Abstract
Suboptimal nutrient intake represents a limiting factor for growth and long-term survival of low-birth weight infants. The objective of this study was to determine if in neonates who can consume only 70 % of their protein and energy requirements for 8 days, enteral leucine supplementation will upregulate the mammalian target of rapamycin (mTOR) pathway in skeletal muscle, leading to an increase in protein synthesis and muscle anabolism. Nineteen 4-day-old piglets were fed by gastric tube 1 of 3 diets, containing (kg body weight−1·day−1) 16 g protein and 190 kcal (CON), 10.9 g protein and 132 kcal (R), or 10.8 g protein + 0.2 % leucine and 136 kcal (RL) at 4-h intervals for 8 days. On day 8, plasma AA and insulin levels were measured during 6 post-feeding intervals, and muscle protein synthesis rate and mTOR signaling proteins were determined at 120 min post-feeding. At 120 min, leucine was highest in RL (P < 0.001), whereas insulin, isoleucine and valine were lower in RL and R compared to CON (P < 0.001). Compared to RL and R, the CON diet increased (P < 0.01) body weight, protein synthesis, phosphorylation of S6 kinase (p-S6K1) and 4E-binding protein (p-4EBP1), and activation of eukaryotic initiation factor 4 complex (eIF4E·eIF4G). RL increased (P ≤ 0.01) p-S6K1, p-4EBP1 and eIF4E·eIF4G compared to R. In conclusion, when protein and energy intakes are restricted for 8 days, leucine supplementation increases muscle mTOR activation, but does not improve body weight gain or enhance skeletal muscle protein synthesis in neonatal pigs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Premature birth rate in the United States has risen by 36 percent over the past 25 years, accounting for almost 8 percent of babies born in 2013 and representing the main cause of newborn deaths (March of Dimes Foundation 2014). Most preterm infants present with low-birth weight (LBW), and some remain small to adulthood (Johnson et al. 2012), increasing their risk of developing chronic metabolic diseases including cardiovascular disease and type 2 diabetes (Whincup et al. 2008; Lapillonne and Griffin 2013). Retrospective data analyses have pointed to nutritional deficiencies as a major contributor to growth faltering in LBW infants during the first weeks of life (Embleton et al. 2001; Rigo et al. 2002). Uncoordinated suckling and swallowing mechanisms and immaturity of the gastrointestinal tract and metabolic pathways have led to the implementation of feeding protocols below optimal infant requirements to minimize the risk of developing feeding-related pathologies (De Curtis and Rigo 2004). Preterm formulas and fortifiers often supplement insufficient nutrients, particularly at the protein level, to prevent growth faltering during these early weeks (De Curtis and Rigo 2004). As a result, more aggressive nutritional support has been advocated for the first days after birth to prevent muscle catabolism and minimize the interruption of growth (Wilson et al. 1997; Ziegler et al. 2002). However, the feeding of high-protein diets to neonates is limited by the concern for producing clinical complications related to hyperammonemia, azotemia and metabolic acidosis (Johnson et al. 1972; Hay 2008).
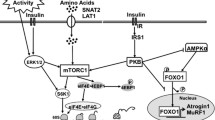
Neonatal growth is largely attributable to high rates of protein synthesis induced by the postprandial rise in insulin and amino acids (AA; Davis et al. 2002; Davis and Fiorotto 2009), with leucine (Leu) being the most effective AA mediator (Escobar et al. 2005, 2007, 2010; Wilson et al. 2010). Data generated from rats (Lynch et al. 2002), cell culture studies (Pham et al. 2001) and in vivo studies with neonatal pigs (Escobar et al. 2005, 2007, 2010; Suryawan et al. 2008; Wilson et al. 2010) indicate that the anabolic effect of Leu in muscle protein synthesis is mediated through upregulation of the mTOR pathway, that regulates both initiation and elongation phases of mRNA translation (Kimball and Jefferson 2004). Recently, we have shown that oral Leu supplementation of a low-protein diet acutely enhances protein synthesis in skeletal muscle and visceral tissues of 4-day-old pigs (Torrazza et al. 2010; Suryawan et al. 2012), and this effect is independent from insulin signaling (Suryawan et al. 2004). As such, inclusion of supplemental Leu in the diet may hold the potential to improve not only muscle protein synthesis but also overall lean growth in premature infants. Given that the clinical condition of LBW infants frequently precludes their consumption of full feeds (Carlson and Ziegler 1998), in the present study we assessed the potential for using oral Leu supplementation to enhance the lean growth of neonatal pigs when both crude protein (CP) and metabolizable energy (ME) intakes were fed at less than optimal levels. We hypothesized that Leu supplementation of a protein- and energy-restricted diet would enhance the efficiency of protein synthesis in neonate pigs, and that this increase would be mediated via the activation or upregulation of proteins involved in the mTOR pathway.
Materials and methods
Animals and experimental design
An overview of the experimental protocol is depicted in Fig. 1. The protocol was approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. Nineteen male and female (n = 4 and 15, respectively) crossbred piglets (Yorkshire × Landrace × Hampshire × Duroc; Agriculture Headquarters, Texas Department of Criminal Justice) were weaned when 2 days old and 2.1 ± 0.2 kg body weight (BW). All animals were administered iron by intramuscular injection, and then housed in individual stainless steel cages in a temperature-controlled room (28 °C), with additional zone heating provided if required, and bowl-fed ad libitum a commercial milk replacement diet (Soweena® Dry Fat 7-60™; Merrick Animal Nutrition, Middleton, WI, USA). At day 3 or 4 of age (n = 10 and 9, respectively), piglets were anesthetized with isoflurane (PPC, Richmond Hill, Ontario, CAN) after overnight fasting, and a catheter was placed surgically in the left jugular vein with use of sterile technique (Davis et al. 1996). The catheter was flushed with sterile heparinized saline solution (100 IU·mL−1; APP, Lake Zurich, IL, USA) every 48 h, and maintained with a heparin lock. At the same time, a gastric tube was inserted surgically for intra-gastric feeding. On the day after surgery (0730), all piglets were randomly assigned to receive 1 of 3 liquid diets (Tables 1, 2) for 8 consecutive days: (1) Control (CON; n = 7): 16 g CP and 190 kcal ME [kg body weight (BW)−1·day−1]; (2) Restricted (R; n = 6): 10.9 g CP and 132 kcal ME, and; (3) Restricted + Leu (RL; n = 6): 10.8 g CP supplemented with 0.2 % L-Leu and 136 kcal ME. Animals were fed 40 mL·kg BW−1 at 4-h intervals 6 times per day starting at 0730, with the liquid diet delivered over a 20-min period. Feed was increased gradually until day 3 post-surgery. The day of surgery was considered as day 0 for the remainder of the study. Body weights were recorded before the beginning of the study and on day 0, 3, 6, and 8. Average daily gain (ADG) was calculated between day 0 and 8. On day 8, protein synthesis was measured on all animals after which they were euthanized with an injection of pentobarbital sodium (0.4 mL·kg BW−1). Tissue samples were obtained from the longissimus dorsi (LD), gastrocnemius, and soleus muscles, heart and liver. Tissue was weighed, frozen in liquid N and stored at −80 °C until further use.
Outline of the experimental protocol. Three groups of 2-day-old piglets were assigned to 1 of 3 diets fed at 4-h intervals for 8 days: 1 control (CON; n = 7): 16 g crude protein (CP) and 190 kcal metabolizable energy (ME)·kg body weight (BW)−1·d−1, 2 Restricted (R; n = 6): 10.9 g CP and 132 kcal ME·kg BW−1·day−1, or 3 restricted + Leucine (Leu) (RL; n = 6): 10.8 g CP + 0.2 % Leu and 136 kcal ME·kg BW−1·day−1
Diets
The CON diet was formulated to meet or exceed nutrient requirements according to NRC (2012), whereas R and RL were formulated to be 30 % below CP and ME requirements, but to meet requirements for all other nutrients (Tables 1, 2). Desired CP reductions were achieved by reducing both whey and casein in the diet, but maintaining their ratio. A sufficient amount of crystalline Leu was added to RL to achieve a twofold increase in postprandial plasma Leu (Escobar et al. 2005), whereas alanine (Ala) was added to R to make R and RL diets isonitrogenous. Desired ME reductions were achieved by reducing the dietary fat sources. Lactose levels were maintained constant across the 3 diets. The ratio of protein to energy and the lactose content was kept constant across all diets.
Plasma insulin, glucose, urea and amino acids
Blood levels of circulating insulin, glucose, urea and free AA were measured on day 8. Blood was sampled from the jugular vein immediately before feeding, and at 30, 60, 90, 120, 180, and 240 min post-feeding. Samples were centrifuged at 12000×g for 2 min within 10 min of collection, and plasma was stored at −20 °C for subsequent analyses. Plasma insulin concentrations were measured using a porcine insulin radioimmunoassay kit (Millipore, St. Charles, MO, USA). Plasma glucose levels were determined with the glucose oxidase method (Thermo Scientific, Waltham, MA, USA). Determination of free AA concentration in plasma was performed by high-performance liquid chromatography (PICO-TAG reverse-phase column; Waters, Milford, MA, USA) using an analytical method based on deproteinization and derivatization of AA with phenylisothiocyanate (Burrin et al. 1995). Plasma urea levels were measured at 0 and 120 min using a commercial urea assay kit (Sigma-Aldrich, St Louis, MO, USA).
Protein synthesis rate
Ninety minutes after feeding, animals were injected via the jugular vein catheter with 10 mL·kg BW−1 of a flooding dose of L-[4-3H]Phe (American Radiolabeled Chemicals, St. Louis, MO, USA), which provided 1.5 mmol Phe·kg BW−1 and 1 mCi of L-[4-3H] Phe·kg BW−1. Blood samples were collected at 5, 15, and 30 min after injection, and stored at −20 °C for measurement of the radioactivity of the Phe blood pool. Piglets were euthanized immediately after the last blood sample, and tissues were collected and immediately frozen. Free and protein-bound phenylalanine in tissues was measured by HPLC using an anion exchange column (PA1 column; Dionex, Sunnyvale, CA, USA) followed by post-column derivatization, as previously described (Davis et al. 1999). Fractions were collected, and the radioactivity associated with the phenylalanine peak was measured in a liquid scintillation counter (Tri-Carb 2500TR; Packard Instrument, Meriden, CT, USA).
The fractional rate of protein synthesis (KS: % of protein mass synthesized per day; %·day−1) for each tissue was calculated as:
where S b (dpm·min−1) is the specific radioactivity of the protein-bound Phe, S a (dpm·min−1) is the average specific radioactivity of the tissue-free Phe calculated from the specific radioactivity at the time of the tissue collection corrected for the change over the labeling period from the linear regression of the blood specific radioactivity of the pig at 5, 15 and 30 min against time, and t is the time of labeling in min of the specific tissue. Previous studies have demonstrated that, after a flooding does of 3H-Phe is administered, the specific radioactivity of tissue-free Phe is in equilibrium with the aminoacyl-tRNA-specific radioactivity, and therefore the tissue-free Phe is a valid measure of the precursor pool-specific radioactivity (Davis et al. 1999).
Translational efficiency and capacity
To assess whether changes in rate of protein synthesis are due to changes in ribosomal abundance and/or translational efficiency, total tissue protein was quantified using the Pierce BCA assay (Lowry et al. 1951) and RNA content was quantified according to Munro and Fleck (1966 ). Ribosomal abundance, namely protein synthetic capacity (CS), was estimated as the total RNA-to-protein ratio (i.e., mg RNA·g protein−1), given that the majority of RNA in the tissue is ribosomal (Fiorotto et al. 2000). Ribosomal translational efficiency, namely protein synthetic efficiency (KRNA), was estimated as the total protein synthesized per total RNA (i.e., g protein·g RNA−1).
Western Blot analysis
Equal amounts of protein samples were electrophoretically separated on polyacrylamide gels (PAGE; C.B.S Scientific, Del Mar, CA, USA) and transferred to activated polyvinylidene difluoride membranes (Pall Corporation, Pensacola, FL, USA) as previously described (Suryawan et al. 2001). The membranes were incubated overnight with primary antibodies against ribosomal protein S6 kinase (S6K1; Thr389; Cell Signaling Technology), eukaryotic initiation factor 4 (eIF4E)-binding protein-1 (4EBP1; Thr70; Cell Signaling Technology), followed by 1-h incubation with secondary antibody (horseradish peroxidase-conjugated IgG fraction of goat anti-rabbit IgG or goat anti-mouse IgG). Blots were developed using an enhanced chemiluminescence kit (GE Health Sciences, Buckinghamshire, UK), visualized, and analyzed using a ChemiDoc-It Imaging System® (UVP, Upland, CA, USA). For normalization, immunoblots performed with anti-phosphospecific antibodies were exposed to stripping buffer (Pierce Biotechnology, Rockford, IL, USA), and reprobed with nonphosphospecific antibodies against S6K1 (Total; Cell Signaling Technology), and 4EBP1 (total; Bethyl Laboratories, Montgomery, TX, USA), as previously described (Davis et al. 2000).
The eukaryotic initiation factor 4 complex (eIF4E·eIF4G) was immunoprecipitated using an anti-eIF4E monoclonal antibody obtained from aliquots of fresh tissue homogenates (gift of Dr. Leonard Jefferson, Pennsylvania State University, College of Medicine, Hershey, PA, USA). Briefly, muscle homogenates were processed as previously described (Suryawan et al. 2001) and immediately subjected to protein immunoblot analysis using rabbit anti-eIF4G (Bethyl Laboratories, Montgomery, TX, USA). Amounts of eIF4G were corrected by the eIF4E recovered from the immunoprecipitates.
Statistical analyses
Data were analyzed by ANOVA using a linear mixed model in SAS 9.2 (SAS Institute Inc., Cary, NC, USA) that include diet, muscle, and their interaction as fixed effects, and initial body weight as covariate. A repeated measurement statement was included for parameters measured over time, with the structure of the covariance selected based on smallest Akaike information criterion. Normality of the residuals and presence of outliers were assessed in SAS. When necessary, data were power transformed by a parameter φ whose optimal value was estimated using the maximum likelihood (ML) method (Piepho 2009). P values for pre-planned pairwise comparisons were calculated using Student’s t tests. Data were presented as least square mean ± SE. Significant effects were considered at P ≤ 0.05 and trends at P ≤ 0.1.
Results
The interaction of diet × muscle was not significant for any of the parameters analyzed on day 8. Hence, only the effect of the diet will be discussed.
Body weight, average daily gain, and tissue weights
There were no differences in initial BW among the 3 groups (Fig. 2). Piglet BW was higher for CON compared to RL and R on day 6 (P ≤ 0.05) and day 8 (P ≤ 0.001). Average daily gain (ADG) was also higher for CON compared to RL and R (P ≤ 0.001; Table 3). Compared to RL and R, the CON diet increased or tended to increase the weight of LD (P = 0.05), gastrocnemius (P ≤ 0.1), soleus (P ≤ 0.1), heart (P ≤ 0.01), and liver (P ≤ 0.01). There were no differences between RL and R treatment groups in BW, ADG or tissue weights.
Insulin, glucose, urea and free AA levels
Compared to CON, plasma insulin concentration tended to be higher in R (P ≤ 0.1), and was lower in RL (P ≤ 0.05) at 30 min post-feeding (Fig. 3a). Between 90 and 120 min post-feeding, both RL and R had lower insulin levels than CON (P ≤ 0.05). There were no differences in plasma glucose and urea concentrations among groups (Fig. 3b; Table 3). Supplementation of Leu in the RL diet increased plasma Leu levels compared to CON and R between 30 and 180 min post-feeding (P ≤ 0.001; Fig. 4a); values for CON and R were similar. Compared to CON, Ile levels decreased in R between 0 and 120 min (P ≤ 0.05) and decreased further in RL (P ≤ 0.01, Fig. 4b), whereas Val decreased only in RL at 60 min post-feeding (P ≤ 0.01; Fig. 4c). There were no differences in the total BCAA concentration among diets (Fig. 4d). Postprandial plasma concentrations of Ala, Lys, Thr, Arg, Cit, Pro, Met, Phe, Gly, Cyst, His, Tyr, Glu, Gln, Asn, Asp, Trp, and Ser are included as Online Resource Material (OR1-18).
Protein synthesis rates and translation initiation signaling in skeletal muscle
Values for KS, CS, and KRNA are shown in Fig. 5a–c. The average KS value was higher in CON compared to R and RL (P < 0.01; Fig. 5a). Likewise, CS was higher in CON compared to R and RL (P ≤ 0.05; Fig. 5b). The KRNA increased in CON compared to R, but not to RL (P ≤ 0.01; Fig. 5c). There were no differences between R and RL in KS, CS or KRNA.
Fractional rates of protein synthesis (KS) (a), protein synthetic capacity (CS) (b) and protein synthetic efficiency (KRNA) (c) in skeletal muscle of 12-day-old piglets fed Control (CON), Restricted + Leu (RL), or Restricted (R) diets for 8 days. Values are least square mean ± SE; n = 7, 6 and 6, respectively
Protein abundance or phosphorylation values for 4EBP1, S6K1 and eIF4G·eIF4E are represented in Fig. 6a–c. The phosphorylation of S6K1 and 4EBP1 was greater in RL compared to R (P ≤ 0.01), and in CON compared to RL (P ≤ 0.0001; Fig. 6a, b). The formation of the active eIF4E·eIF4G complex was greater in RL compared to R (P ≤ 0.01; Fig. 6c), and increased further in CON group (P ≤ 0.001).
Discussion
Despite significant advances in the nutritional management of LBW infants, their extrauterine growth is often restricted due to insufficient nutrient intake (Embleton et al. 2001; Rigo et al. 2002; de Curtis and Rigo 2004), resulting in long-term adverse effects on their health with important economic implications for society (Behrman and Butler 2007; Neubauer et al. 2008; Yliharsila et al. 2007). To our knowledge, the present study is the first to address the effect of chronic dietary Leu supplementation on skeletal muscle protein synthesis in neonates when both protein and energy intakes are suboptimal. In the neonate, the primary driving force for the elevated rate of protein accretion is the capacity to attain high rates of protein synthesis after feeding (Davis et al. 2002; Davis and Fiorotto 2009). Accordingly, we and others have shown that inclusion of Leu in a protein restricted diet enhances KS in skeletal muscle of young pigs (Torraza et al. 2010; Yin et al. 2010; Suryawan et al. 2012), and adult humans (Churchward-Venne et al. 2014). However, data from the present study suggest that supplementation of Leu does not improve muscle KS or body growth when both CP and ME intakes are chronically restricted. It can be argued that dietary Leu supplementation in the present study may have been insufficient for exerting a beneficial effect on piglet growth. In fact, Leu intakes of 4.18 g·kg BW−1·day−1 have been shown to increase ADG in suckling piglets (Sun et al. 2015). However, albeit the overall dietary Leu intake in our study was lower, postprandial Leu concentrations in plasma were more than twofold higher compared to the values reported by Sun et al. (2015), hence rendering unlikely that Leu intake was insufficient for exerting beneficial effects in our pigs. In addition, previous studies have demonstrated that a twofold increase above post-absorptive levels in plasma Leu levels was required to maximize protein synthesis in neonatal pigs, (Escobar et al. 2005, 2007; Columbus et al. 2015b), values that were achieved in both RL and BCAA groups.
Previous studies of chronic Leu supplementation in adult humans following exercise suggest that the anabolic effect of Leu may depend upon the availability of substrate for protein synthesis, and therefore it needs to be associated with other AA to be efficient (Crowe et al. 2006; Balage and Dardevet 2010). Similarly, Wilson et al. (2010) and Escobar et al. (2007) observed that the increase in muscle KS in neonatal pigs induced by the short- and long-term infusion of Leu diminished when circulating EAA were reduced, and KS was restored when EAA were readjusted to baseline levels. Analysis of individual plasma AA concentrations in the current study indicates that Lys, Thr, Cys, and Pro were significantly lower in both RL and R compared to CON, which may have rendered them rate-limiting for protein synthesis. In addition, the levels of circulating Val and Ile also were decreased in the RL diet, likely due to the Leu-induced upregulation of the activity of the branched-chain-keto acid dehydrogenase enzymatic complex in BCAA oxidation (Tannous et al. 1963; Harper et al. 1970). Thus, the limited supply of some amino acids may have limited the protein synthetic response to leucine supplementation. We have shown previously that oral Leu supplementation of a low-protein meal stimulates protein synthesis to the level achieved with feeding a high-protein meal over the short term (Torraza et al. 2010). However, the oral leucine-induced stimulation of protein synthesis was blunted, but not blocked, after 24 h and it was associated with the fall in the circulating levels of Ile and Val (Suryawan et al. 2012), which may have become limiting for protein synthesis. Therefore, an insufficient supply of some AA may limit the protein synthetic response to Leu supplementation.
It is known that the efficiency of dietary protein utilization for growth is dependent not only on absolute protein intake, but also on energy intake. This has been demonstrated in LBW infants fed different energy intakes at the same protein level, where a higher energy intake was associated with an increase on overall N utilization (Duffy et al. 1981). In addition, studies in young pigs (Campbell and Dunkin 1983) and lambs (Black and Griffiths 1975) have shown a positive relationship between energy intake levels and N retention. More recently, long-term supplementation of Leu in 30 % CP restricted diets with normal ME content resulted in an increase in mTOR pathway activation and muscle protein synthesis (Yin et al. 2010; Columbus et al. 2015a), as well as greater daily body weight gain (Yin et al. 2010) in both neonatal and weaned pigs. Similarly, supplementation of Leu twice a day in suckling piglets with unrestricted access to the sow increased ADG between day 7 and 21 of lactation (Sun et al. 2015). As such, the energy deficit conditions in the present study may also have contributed to the lack of anabolic effect of Leu on muscle protein synthesis, as AA may have been redirected towards energy production instead of being used as substrates for protein synthesis.
Previous studies have shown that the Leu-induced upregulation of protein synthesis occurs via the insulin-independent phosphorylation of the mTOR complex and downstream effectors, namely S6K1 and 4EBP1 (Kimball and Jefferson 2004; Avruch et al. 2005). Once phosphorylated, 4EBP1 releases eIF4E from the inactive eIF4E·4EBP1 complex to form the active eIF4G·eIF4E complex that binds to mRNA and initiates translation (Kimball and Jefferson 2004). Likewise, activated S6K1 phosphorylates ribosomal protein S6 that also participates in translation initiation (Kimball and Jefferson 2004). Accordingly, data from the present study indicate that supplementation of the restricted diet with Leu increased the phosphorylation of S6K1 and 4EBP1, and the activation of eIF4G·eIF4E, albeit not as much as in the CON group. As the postprandial increase in insulin activates mTOR signaling in neonatal pigs (Davis et al. 2002), it is possible that the higher insulin levels in CON compared to RL during the 30- to 120-min post-feeding interval may have contributed to the greater activation of S6K1, 4EBP1 and eIF4G·eIF4E. In fact, Suryawan et al. (2012) observed that higher S6K1 and 4EBP1 phosphorylation levels in muscle of control pigs compared to pigs fed a Leu-supplemented low-protein diet were associated with higher levels of plasma insulin. In addition, in an in vitro study, insulin enhanced the activation of mTOR when human myotubes were treated with Leu (Gran and Cameron-Smith 2011).
A significant factor responsible for the higher KS observed in CON compared to RL and R was the greater value for the RNA-to-total protein ratio, which reflects a higher muscle protein synthetic capacity (i.e., ribosomal abundance). These data are consistent with previous observations in young rats (Millward et al. 1973; Yahya et al. 1994) which showed that in response to a chronic suboptimal protein and energy intake, there is a reduction in the muscle’s CS which, together with the reduction in protein synthetic efficiency, limits Ks. In addition to regulating translation initiation, the mTOR pathway is a key regulator of ribosome biogenesis, by controlling the activity of RNA polymerase involved in the synthesis of rDNA (Mayer and Grummt 2006). Although we did not measure the phosphorylation status of mTOR complex 1 (mTORC1) per se, we did find a significant correlation across groups between skeletal muscle CS and the activation of S6K1 (P = 0.02; data not shown), which upregulates ribosomal RNA transcription (Hannan et al. 2003). The response of the RL group in which supplementation with leucine was unable to prevent the decrease in protein synthetic capacity even though there was a small enhancement in mTOR activation, suggests that this response alone is not sufficient to maintain ribosome biogenesis in the presence of a chronic deficit in energy and protein. However, the higher degree of activation of S6K1, 4EBP1 and eIF4G·eIF4E in the RL group was able to augment translation initiation so that the translational efficiency of the ribosomes was not as severely compromised as in the R group. These data suggest that a decrease in the muscle protein synthetic capacity, rather than altered translational efficiency (ribosomal activity), contributed to the decline in KS in response to lower protein and energy diet.
In conclusion, chronic supplementation of Leu to a 30 % protein and energy-restricted diet did not improve the protein synthesis rate in skeletal muscle nor the body weight gain of the piglets, even though it increased the activation of key mTOR signaling proteins involved in protein translation initiation. This lack of effectiveness of leucine supplementation may be due to substrate limitation and/or reduced insulin levels. Further study is needed to determine whether availability of Val and Ile limits the ability of leucine supplementation to enhance muscle protein synthesis and growth when protein and energy intakes are suboptimal.
Abbreviations
- AA:
-
Amino acid
- ADG:
-
Average daily gain
- BCAA:
-
Branched-chain amino acid
- BUN:
-
Blood urea nitrogen
- BW:
-
Body weight
- BWG:
-
Body weight gain
- CP:
-
Crude protein
- CS :
-
Protein synthetic capacity
- CON:
-
Control diet
- EAA:
-
Essential amino acids
- eIF4E·eIF4G:
-
Eukaryotic initiation factor 4 complex
- KS :
-
Fractional rate of protein synthesis
- KRNA :
-
Protein synthetic efficiency
- mTOR:
-
Mammalian target of rapamycin
- ME:
-
Metabolizable energy
- NEAA:
-
Non-essential amino acids
- R:
-
Restricted diet
- RL:
-
Restricted diet supplemented with leucine
- S6K1:
-
Ribosomal protein S6 kinase 1
- SID:
-
Standardized ileal digestible
- 4EBP1:
-
Eukaryotic initiation factor 4-binding protein 1
References
Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S (2005) Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care 8:67–72
Balage M, Dardevet D (2010) Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care 13(3):265–270
Behrman RE, Butler AS (2007) Societal costs of preterm birth. Preterm birth: causes, consequences, and prevention. National Academies Press, Washington, pp 398–429
Black JL, Griffiths DA (1975) Effects of live weight and energy intake on nitrogen balance and total N requirement of lambs. Br J Nutr 33(3):399–413
Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S (1995) Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res 37:593–599
Campbell RG, Dunkin AC (1983) The effects of energy intake and dietary protein on nitrogen retention, growth performance, body composition and some aspects of energy metabolism of baby pigs. Br J Nutr 49(2):221–230
Carlson SJ, Ziegler EE (1998) Nutrient intakes and growth of very low birth weight infants. J Perinatol 18(4):252–258
Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, Phillips SM (2014) Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 99:276–286
Columbus DA, Steinhoff-Wagner J, Suryawan A, Nguyen HV, Hernandez-Garcia A, Fiorotto ML, Davis TA (2015a) Impact of prolonged leucine supplementation on protein synthesis and lean growth in neonatal pigs. Am J Physiol Endocrinol Metab. doi:10.1152/ajpendo.00089.2015
Columbus DA, Fiorotto ML, Davis TA (2015b) Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 47(2):259–270
Crowe MJ, Weatherson JN, Bowden BF (2006) Effects of dietary leucine supplementation on exercise performance. Eur J Appl Physiol 97(6):664–672
Davis TA, Fiorotto MF (2009) Regulation of muscle growth in neonates. Curr Opin Clin Nutr 12:78–85
Davis TA, Burrin DG, Fiorotto ML, Nguyen HV (1996) Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7-than in 26-day-old pigs. Am J Physiol 270:E802–E809
Davis TA, Fiorotto ML, Nguyen HV, Burrin DG (1999) Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol 277:E103–E109
Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR (2000) Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab 279(6):E1226–E1234
Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O’Connor PMJ (2002) Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282:E880–E890
De Curtis M, Rigo J (2004) Extrauterine growth restriction in very-low-birthweights infants. Acta Pediatr 93:1563–1568
Duffy B, Gunn T, Collinge J, Pencharz P (1981) The effect of varying protein quality and energy intake on the nitrogen metabolism of parenterally fed very low birthweight (less than 1600 g) infants. Pediatr Res 15(7):1040–1044
Embleton NE, Pang N, Cooke RJ (2001) Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? J Pediatr 107(2):270–273
Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA (2005) Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab 288:E914–E921
Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA (2007) Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab 293:E1615–E1621
Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, Davis TA (2010) Leucine and {alpha}-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr 140:1418–1424
Fiorotto ML, Davis TA, Reeds PJ, Burrin DG (2000) Nonnutritive factors in colostrum enhance myofibrillar protein synthesis in the newborn pig. Pediatr Res 48:1–7
Gran P, Cameron-Smith D (2011) The actions of exogenous leucine on mTOR signaling and amino acid transporters in human myotubes. BMC Phys 11:10–16
Hannan KM, Brandenburger Y, Jenkins A, Sharkey K, Cavanaugh A, Rothblum L et al (2003) mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol 23:8862–8877
Harper AE, Benevenga NJ, Wohlhueter RM (1970) Effects of ingestion disproportionate amounts of amino acids. Physiol Rev 50:428–558
Hay WW (2008) Strategies for feeding the preterm infant. Neonatology 94:245–254
Johnson JD, Albritton WL, Sunshine P (1972) Hyperammonemia accompanying parenteral nutrition in newborn infants. J Pediatr 81:154–161
Johnson MJ, Wootton SA, Leaf AA, Jackson AA (2012) Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics 130(3):E640–E649
Kimball SR, Jefferson LS (2004) Regulation of global and specific mRNA translation by oral administration of branched-chain amino acids. Biochem Biophys Res Commun 313:423–427
Lapillonne A, Griffin IJ (2013) Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr 162(3 Suppl):S7–S16
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Lynch CJ, Patson BJ, Anthony J, Vaval A, Jefferson LS, Vary TC (2002) Leucine is a direct-acting nutrient signal that regulates protein synthesis. Am J Physiol 283:E503–E513
March of Dimes Foundation (2014) http://www.marchofdimes.org/mission/prematurity-campaign.aspx. Accessed 6 Oct 2014
Mayer C, Grummt I (2006) Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 25:6384–6391
Millward DJ, Garlick PJ, James WP, Nnanyelugo DO, Ryatt JS (1973) Relationship between protein synthesis and RNA content in skeletal muscle. Nature 241(5386):204–205
Munro N, Fleck A (1966) The determination of nucleic acids. Meth Biochem Anal 14:113–176
Neubauer AP, Voss W, Kattner E (2008) Outcome of extremely low birth weight survivors at school age: the influence of perinatal parameters on neurodevelopment. Eur J Pediatr 167:87–95
Pham PT, Heydrick SJ, Fox HL, Kimball SR, Jefferson LS Jr, Lynch CJ (2001) Assesment of cell-signaling pathways in the regulation of mammalian target of rapamycin (mTOR) by amino acids in rat adipocytes. J Cell Biochem 79:427–444
Piepho HP (2009) Data transformation in statistical analysis of field trials with changing treatment variance. Agron J 101(4):865–869
Rigo J, De Curtis M, Pieltain C (2002) Nutritional assessment and body composition of preterm infants. Semin Neonatol 6:383–391
Sun Y, Wu Z, Li W, Zhang C, Sun K, Ji Y, Wang B, Jiao N, He B, Wang W, Dai Z, Wu G (2015) Dietary l-leucine supplementation enhances intestinal development in suckling piglets. Amino Acids 47(8):1517–1525
Suryawan A, Nguyen HV, Bush JA, Davis TA (2001) Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab 281(5):E908–E915
Suryawan A, O’Connor PM, Kimball SR, Bush JA, Nguyen HV, Jefferson LS, Davis TA (2004) Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. J Nutr 134:24–30
Suryawan A, Jeyapalan AS, Orellana RA, Wiilson FA, Nguyen FA, Davis TA (2008) Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 295:E868–E875
Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA, Fiorotto ML, El-Kadi SW, Srivastava N, Nguyen HV, Davis TA (2012) Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res 71:324–331
Tannous RE, Rogers QR, Harper AE (1963) Effect of leucine-isoleucine and valine antagonism on the pattern of free amino acids in blood plasma and several tissues of the rat. Fed Proc 22:202–210
Torrazza RM, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA (2010) Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr 140(12):2145–2152
Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE, Carlsson S et al (2008) Birth weight and risk of type 2 diabetes: a systematic review. J Am Med Assoc 300:2886–2897
Wilson DC, Cairns P, Halliday HL, Reid M, McClure G, Dodge JA (1997) Randomized controlled trial of aggressive nutritional regiment in sick very low birthweight infants. Arch Dis Child 77:F4–F11
Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA (2010) Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 140:264–270
Yahya ZA, Tirapegui JO, Bates PC, Millward DJ (1994) Influence of dietary protein, energy and corticosteroids on protein turnover, proteoglycan sulphation and growth of long bone and skeletal muscle in the rat. Clin Sci (Lond) 87(5):607–618
Yin YL, Yao K, Liu ZJ, Gong M, Ruan Z, Deng D, Tan BE, Liu ZQ, Wu G (2010) Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486
Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG (2007) Birth size, adult body composition and muscle strength in later life. Int J Obesity 31:1392–1399
Ziegler EE, Thureen PJ, Carlson SJ (2002) Aggressive nutrition of very low birthweight infant. Clin Perinatol 29:225–244
Acknowledgments
The work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR-044474 (Davis) and AR-46308 (Fiorotto), National Institute of Child Health and Human Development HD-072891 (Davis), United States Department of Agriculture National Institute of Agriculture grant 2013-67015-20438 (Davis), and by the USDA/ARS under Cooperative Agreement no. 6250-510000-055 (Davis). This work is a publication of the USDA, Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. The contents of this publication do not necessarily reflect the views or politics of the USDA, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
R. Manjarín and D. A. Columbus contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manjarín, R., Columbus, D.A., Suryawan, A. et al. Leucine supplementation of a chronically restricted protein and energy diet enhances mTOR pathway activation but not muscle protein synthesis in neonatal pigs. Amino Acids 48, 257–267 (2016). https://doi.org/10.1007/s00726-015-2078-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2078-y