Abstract
This study was conducted to test the hypothesis that dietary L-arginine supplementation enhances immunity in early weaned piglets. Seventy piglets weaned at 7 days of age were assigned to five groups (14 pigs/group), representing supplementation of 0.0, 0.2, 0.4, 0.6, and 0.8% l-arginine to a milk-based formula. On Day 7 after initiation of treatment, spleen weight in piglets supplemented with 0.2 and 0.8% arginine was heavier and thymus size was larger in piglets supplemented with 0.6% arginine, whereas serum concentration of immunoglobulin (Ig) M was higher but that of IL-8 was lower in piglets supplemented with 0.6 and 0.8% arginine, compared with the control group. Dietary supplementation with 0.8% arginine increased the numbers of white blood cells and granulocytes, and gene expression of interleukin (IL)-8 in spleen. On Day 14, compared with control piglets, granulocyte numbers were greater but lymphocyte numbers were lower in piglets supplemented with 0.2 and 0.4% arginine, whereas splenic expression of IL-8 and tumor necrosis factor-α genes was increased in piglets supplemented with 0.8% arginine. Additionally, IgG and IgM concentrations in serum and growth performance were greater in piglets supplemented with 0.4–0.8% arginine, compared with unsupplemented piglets. Collectively, dietary supplementation with 0.4–0.8% l-arginine for 2 weeks enhances both cellular and humoral immunity in piglets by modulating the production of leukocytes, cytokines and antibodies. These results indicate that increasing l-arginine provision is beneficial for optimal immune responses in young pigs and also have important implications for designing the next generation of improved formula for human infants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The current swine industry strives to wean piglets at an early age to prevent sow-originated infectious diseases and maximize sows’ production potential (Dong and Pluske 2007; Kim et al. 2007). This practice presents a tremendous challenge to neonatal pigs, whose immune system is not well developed in the first 4 weeks of life (Yang and Schultz 1986). Nutritional means, including dietary supplementation with glutamine (Johnsona et al. 2006), zinc (Ou et al. 2007), or herbs (Kong et al. 2007), have been used to improve the immunity of early-weaned piglets. However, due to low feed intake in the first week postweaning, these approaches cannot provide piglets with sufficient arginine, an amino acid crucial for optimal immune responses (Li et al. 2007).
Arginine, which is an abundant amino acid in tissue proteins (Wu et al. 1999), is utilized by multiple pathways, including the synthesis of protein, nitric oxide (NO), polyamines and creatine (Wu and Morris 1998). Through cGMP-dependent signaling pathways, NO regulates a variety of physiological and biochemical processes (Jobgen et al. 2006). Of particular interest, weanling piglets have a particularly high requirement for dietary arginine (Kim and Wu 2004; Wu et al. 2004). A large body of evidence from studies with rats and mice indicates that adequate provision of arginine is required for lymphocyte development and that dietary arginine supplementation enhances immune function in various models of immunological challenges (Li et al. 2007). However, little is known about the effect of dietary arginine supplementation on the immune status of early-weaned piglets. Therefore, the major objective of the present study was to determine an immunoregulatory role for arginine in young pigs.
Materials and methods
Animals and feeding
Seventy piglets (Landrace × Yordshire) weaned at 7 days of age were assigned randomly to five treatment groups (14 pigs/group), representing supplementation with 0.0% (control), 0.2, 0.4, 0.6 and 0.8% l-arginine (Ajinomoto, Tokyo, Japan) to a milk-based diet (Table 1). All diets were made isonitrogenous with addition of appropriate amounts of alanine at the expense of lactose and glucose, as described by Kim and Wu (2004). Diets were formulated to meet National Research Council-recommended nutrient requirements for weanling piglets (NRC 1998). The piglets were housed individually in an environmentally controlled nursery with hard plastic slatted flooring and fed their respective liquid diets (water/diet = 4:1) 4 times per day at 7:00, 11:00, 15:00 and 19:00. All animals had free access to drinking water.
Sample collection
On Days 7 and 14 after initiation of treatment, 6 piglets in each treatment group were selected randomly for obtaining blood samples from the jugular vein at 2 h after feeding. Approximately 1 mL of the blood was placed in ethylene-diamine-tetraacetic acid-coated tubes for the determination of total and differential counts of leucocytes. In addition, 8 mL blood samples were contained in heparinized tubes, followed immediately by centrifugation at 3,000×g for 10 min. The resultant sera were transferred to polystyrene tubes and stored at −80°C for analysis of immunoglobulins and cytokines. When blood sampling was completed, piglets were anesthetized with sodium pentobarbital and killed by jugular puncture (Kong et al. 2007). Thymus and spleen were obtained and weighed. Spleens were immediately snap-frozen in liquid nitrogen and stored at −80°C for the extraction of total RNA. This study was carried out in accordance with the Chinese guidelines for animal welfare and was approved by the Animal Care and Use Committee of the Chinese Academy of Sciences.
Determination of total and differential counts of blood leucocytes
Total and differential numbers of blood leucocytes were determined using an Auto-Hemocytometer (Cell Dyn BT-2100, Abbott Diagnostics, Abbott Park, IL, USA) within 2 h after blood samples were obtained. The procedures were performed according to the manufacturer’s instructions (Kong et al. 2007).
Determination of serum IgG, IgM and cytokines
Serum concentrations of IgG and IgM were measured using immunoassay kits from Triple J Farms (Bellingham, WA, USA) according to the manufacturer’s instructions. Serum concentrations of interleukin (IL)-1β, IL-2, IL-6, IL-8, and tumor necrosis factor (TNF)-α were determined by radioimmunoassays using kits provided by Beijing Chemclin Biotech (Beijing, China).
Analysis of IL-1β, IL-6, IL-8 and TNF-α gene expression in spleen
Total RNA was extracted from spleen using the Trizol Reagent (Invitrogen) and concentration was measured by optical density at 260 nm (Deng et al. 2007). The cDNA was reverse-transcribed from 0.2 μg of eluted RNA using the First Strand cDNA Synthesis Kit (MBI Fermentas, Newington, NH, USA) based on the manufacturer’s instruction manual. The real-time PCR for IL-1β, IL-6, IL-8 and TNF-α, as well as β-actin as internal control, was performed using their respective primer pairs (Table 2). The amplification mixture contained 5 μL cDNA, 0.5 μL of each primer, and components of the iQ SYBR Green Supermix (Bio-Rad, Richmond, CA, USA) in a final volume of 25 μL. The PCR analysis was performed at 94°C for 10 s, followed by 35 cycles of denaturation at 94°C for 10 s, annealing at 54.7°C for 30 s, and extension at 72°C for 15 s. The relative quantification of gene amplification by RT-PCR was performed using cycle threshold (C t) values (Deng et al. 2007). The comparative C t value method was employed to quantitate expression levels for cytokine genes relative to those for β-actin, as described by Fu et al. (2006).
Calculation and statistical analysis
The relative weights of lymphoid organs were calculated as the organ weights divided by body weights (g/kg). All data, expressed as mean ± SEM, were subjected to ANOVA analysis using the SPSS 13.0 Programme (Chicago, IL, USA). The differences among group means were compared using the Duncan multiple comparison test. Probability values <0.05 were taken to indicate statistical significance.
Results
Effects of dietary arginine supplementation on growth performance of piglets
Feed intake did not differ (P > 0.05) among all the groups of piglets (63.5 ± 2.4 g dry matter/kg body weight per day) during the 14-day-period of experiment. On Days 7 and 14 after initiation of treatment, piglets supplemented with 0.6 and 0.8% arginine had heavier body weights (P < 0.05) than piglets in the other groups (Table 3). On Day 14, dietary supplementation with 0.4% arginine increased (P < 0.05) the body weight of piglets by 8%, compared with the control group. Over the entire 2-week of study, the daily weight gain of piglets supplemented with 0.4, 0.6 and 0.8% arginine was 20, 49 and 48% greater (P < 0.05) than that for the control group, respectively (Table 3).
Effects of dietary arginine supplementation on relative organ weights of piglets
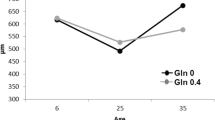
The relative weights of spleen in piglets supplemented with 0.2 and 0.8% arginine were 32 and 14% greater (P < 0.01), respectively, on Day 7 after initiation of treatment, when compared with the control group (Fig. 1). In addition, dietary supplementation with 0.6% arginine increased (P < 0.05) the relative weights of thymus by approximately 150% on Day 7, in comparison with the control and other groups of piglets, whereas a higher dose of arginine (0.8%) reduced (P < 0.05) the relative weight of thymus by 24%, compared with the control group. On Day 7, dietary supplementation with 0.4 and 0.6% arginine had no effect (P > 0.05) on the relative weight of spleen, and dietary supplementation with 0.2% and 0.4% arginine did not affect (P < 0.05) thymus size. On Day 14, the relative weights of the spleen and thymus were similar (P > 0.05) among all the treatment groups (Fig. 1).
Effects of dietary arginine supplementation on total and differential counts of blood leucocytes
Dietary supplementation with 0.8% arginine increased (P < 0.05) the numbers of white blood cells and granulocytes, as well as the percentage of blood granulocytes by 60–80%, on Day 7 after initiation of treatment, in comparison with all other groups of piglets (Table 4). On Day 7, the numbers of white blood cells or granulocytes did not differ (P > 0.05) among piglets supplemented with 0.0–0.6% arginine, and the number of lymphocytes and middle cells did not differ (P > 0.05) among all groups of pigs. Dietary arginine supplementation had no effect (P > 0.05) on the percentage of blood lymphocytes or middle cells on Day 7, except that the percentage of granulocytes was higher (P < 0.05) in piglets supplemented with 0.8% arginine than that in the control group.
On Day 14, dietary supplementation with 0.4 and 0.6% arginine reduced (P < 0.05) the number of white blood cells, compared with the other groups of piglets (Table 4). The number of total blood lymphocytes was lower (P < 0.05) in piglets supplemented with 0.2–0.6% arginine, compared with piglets supplemented with 0.0 and 0.8% arginine. On Day 14, the percentage of lymphocytes were lower (P < 0.05) but the number and percentage of granulocytes were higher (P < 0.05) in piglets supplemented with 0.2 and 0.4% arginine, when compared with the other groups of piglets (Table 4). Dietary supplementation with 0.2–0.8% arginine had no effect (P > 0.05) on the total number or percentage of blood middle cells.
Effects of dietary arginine supplementation on serum concentrations of immunoglobulins and cytokines
On Day 7, dietary supplementation with 0.2–0.8% arginine did not affect (P > 0.05) serum concentrations of IgG, but dietary supplementation with 0.6–0.8% arginine increased (P < 0.05) serum concentrations of IgM by approximately 150–200%, compared with the other three groups (0.0, 0.2, and 0.4% arginine) (Table 5). In contrast, on Day 14, dietary supplementation with 0.2% arginine enhanced (P < 0.05) serum concentrations of IgG by 12%, but a higher dose of arginine (0.4–0.8%) did not result in a further increase in this parameter. Supplementing 0.4, 0.6 and 0.8% arginine to the diet increased (P < 0.05) serum concentrations of IgM by 62, 77 and 91%, respectively, in comparison with the control group.
Dietary supplementation with 0.2–0.8% arginine had no effect (P > 0.05) on serum concentrations of IL-1β and TNF-α on Day 7 or IL-2 and IL-6 on either Day 7 or Day 14 (Table 5). However, on Day 14, dietary supplementation with 0.4% arginine increased (P < 0.05) serum concentration of IL-1β by 32–50%, in comparison with all other groups of piglets. Serum concentration of TNF-α was highest (P < 0.05) in piglets supplemented with 0.2% arginine among the treatment groups and was not affected (P > 0.05) by dietary supplementation with higher doses of arginine (Table 5). Interestingly, IL-8 was the only measured cytokine that exhibited a change in serum concentrations on both Days 7 and 14 in response to the arginine treatment. Particularly, compared with the control group, serum concentrations of IL-8 were 72 and 61% lower (P < 0.05), respectively, in piglets supplemented with 0.6 and 0.8% arginine on Day 7, and were 36 and 40% lower (P < 0.05), respectively, in piglets supplemented with 0.2 and 0.8% arginine on Day 14 (Table 5).
Effects of dietary arginine supplementation on cytokine gene expression in spleen
Dietary supplementation with 0.2–0.8% arginine did not affect (P > 0.05) splenic expression of IL-1β and IL-6 genes on Days 7 and 14 or TNF-α on Day 7 (Fig. 2). Compared with the control group, splenic expression of the IL-8 gene was higher (P < 0.05) in piglets supplemented with 0.4–0.8% arginine on Day 7 and in piglets supplemented with 0.8% arginine on Day 14. Dietary supplementation with 0.8% arginine enhanced (P < 0.05) TNF-α gene expression in spleen, compared with piglets supplemented with 0.0, 0.2 and 0.4% arginine.
Discussion
Studies with rodents and humans demonstrate that arginine is an important immunomodulatory nutrient (Li et al. 2007; Popovic et al. 2007). Its immunostimulatory effect is most promising in immunocompromised hosts under such stress conditions as trauma, surgery and or viral infection (Daly et al. 1988; Kelly et al. 1995; Rodriguez et al. 2003). In laboratory animals, administration of arginine increased thymus size and cellularity, stimulated lymphocyte proliferation in response to mitogen and alloantigen, augmented macrophage and natural killer cell-mediated lysis of tumor cells, and enhanced IL-2 production by lymphocytes (Reynolds et al. 1990; Yeh et al. 2002). At present, there is a paucity of information in the literature regarding the effects of supplemental arginine on the immune status in neonates. Because the pig is an established animal model for studying human nutrition (Wu et al. 2004), findings from this work may have important implications for designing the next generation of improved formula for infants.
Arginine, which is synthesized from glutamine/glutamate and proline via the intestinal-renal axis (Wu 1997; Hu et al. 2008), is a nutritionally essential amino acid for neonatal pigs (Kim and Wu 2004). It also stimulates the secretion of growth hormone and insulin that beneficially modulate the immune response (Evoy et al. 1998; Flynn et al. 2002). Compelling evidence shows that the supply of arginine from the milk-based diet and endogenous synthesis is inadequate for supporting maximal growth of 7- to 21-day-old piglets (Frank et al. 2007; Wu et al. 2004; Yao et al. 2008). During the weaning period, the problem of low feed intake by piglets is exacerbated by high concentrations of cortisol (Wu et al. 2000). This stress hormone induces expression of arginase in multiple organs (including the small intestine) for the hydrolysis of arginine (Flynn et al. 1999; Morris 2002). The outcome is an arginine deficiency as indicated by elevated levels of ammonia and reduced levels of arginine in plasma (Wu et al. 1996). Thus, the weanling piglet is challenged by a variety of hormonal and environmental factors (such as pathogens and diet), which compromises its immune function (Lalles et al. 2007). Immune-enhancing diets, which can improve development of the immune system in young animals, may be an effective means to enhance the immune status (Li et al. 2007). Therefore, the early-weaned piglet provides an established animal model to test this hypothesis.
As key lymphoid organs, the thymus and spleen play an important role in the host immune response (Li et al. 2007). Kwak et al. (1999) reported that dietary supplementation with of 0.2% arginine increased the weights of these two organs in chickens, but feeding an arginine-deficient diet impaired their growth and development. Similarly, extensive studies with mammals indicate that dietary arginine supplementation increased mitogenesis and functional reactivities of thymic and splenic lymphocytes (Barbul et al. 1980; Reynolds et al. 1990; Kelly et al. 1995). Consistent with these findings, dietary supplementation of 0.2 and 0.8% arginine to piglets increased the relative weight of spleen, whereas dietary supplementation of 0.6% arginine increased the relative weight of thymus in the first week post weaning (Fig. 1), the most critical period for the survival of weanling piglets (Dong and Pluske 2007). Notably, the stimulatory effect of the arginine treatment on growth and development of lymphoid organs appears to depend on its dose, as either a low or a high dose of arginine was ineffective (Fig. 1). These results may be explained by a complex interaction between arginine and other dietary nutrients in regulating the synthesis of NO and polyamines (Wu and Meininger 2002; Flynn et al. 2002), whose biological actions are now known to vary with their cellular concentrations (Montanez et al. 2007; Nikolic et al. 2007).
The total and differential counts of leukocytes may indicate an inflammatory status of animals (Holtenius et al. 2004). Generally, a reduction in the number of blood leukocytes under inflammatory conditions may be an indicator of reduced infections in hosts (Evoy et al. 1998). In addition, both IgG and IgM play an important role in defending the host from infectious diseases (Li et al. 2007). Further, cytokines are crucial protein mediators in humoral immunity (Ma et al. 2007). Of particular interest, TNF-α regulates leukocyte recruitment through both upregulation of adhesion molecules on vascular endothelial cells and induction of cytokine and chemokine synthesis (Kips 2001). Furthermore, IL-6 plays an important role under inflammatory conditions, including bacterial infections (Song and Kellum 2005), whereas IL-8 is a potent neutrophil chemotactic factor, which reinforces the recruitment of additional neutrophils to inflammatory sites (Evoy et al. 1998). As components of the immune system, these cytokines are crucial for the immune response (Escobar et al. 2004; Li et al. 2007).
Several lines of evidence from the present study supports the notion that dietary arginine supplementation enhances the immune status in early-weaned piglets. First, dietary supplementation with 0.8% arginine increased the relative weight of spleen on Day 7 (Fig. 1). Second, on Day 14, dietary supplementation with 0.4–0.6% arginine reduced the number of white blood cells and lymphocytes, while dietary supplementation with 0.6% arginine increased thymus size (Fig. 1) and the number of granulocytes (Table 4). Third, dietary supplementation with 0.6–0.8% arginine increased serum concentrations of IgM on Days 7 and 14, as well as IgG on Day 14 (Table 4). Fourth, on Day 14, dietary supplementation with 0.2 and 0.4% arginine augmented serum concentrations of TNF-α and IL-1β, respectively (Table 5). Also, dietary supplementation with 0.8% arginine decreased serum concentrations of IL-8 on both Days 7 and 14 (Table 5), which may reflect a reduction in systemic infection (Evoy et al. 1998), while increasing splenic expression of the IL-8 gene (Fig. 2) as a possible mechanism for beneficially regulating cell proliferation and differentiation in the spleen (Broxmeyer et al. 1996). Finally, supplementing 0.4–0.8% arginine to the diet did not affect feed intake but markedly improved the growth performance of young pigs (Table 3). On the basis of changes in the measured parameters, dietary supplementation with 0.8% arginine appears to yield optimal immune responses in piglets.
Results of this study indicate that a gradual increase in the doses of supplemental arginine did not result in gradual response in most of the immune parameters measured. Rather, some changes were observed stochastically. Additionally, in certain cases, only one dose yielded an influence, while both lower and higher levels did not have an effect on the same parameter. Clearly, there are complex immunological responses to dietary arginine supplementation, which likely depend on multiple factors (including developmental stage, target cells, metabolic pathways, balance among basic amino acids, levels of NO and polyamine production, and endocrine status in animals), as recently reported for muscle mTOR signaling (Yao et al. 2008), growth performance (Table 3), secretion of insulin and growth hormone (Kim and Wu 2004; Wu et al. 2007), reproduction (Mateo et al. 2007; Wu et al. 2008), and the digestive system (Zhan et al. 2008) in pigs. This underscores the need of the current work to establish an optimal dose of supplemental arginine for enhancing immune responses in neonates.
Abbreviations
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- NO:
-
Nitric oxide
- RT-PCR:
-
Real-time polymerase-chain reaction
- TNF:
-
Tumor necrosis factor
References
Barbul A, Wasserkrug HL, Sisto DA, Seifter E, Rettura G, Levenson SM, Efron G (1980) Thymic stimulatory actions of arginine. J Parenter Enteral Nutr 4:446–449
Broxmeyer HE, Cooper S, Cacalano G, Hague NL, Baillish E, Moore MW (1996) Involvement of interleukin (IL) 8 receptor in negative regulation of myeloid progenitor cells in vivo: evidence from mice lacking the murine IL-8 receptor homologue. J Exp Med 184:1825–1832
Daly JM, Reynolds J, Thom A, Kinsley L, Dietrick-Gallagher M, Shou J, Ruggieri B (1988) Immune and metabolic effects of arginine in the surgical patient. Ann Surg 208:512–523
Deng ZY, Zhang JW, Wu GY, Yin YL, Ruan Z, Li TJ, Chu WY, Kong XF, Zhang YM, Fan YW, Liu R, Huang RL (2007) Dietary supplementation with polysaccharides from Semen cassiae enhances immunoglobulin production and interleukin gene expression in early-weaned piglets. J Sci Food Agric 87:1868–1873
Dong GZ, Pluske JR (2007) The low feed intake in early-weaned pigs: problems and possible solutions. Asian Aust J Anim Sci 20:440–452
Escobar J, Van Alstine WG, Baker DH, Johnson RW (2004) Decreased protein accretion in pigs with viral and bacterial pneumonia is associated with increased myostatin expression in muscle. J Nutr 134:3047–3053
Evoy D, Fahey TJ, Daly JM (1998) Immunonutrition: the role of arginine. Nutrition 14:611–617
Flynn NE, Meininger CJ, Kelly K, Ing NH, Morris SM Jr, Wu G (1999) Glucocorticoids mediate the enhanced expression of intestinal type II arginase and argininosuccinate synthase in postweaning pigs. J Nutr 129:799–803
Flynn NE, Meininger CJ, Haynes TE, Wu G (2002) The metabolic basis of arginine nutrition and pharmacotherapy. Biomed Pharmacother 56:427–438
Frank JW, Escobar J, Hguyen HV, Jobgen SC, Jobgen WS, Davis TA, Wu G (2007) Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J Nutr 137:315–319
Fu WJ, Hu J, Spencer T, Carroll R, Wu G (2006) Statistical models in assessing fold changes of gene expression in real-time RT-PCR experiments. Comput Biol Chem 30:21–26
Holtenius K, Persson-Waller K, Essen-Gustavsson B, Holtenius P, Hallen SC (2004) Metabolic parameters and blood leukocyte profiles in cows from herds with high or low mastitis incidence. Vet J 168:65–73
Hu CA, Khalil S, Zhaorigetu S, Liu Z, Tyler M, Wan G, Valle D (2008) Human ∆1-pyrroline-5-carboxylate synthase: function and regulation. Amino Acids. doi:10.1007/s00726-008-0075-0
Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G (2006) Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17:571–588
Johnsona IR, Ball RO, Baracos VE, Field CJ (2006) Glutamine supplementation influences immune development in the newly weaned piglet. Dev Comp Immunol 30:1191–1202
Kelly E, Morris SM Jr, Billiar TR (1995) Nitric oxide, sepsis, and arginine metabolism. J Parenter Enteral Nutr 19:234–238
Kim SW, Wu G (2004) Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr 134:625–630
Kips JC (2001) Cytokines in asthma. Eur Respiratory J 18:24s–33s
Kong XF, Wu GY, Liao YP, Hou ZP, Liu HJ, Yin FG, Li TJ, Huang RL, Zhang YM, Deng D, Xie MY, Kang P, Yang CB, Yin YL, Fan MZ (2007) Dietary supplementation with Chinese herbal ultra-fine powder enhances cellular and humoral immunity in early-weaned piglets. Livest Sci 108:94–98
Kwak H, Austic RE, Dietert RR (1999) Influence of dietary arginine concentration on lymphoid organ growth in chickens. Poult Sci 78:1536–1541
Lalles JP, Bosi P, Smidt H, Stokes CR (2007) Weaning—a challenge to gut physiologists. Livest Sci 108:82–93
Li P, Yin YL, Li DF, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98:237–252
Ma CS, Nichols KE, Tangye SG (2007) Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol 25:337–379
Mateo RD, Wu G, Bazer FW, Park JC, Shinzato I, Kim SW (2007) Dietary L-arginine supplementation enhances the reproductive performance of gilts. J Nutr 137:652–656
Montanez R, Sanchez-Jimenez F, Aldana-Montes JF, Medina MA (2007) Polyamines: metaboolism to systems biology and beyond. Amino Acids 33:283–289
Morris SM Jr (2002) Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr 22:87–105
National Research Council (NRC) (1998) Nutrient requirements of swine. National Academy Press, Washington, DC
Nikolic J, Stojanovic I, Pavlovic R, Sokolovic D, Bjelakovic G, Beninati S (2007) The role of l-arginine in toxic liver failure: interrelation of arginase, polyamine catabolic enzymes and nitric oxide synrhase. Amino Acids 32:127–131
Ou DY, Li DF, Cao YH, Li XL, Yin JD, Qiao SY, Wu G (2007) Dietary supplementation with zinc oxide decreases expression of the stem cell factor in the small intestine of weanling pigs. J Nutr Biochem 18:820–826
Popovic PJ, Zeh HJ, Ochoa JB (2007) Arginine and immunity. J Nutr 136:1681S–1686S
Reynolds JV, Daly JM, Shou J, Sigal R, Ziegler MM, Naji A (1990) Immunologic effects of arginine supplementation in tumor-bearing and non-tumor-bearing hosts. Ann Surg 211:202–210
Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC (2003) l-Arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol 171:1232–1239
Song M, Kellum JA (2005) Interleukin-6. Crit Care Med 33:463s–465s
Wu G (1997) Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol Gastrointest Liver Physiol 272:G1382–G1390
Wu G, Bazer FW, Datta S, Johnson GA, Li P, Satterfield MC, Spencer TE (2008) Proline metabolism in the conceptus: Implications for fetal growth and development. Amino Acids. doi: 10.1007/s00726-008-0052-7
Wu G, Bazer FW, Davis TA, Jaeger LA, Johnson GA, Kim SW, Knabe DA, Meininger CJ, Spencer TE, Yin YL (2007) Important roles for the arginine family of amino acids in swine nutrition and production. Livest Sci 112:8–22
Wu G, Flynn NE, Knabe DA, Jaeger LA (2000) A cortisol surge mediates the enhanced polyamine synthesis in porcine enterocytes during weaning. Am J Physiol Regul Integr Comp Physiol 279:R554–R559
Wu G, Meier SA, Knabe DA (1996) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 126:2578–2584
Wu G, Meininger CJ (2002) Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr 22:61–86
Wu G, Ott TL, Knabe DA, Bazer FW (1999) Amino acid composition of the fetal pig. J Nutr 129:1031–1038
Wu G, Knabe DA, Kim SW (2004) Arginine: nutrition in neonatal pigs. J Nutr 134:2783S–2790S
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Zhan ZF, Ou DY, Piao XS, Kim SW, Liu YH, Wang JJ (2008) Dietary arginine supplementation affects microvascular development in the small intestine of early-weaned pigs. J Nutr 138:1304–1309
Yang WC, Schultz RD (1986) Ontogeny of natural killer cell activity and antibody dependent cell-mediated cytotoxicity in pigs. Dev Comp Immunol 10:405–418
Yao K, Yin YL, Chu WY, Liu ZQ, Deng D, Li TJ, Huang RL, Zhang JS, Tan BE, Wang W, Wu G (2008) Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr 138:867–872
Yeh CL, Yeh SL, Lin MT, Chen WJ (2002) Effects of arginine-enriched total parenteral nutrition on inflammatory-related mediator and T-cell population in septic rats. Nutrition 18:631–635
Acknowledgments
This research was supported by the Outstanding Overseas Chinese Scholars Fund of Chinese Academy of Sciences (CAS; no. 2005-1-4 and 2005-1-7), CAS Knowledge Innovation Project (no. YW-N-022 and KSCX2-SW-323), the National Basic Research Program of China (no. 2004CB117502), National Natural Science Foundation of China (no. 30671517, 30528006 and 30371038), Texas AgriLife Research (H-8200), and National Research Initiative Competitive Grant (no. 2008-35206-18764) from the USDA Cooperative State Research, Education, and Extension Service.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tan, B., Li, X.G., Kong, X. et al. Dietary l-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids 37, 323–331 (2009). https://doi.org/10.1007/s00726-008-0155-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0155-1