laboratory chow and water. All procedures involving animal manipulation were performed in accordance with guidelines set by the Institutional Animal Care and Use Committee of Charles University. The Animal Care and Use Committee of Charles University, Faculty of Medicine in Hradec Kralove specifically approved this study (License No.144879/2011-MZE-17214). Animals were treated carefully by animal experts educated and trained in how to manipulate animals to maintain a healthy environment and to reduce distress and minimize potential pain and suffering.
Materials
L-[1-14C]leucine was purchased from GE Healthcare Life Sciences (Buckinghamshire, UK), amino acids, Folin-Ciocalteu’s phenol reagent, N-succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (Suc-LLVY-MCA), and albumin were purchased from Sigma Chemical (St Louis, MO, USA). Hyamine hydroxide was obtained from Packard Instrument (Meriden, CT, USA). The remaining chemicals were obtained from Sigma Chemicals (St Louis, MO, USA), Waters (Milford, MA, USA), and Lachema (Brno, CZ).
Experimental design and methods
The studies were performed using isolated soleus (SOL) and extensor digitorum longus (EDL) muscles obtained from rats weighing 40–60 g. Young animals weighing less than 75 g must be used to avoid inadequate diffusion of gases and metabolites between the tissue and medium [20]. The animals were sacrificed through pentobarbital narcosis (6 mg/100 g body weight, intraperitoneally) by exsanguination via the abdominal aorta. SOL and EDL muscles from both legs were dissected according to Maizels et al. [20] and fixed via the tendons to stainless steel clips to provide slight tension (at approximately resting length). Thereafter, the muscles were transferred into 2.5 ml of modified Krebs-Henseleit bicarbonate buffer (pH 7.4, 37 °C) with 6 mM glucose, 2 mU/ml insulin, and amino acids at approximately physiological concentrations, and saturated with O2:CO2 (19:1). GLN was not added to the media to enable the examination of the effects of ammonia and BCAAs on GLN release from muscles. The muscles were preincubated for 30 min in a thermostatically controlled bath (37 °C) on a shaking device (70 cycles/min).
After preincubation, the muscles were quickly rinsed in 0.9% NaCl, blotted, and transferred to a second set of vials. The muscles from the right leg were incubated in the medium used for preincubation and served as paired controls to the left leg muscles. The left leg muscles were incubated in a medium containing 1.0 mM ammonia (NH3 group), the medium containing BCAAs at approximately four times the concentration in the controls (the BCAA group) or high concentrations of both ammonia and BCAA (NH3 + BCAA group).
The ammonia concentration in the NH3 and NH3 + BCAA group media were based on reports of ammonia concentrations in muscles from cirrhotic subjects [4]. In a preliminary experiment, we determined that fourfold increase in the BCAA concentration in medium doubles the BCAA concentration in muscles; this is similar to animals fed a diet with high BCAA content [15]. The viability of the incubated muscles was previously confirmed in our laboratory [25].
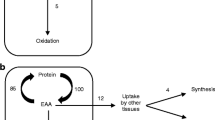
Leucine oxidation and protein synthesis (Fig. 2)
Leucine oxidation and protein synthesis were measured after a 2-h incubation of the muscles in medium containing L-[1-14C]leucine (0.6 μCi/ml). At the end of the incubation period, 0.4 ml of hyamine hydroxide was added to the suspended well above the incubation medium. The incubation was stopped by the addition of 35% (v/v) perchloric acid solution (0.2 ml) to the medium, and the flasks were shaken for 1 h to ensure complete absorption of 14CO2 into the hyamine hydroxide. The suspended wells containing hyamine were placed in scintillation vials containing 10 ml of scintillation mixture, and the radioactivity was measured using an LS 6000 liquid scintillation radioactivity counter (Beckman Instruments, CA, USA).
The leucine oxidation rates were calculated based on the radioactivity of the released 14CO2, the efficiency of 14CO2 recovery, and the specific activity of leucine in the incubation medium. The results are expressed as nmol of oxidized leucine/g muscle per hour.
Protein synthesis rates were calculated using the radioactivity of leucine incorporated into muscle protein and leucine specific activity in the incubation medium. The muscles were removed from the incubation flasks, quickly rinsed in cold 6% (v/v) HClO4, blotted, and homogenized in 0.6 ml of 6% (v/v) HClO4. The homogenate was centrifuged for 5 min at 12,000×g. The supernatant was collected to determine the free amino acid concentrations in the muscle via liquid chromatography. The pellet was washed three times and then hydrolyzed in 1 M NaOH. Aliquots were taken to measure the protein content [19] and L-[1-14C]leucine radioactivity. The results are expressed as nmol of leucine incorporated in mg of muscle protein per hour.
Amino acid concentrations in muscles
Amino acid concentrations in the supernatants of muscles homogenized for protein synthesis measurements were determined after derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate using a high-performance liquid chromatography technique (Alliance 2695, Waters, Milford, MA, USA) with norleucine as internal standard. The results are expressed as μmol/g wet muscle.
Release of GLN, BCKA, and 3-methylhistidine (3-MH) into incubation medium
The release of GLN, BCKA, and 3-MH from muscles was calculated based on their concentrations in medium after 2 h of the incubation, the volume of the medium, and the weight of the muscles. GLN, BCKA, and 3-MH concentrations in incubation medium were measured using HPLC (Alliance 2695, Waters, Milford, MA, USA). Derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate was used to determine GLN concentrations, o-phenylenediamine for BCKA (KIC, α-ketoisocaproate; KIV, α-ketoisovalerate; and KMV, α-keto-β-methylvalerate) concentrations, and fluorescamine for 3-MH concentrations. The results are expressed as μmol or nmol/g wet muscle per hour.
TCA cycle intermediates in muscles
TCA cycle components including α-ketoglutarate (α-KG), citrate, fumarate, cis-aconitate, and succinate in supernatants from deproteinized EDL and SOL muscle samples were quantified by liquid chromatography (Alliance 2695, Waters, Milford, MA, USA) equipped with C18 YMC-Triart analytical column in isocratic mode with 20 mM potassium phosphate buffer (pH 2.9). The wavelength for detection was set at 210 nm. The results are expressed as μmol/g wet muscle.
Statistical analysis
The Wilcoxon signed-rank test was used to determine the effects of ammonia, BCAAs, or ammonia + BCAAs on paired muscles from the same animal. Analysis of variance (ANOVA) followed by Bonferroni multiple comparison post hoc analysis was used to detect differences between multiple independent groups. Differences were considered significant at p < 0.05. The NCSS 2001 statistical software (Kaysville, UT, USA) was applied for the analyses. The results are expressed as the means ± SE.
Results
Alterations in leucine oxidation, protein synthesis, and myofibrillar proteolysis (Figs. 3, 4, and 5)
Ammonia increased LEU oxidation and decreased protein synthesis in both muscles. The addition of BCAAs to incubation medium increased LEU oxidation in both muscles and protein synthesis in EDL muscle. The presence of high BCAA concentrations in the medium adjusted the decrease in protein synthesis observed in the NH3 group. The effects of ammonia and BCAA on 3-MH release from muscles were not observed.
Alterations in amino acid concentrations in muscles (Table 1)
Increased GLN concentrations were observed in both muscles and decreased VAL, ILE, and GLU concentrations were observed in EDL muscles of NH3 group. The addition of BCAAs to the incubation medium doubled intracellular BCAA concentrations in both muscles and increased ALA and GLU concentrations in EDL muscles. Increased BCAA and GLN concentrations were observed in muscles of NH3 + BCAA group. However, the GLU concentration in the BCAA + NH3 group was lower than in the BCAA group.
Release of BCKA from muscles (Table 2)
Ammonia had no effect on BCKA release from muscles. However, a significant increase in BCKA release was observed in muscles after the addition of BCAA. The release of KIC from SOL muscles in the NH3 + BCAA group was lower than in SOL muscles in the BCAA group.
Release of GLN from muscles (Fig. 6)
Ammonia and BCAAs enhanced the release of GLN from both muscles. However, this effect was not synergistic, and we did not observe differences among the experimental groups.
TCA cycle intermediates (Table 3)
A decrease in α-KG levels was observed in muscles incubated in medium with a high ammonia concentration. The influence of high levels of ammonia, BCAAs, and/or a combination of ammonia and BCAAs on the concentrations of other TCA cycle intermediates was insignificant.
Discussion
Effects of ammonia on muscle
Our data clearly demonstrate that enhanced ammonia concentrations impair protein synthesis, activate BCAA catabolism, and enhance GLN synthesis in muscles. These findings are in line with opinions that ammonia is the main cause for the decrease in BCAAs in the blood and plays a role in the pathogenesis of muscle wasting in patients with liver cirrhosis.
Insignificant effect of ammonia on release of 3-MH from muscles is in line with observation that ubiquitin-proteasome components are not increased in skeletal muscle from patients with cirrhosis [24]. However, it should be noted that measuring of proteolysis by 3-MH may not evaluate all proteolytic pathways, particularly autophagy that has been reported to be increased during hyperammonemia [24].
The increased GLN and decreased GLU and α-KG levels in muscles incubated in medium with high ammonium concentrations indicate more rapid detoxification of ammonia by conversion of GLU to GLN and cataplerotic efflux of α-KG from TCA cycle. Because the concentrations of other TCA cycle intermediates were not altered, we believe that ammonia did not have any adverse effects on the TCA cycle in this case. The cause of the reduction of α-KG but no other intermediates of TCA cycle could be compensatory anaplerotic reactions including ILE and VAL that can enter the TCA cycle via succinyl-CoA.
The explanation of the effect of ammonia on BCAA concentration in muscles should be sought in terms of their enhanced catabolism and altered transport across the cell membrane. It has been shown that ammonia increases expression of the leucine/glutamine exchanger SLC7A5 [5], which can couple the uptake of BCAA with the efflux of GLN. Therefore, the enhanced efflux of GLN could prevent intracellular depletion of BCAA due their enhanced catabolism induced by high ammonia levels. In the present study, ammonia reduced BCAA levels in EDL but not in SOL. Significantly lower BCAA concentrations were found in the muscle of cirrhotic patients [16], but not in our previous study, in which we used half the ammonia concentration in the medium [14].
The data of our studies indicate more pronounced adverse effects by 1 mM ammonia (present study) than 0.5 mM ammonia (previous study). In the present study, ammonia reduced protein synthesis in both muscles and BCAA levels in EDL while insignificant effects were found in previous study. The lack of increase in BCKA release into the medium, which was significant in our previous study, is probably due to their increased oxidation. The concentration-dependent differences in effects of ammonia on protein synthesis and tissue amino acid concentrations may be due to clinically relevant differences in perturbations in amino acid transporter expression and mitochondrial functions. Arterial ammonia concentration was significantly inversely correlated with skeletal muscle ATP content in rats with portacaval anastomosis [4].
Effects of BCAAs on muscles
The increased rates of protein synthesis observed in muscles incubated in medium with high BCAA levels are in agreement with a number of studies demonstrating the stimulatory effects of BCAAs on protein synthesis [6, 15]. The differences in the responses by SOL and EDL muscles are due to the higher susceptibility of fast-twitch fibers to various signals, as has been shown in our previous studies [12, 17].
The increased rates of LEU oxidation and enhanced release of all three BCKAs into incubation medium by muscles incubated in medium with high concentrations of BCAAs demonstrate that BCAA catabolism increases in skeletal muscle proportionally to their supply. Half a century ago, Manchester [21] demonstrated with isolated rat diaphragm that the fraction of labeled LEU taken up by the diaphragm, which appeared as labeled CO2, did not decrease as the concentration of supplied amino acids increased, while the amount of isotope incorporated into proteins dropped steadily as the specific activity fell. The consequences of this phenomenon are that the primary effect of a BCAA-enriched diet is activated BCAA catabolism and enhanced BCKA, ALA, and GLN levels in the blood [15].
Effects of the BCAA under hyperammonemic conditions
The effects of BCAAs on muscles incubated in medium with 1 mM ammonia indicate the potential benefits of their enhanced intake. First, the addition of BCAAs to the medium enhanced GLN formation and completely eliminated the adverse effects of high ammonia levels on protein synthesis. Another positive finding is the adjustment in the decline in α-KG in the ammonia group which reduces the concern that excessive BCAA catabolism after BCAA consumption may drain α-KG from the TCA cycle (cataplerosis) and disrupt aerobic oxidation [11, 28]. Perhaps anaplerotic effect of succinyl-CoA originating in catabolism of VAL and ILE is involved in beneficial effects of BCAA on α-KG levels, which decrease in the skeletal muscle of cirrhotic patients [26]. Enhanced LEU availability can provide acetyl-CoA as a TCA cycle substrate and activate mTORC1 to restore proteostasis [2].
It should be noted that positive effect of BCAA on ammonia detoxification to GLN in muscles may also have adverse side effects. Physiologically, most GLN is released from muscles and subsequently catabolized in visceral tissues to ammonia, which is detoxified to urea in the liver. Under conditions where hepatic function is impaired, GLN breakdown in visceral tissues can result in increased ammonia concentrations in the blood. A vicious cycle characterized by enhanced ammonia detoxification to GLN in muscles and subsequent GLN breakdown to ammonia in the kidneys and enterocytes has been suggested in subjects with liver disease [10].
Conclusions
We conclude that high levels of ammonia impair protein synthesis, activate BCAA oxidation, and enhance GLN synthesis and decrease GLU and α-KG levels in skeletal muscle. Enhanced BCAA availability attenuates the adverse effects of ammonia on protein synthesis and ameliorates the decline in α-KG.
Abbreviations
- ALA:
-
Alanine
- BCAAs:
-
Branched-chain amino acids
- BCKA:
-
Branched-chain keto acids
- EDL:
-
Musculus extensor digitorum longus
- GLN:
-
Glutamine
- GLU:
-
Glutamate
- ILE:
-
Isoleucine
- KIC:
-
α-Ketoisocaproate (ketoleucine)
- KIV:
-
α-Ketoisovalerate (ketovaline)
- KMV:
-
α-Keto-β-methylvalerate (ketoisoleucine)
- LEU:
-
Leucine
- SOL:
-
Musculus soleus
- TCA cycle:
-
Tricarboxylic acid cycle
- VAL:
-
Valine
- α-KG:
-
α-Ketoglutarate
References
Als-Nielsen B, Koretz RL, Kjaergard LL, Gluud C (2003) Branched-chain amino acids for hepatic encephalopathy. Cochrane Database Syst Rev 2:CD001939
Dasarathy S (2017) Myostatin and beyond in cirrhosis: all roads lead to sarcopenia. J Cachexia Sarcopenia Muscle 8:864–869
Dasarathy S, Hatzoglou M (2018) Hyperammonemia and proteostasis in cirrhosis. Curr Opin Clin Nutr Metab Care 21:30–36
Davuluri G, Allawy A, Thapaliya S, Rennison JH, Singh D, Kumar A, Sandlers Y, Van Wagoner DR, Flask CA, Hoppel C, Kasumov T, Dasarathy S (2016) Hyperammonaemia-induced skeletal muscle mitochondrial dysfunction results in cataplerosis and oxidative stress. J Physiol 594:7341–7360
Davuluri G, Krokowski D, Guan BJ, Kumar A, Thapaliya S, Singh D, Hatzoglou M, Dasarathy S (2016) Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J Hepatol 65:929–937
Garlick PJ (2005) The role of leucine in the regulation of protein metabolism. J Nutr 135:1553S–1556S
Gluud LL, Dam G, Les I, Córdoba J, Marchesini G, Borre M, Aagaard NK, Vilstrup H (2015) Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev 9:CD001939
Graham TE, MacLean DA (1992) Ammonia and amino acid metabolism in human skeletal muscle during exercise. Can J Physiol Pharmacol 70:132–141
Hayashi M, Ikezawa K, Ono A, Okabayashi S, Hayashi Y, Shimizu S, Mizuno T, Maeda K, Akasaka T, Naito M, Michida T, Ueshima D, Nada T, Kawaguchi K, Nakamura T, Katayama K (2007) Evaluation of the effects of combination therapy with branched-chain amino acid and zinc supplements on nitrogen metabolism in liver cirrhosis. Hepatol Res 37:615–619
Holecek M (2014) Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy-therapeutic perspectives. Metab Brain Dis 29:9–17
Holeček M (2017) Branched-chain amino acid supplementation in treatment of liver cirrhosis: updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition 41:80–85
Holeček M, Mičuda S (2017) Amino acid concentrations and protein metabolism of two types of rat skeletal muscle in postprandial state and after brief starvation. Physiol Res 66:959–967
Holeček M, Šprongl L, Tichý M (2000) Effect of hyperammonemia on leucine and protein metabolism in rats. Metabolism 49:1330–1334
Holecek M, Kandar R, Sispera L, Kovarik M (2011) Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids 40:575–584
Holecek M, Siman P, Vodenicarovova M, Kandar R (2016) Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr Metab (Lond) 13:12
Iob V, Coon WW, Sloan M (1967) Free amino acids in liver, plasma, and muscle of patients with cirrhosis of the liver. J Surg Res 7:41–43
Kadlcikova J, Holecek M, Safranek R, Tilser I, Kessler BM (2004) Effects of proteasome inhibitors MG132, ZL3VS and AdaAhx3L3VS on protein metabolism in septic rats. Int J Exp Pathol 85:365–371
Leweling H, Breitkreutz R, Behne F, Staedt U, Striebel JP, Holm E (1996) Hyperammonemia-induced depletion of glutamate and branched-chain amino acids in muscle and plasma. J Hepatol 25:756–762
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maizels EZ, Ruderman NB, Goodman MN, Lau D (1977) Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem J 162:557–568
Manchester KL (1965) Oxidation of amino acids by isolated rat diaphragm and the influence of insulin. Biochim Biophys Acta 100:295–298
Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R, Italian BCAA Study Group. Italian BCAA Study Group (2003) Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology 124:1792–1801
Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y, Shiraishi K, Okuda H, Onji M, Kanazawa H, Tsubouchi H, Kato S, Kaito M, Watanabe A, Habu D, Ito S, Ishikawa T, Kawamura N, Arakawa Y, Hepatic Nutritional Therapy (HNT) Study Group. Hepatic Nutritional Therapy (HNT) Study Group (2007) BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition 23:113–120
Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, Eghtesad B, Singh K, Fu X, Dubyak G, McDonald C, Almasan A, Hazen SL, Naga Prasad SV, Dasarathy S (2012) Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab 303:E983–E993
Safranek R, Holecek M, Kadlcikova J, Sprongl L, Mislanová C, Kukan M, Chládek J (2003) Effect of acute acidosis on protein and amino acid metabolism in rats. Clin Nutr 22:437–443
Tsien C, Davuluri G, Singh D, Allawy A, Ten Have GA, Thapaliya S, Schulze JM, Barnes D, McCullough AJ, Engelen MP, Deutz NE, Dasarathy S (2015) Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology 61:2018–2029
Urata Y, Okita K, Korenaga K, Uchida K, Yamasaki T, Sakaida I (2007) The effect of supplementation with branched-chain amino acids in patients with liver cirrhosis. Hepatol Res 37:510–516
Wagenmakers AJ, Coakley JH, Edwards RH (1990) Metabolism of branched-chain amino acids and ammonia during exercise: clues from McArdle's disease. Int J Sports Med 11:S101–S113
Acknowledgements
The authors wish to thank R. Fingrova, D. Jezkova, and K. Sildbergerova for their technical assistance.
Funding
This project was supported by the PROGRES Q40/02 program.
Author information
Authors and Affiliations
Contributions
MH outlined the experiments, performed the statistical analysis, interpreted the experimental results, and prepared the manuscript. MV was involved in the data acquisition and the data interpretation. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
All procedures involving animal manipulation were performed in accordance with guidelines set by the Institutional Animal Care and Use Committee of Charles University. The Animal Care and Use Committee of Charles University, Faculty of Medicine in Hradec Kralove, specifically approved this study (license no.144879/2011-MZE-17214).
Competing interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Holeček, M., Vodeničarovová, M. Effects of branched-chain amino acids on muscles under hyperammonemic conditions. J Physiol Biochem 74, 523–530 (2018). https://doi.org/10.1007/s13105-018-0646-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-018-0646-9