Abstract
A specially synthesized nitroxide biradical R6-13C≡C-p-C6H4–C≡13C-R6, B3, where R6 = 1-oxyl-2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridine nitroxide, has been studied by electron paramagnetic resonance spectroscopy, and electron-nuclear double resonance (ENDOR). Spin density distribution and hyperfine splitting (hfs) constant on 13C atoms calculations for biradical B3 were carried out using B3LYP and PBE0 functionals and several different basis sets including N07 family and were compared with the experimental value of the hfs constant on 13C atoms, measured from ENDOR spectra of B3. The mechanism of the intramolecular electron spin exchange in B3 biradical is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Scientists all over the world have been intrigued by the chemistry of stable free radicals since their discovery in 1900 [1]. Stable radicals of different types are presently used in a variety of fields [2], including spin labeling [3–12], the construction of organic magnetic materials [13], MRI contrast agents [14], redox-active components of organic radical batteries [15], co-oxidants [16] and tools for controlled radical polymerization [17]. Some biradicals were found to be promising polarizing agents for dynamic nuclear polarization [18, 19]. Nevertheless, in many cases, biradical or polyradical structure and features, their magnetic properties are still the subject of investigation [20, 21].
Among numerous nitroxide biradicals, those containing acetylene groups in the bridge connecting two nitroxide rings present an interesting specific group [22–31]. In these biradicals, which are structurally rigid enough, spin exchange coupling between the unpaired electrons is realized by the indirect mechanism via the bridge of atoms and bonds connecting two paramagnetic nitroxide rings [27, 30–32]. The intramolecular dynamics and temperature behavior in such biradicals dissolved in various molecular and ionic liquid solvents is usually described well with a two-conformational model [32, 33]. In such biradicals, the intramolecular spin exchange coupling occurs by the indirect mechanism through the bridge of atoms and bonds.
It has been recently shown that such polyacetylene biradicals as R6-C≡C-R6 (B1), R6-C≡C–C≡C-R6 (B2), R6-C≡C-p-C6H4–C≡C-R6 (B3), where R6 is 1-oxyl-2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridine nitroxide [30, 31] with acetylene groups in the bridge are not “rigid” but “flexible”, and biradical B2, due to fast rotations around its molecular axis, demonstrates average values of the exchange integral |J|. Biradical B3 is characterized by a higher rotation barrier in vacuum and is, therefore, more rigid. It was concluded from electron paramagnetic resonance (EPR) data that both biradicals exist in at least two different conformers with a slight influence of temperature on the |J/a| value. It decreases at high temperature, when faster rotation averages the configurations more effectively [30]. A short nitroxide biradical, B1, has been investigated by EPR spectroscopy supplemented with a single-crystal X-ray analysis and density functional theory (DFT) calculations [31]. Structural and EPR data were complemented with quantum chemical calculations, and conformational transitions in biradical B1 were also in a good agreement with quantum chemical computation. Nevertheless, understanding that in such chemically rigid polyacetylene nitroxide biradicals, intramolecular spin exchange can be realized only by the indirect mechanism, we could not measure the spin density distribution on atoms forming the bridge in biradicals and describe quantitatively spin delocalization and its influence on the |J| value.
In this work, we present our results on studying the spin density delocalization and the mechanism of the intramolecular spin exchange in the nitroxide biradical R6-13C≡C-p-C6H4–C≡13C-R6, B3, in which two carbon atoms are substituted by 13C isotopes (I = 1/2). Spin coupling in B3 can be realized only via the bridge of atoms and bonds connecting two 1-oxyl-2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridine nitroxides, and it was investigated using EPR, electron-nuclear double resonance (ENDOR) spectroscopy as well as quantum chemical calculations.
2 Experimental
2.1 Synthesis of B3 Biradical
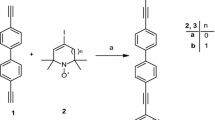
Methyl-13C-triphenylphosphonium iodide in anhydrous THF suspension was treated with butyl lithium solution. To this brown-colored solution was added the terephthalaldehyde (1), and after work-up, we got the 1,4-divinylbenzene, of which bromination with 2.2 equiv. Br2 in CHCl3 gave the tetrabromo product. Elimination with 6 equiv. KOt-Bu in THF generated the isotopically labeled 1,4-diethynyl benzene (2). The Sonogashira coupling with 4-iodo-2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridin-1-yloxyl radical [36] in Et3N and in the presence of CuI and Pd(PPh3)2Cl2 biradical (3) was generated. According to our experience this synthetic methodology resulted no Glaser-coupled product formation was observed (while paramagnetic acetylenes reaction with 1,4-diiodobenzene generated two types of biradicals) (Fig. 1).
Reagents and conditions were: (i) 13CH3P(Ph)3I (2.18 eq.), BuLi (2.18 eq.), THF, 2 h, 0 °C, then −78 °C, terephthalaldehyde (1.0 eq.) 15 min, rt, 3 h, 83 %, then CHCl3, Br2 (2.2 eq.), rt, 12 h, then KOt-Bu (6.0 eq.), THF, reflux, 3 h, 60 %. (ii) 4-iodo-2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridin-1-yloxyl radical (2.1 eq.), Et3N, CuI (0.1 eq.), Pd(PPh3)2Cl2 (0.06 eq.), 50 °C, 20 h, 25 %. Here, rt denotes room temperature.
Melting points were determined with a Boetius micro melting point apparatus and are uncorrected. Elemental analyses (C, H, N) were performed on Fisons EA 1110 CHNS elemental analyzer. Mass spectra were recorded on a Thermoquest Automass Multi. 1H NMR and 13C NMR spectra were recorded with Bruker Avance 3 Ascend 500 spectrometer. Chemical shifts are referenced to Me4Si. The paramagnetic compound was reduced with 5 equiv. pentafluorophenyl hydrazine/radical. Measurements were run at 298 K probe temperature in CDCl3 or CD3OD solution. Flash column chromatography was performed on Merck Kieselgel 60 (0.040–0.063 mm). Qualitative TLC was carried out on commercially available plates (20 × 20 × 0.02 cm) coated with Merck Kieselgel GF254. It is essential that anhydr. THF can be used only, Et3N was distilled from CaH2 prior to use. Terephthalaldehyde and methyl-13C-triphenylphosphonium iodide and butyl lithium were purchased from Aldrich. 4-Iodo-2,2,6,6-tetramethyl-5,6-dihydropyridin-1-yloxyl radical [36] was prepared according to published procedures, other reagents were purchased from Aldrich or Alfa Aesar.
1,4-Diethynylbenzene (2): methyl-13C-triphenylphosphonium iodide (4.86 g, 12 mmol) was suspended in dry THF (50 ml), cooled to 0 °C, and n-BuLi (4.8 ml, 12.0 mmol, 2.5 mol/l solution in hexane) was added. It was stirred for 2 h at the same temperature, then cooled to −78 °C, and the terephthalaldehyde (737 mg, 5.5 mmol) as solution in anhydrous THF was added dropwise. After 15 min of stirring at −78 °C, the mixture was warmed to room temperature and stirred for another 3 h. After stopping the reaction with water (50 ml), it was extracted with Et2O (3 × 20 ml), and the combined organic phases were dried over MgSO4, the pure olefin was obtained by column chromatography to give 1,4-divinylbenzene (600 mg, 83 %). The 1,4-divinylbenzene was dissolved in CHCl3 (20 ml); to this solution, Br2 (1.60 g, 10.0 mmol) was added, and the mixture was allowed to stay overnight. The solvent and bromine were evaporated off in vacuo, the residue was dissolved in THF (30 ml), then KOt-Bu (27.6 mmol, 3.10 g) was added in one portion, and the mixture was stirred and refluxed for 3 h. After cooling, the solvent was evaporated off, and the residue was partitioned between Et2O (30 ml) and 5 % aq. H2SO4 (10 ml). The organic phase was separated, dried (MgSO4), filtered and evaporated, and the residue was purified by flash column chromatography with (hexane/Et2O, 100:1) to give a brownish-white solid (350 mg, 60 %), mp 93–94 °C, R f 0.37 (hexane), MS (70 eV): m/z = 128 (M+, 100), 101 (6) 76 (33). Anal calculated for C 138 C2H6: C 95.28; H 4.72, found C 95.20; H 4.59.
4,4′-(1,4-Phenylenebis(ethyne-2,1-diyl))bis(2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridin-1-yloxyl) biradical B3.
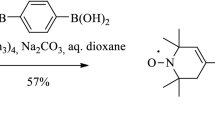
To a degassed solution of 4-iodo-2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridin-1-yloxyl radical (1.40 g, 5.0 mmol) in anhydrous Et3N (10 ml), CuI (47 mg, 0.25 mmol) and PdCl2(PPh3)2 (112 mg, 0.16 mmol) were added, and the mixture was stirred for 15 min, followed by the addition of 13C containing 1,4-diethynylbenzene (300 mg, 2.34 mmol) and was stirred for 20 h at 50 °C under Ar, in a sealed tube. The reaction mixture was diluted with CHCl3 (10 ml), filtered through Celite, and the solvent was evaporated. The residue was partitioned between CHCl3 (30 ml) and aq. sat. NaCl solution (10 ml), the organic phase was separated, washed with 10 % aq. Na2S2O3 (10 ml), water (10 ml), dried (MgSO4) and to this mixture, activated MnO2 (172 mg, 2.0 mmol) was added, and the mixture was set aside for overnight. Next, the mixture was filtered, evaporated and purified by flash column chromatography (hexane/CHCl3) to give the title compound (254 mg, 25 %) as a deep-yellow solid, mp 233–235 °C, R f 0.66 (hexane/EtOAc). MS (70 eV): m/z = 432 (M+, 42), 402 (29), 372(18), 357 (16), 257 (22), 42 (100). Anal calculated for C 1326 C2H34N2O2: C 78.20; H 7.92; N 6.48; found C 78.15; H 7.80; N 6.42.
2.2 EPR Measurements
Toluene was selected as a solvent and was carefully purified as per the literature procedure [37]. Solutions were prepared, bubbled with nitrogen for 20–25 min. 0.5 ml of the solution was taken into a thin capillary and degassed by frieze-pump circle to remove oxygen, and sealed off under vacuum. Radical concentrations were sufficiently low (≤4 × 10−4 mol l−1) to eliminate intermolecular exchange broadening of EPR lines [38].
EPR spectra were recorded at X-band on a Bruker EMX-8 spectrometer with a modulation frequency of 100 kHz. Temperatures were controlled with an accuracy of ±0.5 °C at temperatures between −20 and 75 °C by means of a JEOL JNM-VT-30 temperature control system. EPR parameters: the hyperfine splitting, hfs, constant on nitrogen 14N atom, a, and a value of the exchange integral |J/a|, which is sensitive to any changes of the spin density distribution of the unpaired electron in the system. The exchange integral values |J/a| were calculated in accordance with [32, 39]. EPR spectra of biradicals were simulated with the computer program package created by Dr. A. A. Shubin (Boreskov Institute of Catalysis, Siberian Branch, Russian Academy of Sciences) and described in details in Ref. [39]. For our calculations, we used spin-Hamiltonian parameters of similar nitroxide radicals collected in [40].
In liquid solutions with rather low viscosity, the spin Hamiltonian Ĥ comprises the isotropic hyperfine, the Zeeman interactions and the exchange coupling. In the case when both radical fragments are identical and one nucleus with a nonzero nuclear spin I, the following equation is valid [34, 35]:
The spin Hamiltonian is, here, written in frequency units; superscripts 1 and 2 denote different radical fragments; S (k) are electron spin operators; S (k)z and I (m)z are projections of the electron and nuclear spins to the Z-axis, respectively; g is the isotropic g-factor of the radical fragments; β e is the Bohr magneton; H 0 is the external magnetic field; a ≈ 3 × 108 rad/s denotes the 14N isotropic hyperfine splitting (hfs) constant, and J is the exchange integral. In low-viscous solvents, the tensor of the dipole–dipole coupling is averaged to zero by fast rotation of the biradical molecule [32, 33]. For any individual conformation, the one value of |J| should correctly describe the position and integral intensities of all lines in the EPR spectrum. Values of |J| are usually measured in units of the hfs constant a, i.e., as |J/a|, with an accuracy of ±2–3 %.
For the two-conformational model [32, 34, 35], the Arrhenius plot of |J/a| should be linear for any biradical, and this allows determining the differences in enthalpies, ΔH, and entropies, ΔS, of these two conformations:
The experimental observation of such plot confirms the validity of the two-conformational model for a biradical.
2.3 ENDOR Measurements
For measuring hyperfine splitting (hfs) constants of the interaction between the unpaired electron and 13C nucleus, the ENDOR method suggested by Mims has been used [41]. In this method, three microwave pulses are used, which form the signal of the stimulated echo. Between the second and the third microwave pulses (mw pulses), the radio-frequency pulse (rf pulse) is applied. The signal of the stimulated echo is recorded as a function of the rf pulse frequency (Fig. 2).
Measurements were carried out using a pulsed EPR spectrometer Elexsys E580 at X-band (10 GHz). A spectrometer is equipped with a standard commercial resonator EN 4118X-MD4W1, which was placed into cryostat CF935. Temperature control was carried out by the ITC503 unit. All measurements were done at 80 K. Two biradicals, B3 and its analog B3a with a usual isotope 12C (I = 0), were investigated. Both biradicals were dissolved in toluene at ~1 mmol/l concentration and degassed by pumping. ENDOR study was performed using 16-, 48- and 60-ns pulses as mw π/2-pulse and 10 µs as rf one. It should be noted that no difference in ENDOR spectra using different lengths of the mw pulses was observed. Interpulse time τ was equal to 600 ns.
2.4 Calculation Details
Calculations were performed with ORCA 3.0.3 program package [42]. B3 geometry was optimized on UKS/B3LYP/cc-pVTZ level and is in good agreement with previously reported results. Hfs constants were calculated using density functional theory with B3LYP and PBE0 functionals and variety of full electron basis sets such as EPR-II [43], cc-pVTZ, aug-cc-pVTZ [44], N07D and N07T family (including diffuse functions) [45]. Fine Lebedev 770 angular grid and 10−10 Eh SCF convergence tolerance were used. Solvent effects were simulated with COSMO model [46].
3 Results and Discussion
3.1 EPR Spectroscopy
Typical EPR spectrum of biradical B3 at 298 K is shown in Fig. 3. The comparison of simulated and experimental spectra shows that they are in a very good agreement, and positions of all “exchange” lines in the magnetic field are practically coinciding (Fig. 3). Similar EPR spectra were described in Refs. [25, 30].
The exchange integral value |J/a| measured from spectra simulation at 298 K is equal to 3.4 ± 0.1 mT (a = 15.02 G), which coincides with those published in Refs. [25, 30]. One can see from Fig. 3 that amplitudes of the central and high-field simulated main “radical” lines are bigger than those of experimental ones. This is caused by the “stick” shape of the B3 molecule, which is realized in the anisotropic rotation of B3 performed in the experimental spectrum, while spectrum simulation has been done for the case of the isotropic rotation [39]. Positions of all the lines for both spectra are coinciding.
Temperature dependences both of a and |J/a| values for B3 dissolved in toluene slightly decrease with the increase of temperature and coincide in the error limits with those reported in [30]: ΔH = −0.5 ± 0.2 kJ mol−1 and ΔS = 6.8 ± 0.7 J mol−1 K−1. Such small values were already explained in Ref. [30]: the barrier of rotation, E a, of piperidine rings in biradicals with acetylene groups in the bridge is rather low as shown by results from the DFT calculations: it does not exceed 8.0 kJ mol−1 for B3. Librations of the piperidine planes, relatively the p-C6H4 plane by up to 5°, have a value of E a less than 0.1 kJ mol−1, i.e., negligible. These results correlate well with the experimentally measured values of ΔH, which are equal to −0.5 kJ mol−1 and close to −1.0 kJ mol−1 in the case of B3 dissolved in toluene and ethanol, respectively. It should also be mentioned that such small values of E a for biradical B3 result in rather free intramolecular rotation of the nitroxide rings even at low temperatures, i.e., the EPR spectra and measured values of |J/a| characterize not individual conformations of B3 but an averaged pattern with very fast transitions between several rotamers, and the measured value of |J/a| is averaged by all these conformations.
3.2 ENDOR Investigation
ENDOR spectra of biradicals B3 and B3a are shown in Fig. 4. Signals of both samples are observed in the area of 3 and 5 MHz (and the same lines or two biradicals at the frequency region about 8–20 MHz, data are not shown), and we suppose that they reflect the interaction of the unpaired electron with a nuclear spin of nitrogen 14N. Besides these signals, in the ENDOR spectrum of B3 enriched with 13C, two additional lines are observed in the area of 3.5–4.5 MHz. Subtracting the spectrum of the usual biradical B3a from the spectrum of B3 results in the signal (Fig. 4c), which contains only two peaks with a center at 3.7 MHz, what corresponds to a Larmor frequency of the carbon nucleus precession in the magnetic field, close to ~340 mT. The splitting between peaks is equal to 0.48 ± 0.14 MHz. These results clearly indicate that we observe a rather weak interaction between the unpaired electron and the 13C carbon atom. The hfs constant value in such case is equal to the splitting, i.e., to 0.48 MHz. It should be noted that we could not determine all values of the anisotropic hfs tensor from our study of biradical B3 in frozen toluene solutions.
3.3 DFT Calculations
Important information about B3 structure and features has been obtained from quantum chemical calculations. Calculated geometry of biradical B3 is shown in Fig. 5, where the appropriate values of bond lengths, 13C and other isotropic hfs constants on different atoms in B3 are listed for two cases of calculation methods: B3LYP/N07D and PBE0/N07D. The whole set of 13C isotropic hfs constant values calculated using B3LYP and PBE0 functionals at various basis sets is given in Table 1. The distance between centers of N–O bonds is equal to 17.78 Å.
It should be noted that while calculations of the dipolar components of the hfs tensor can be systematically tuned by going to a much larger basis set of common use, isotropic hfs constants are usually reproduced better using a specialized basis set described in [45]. According to this note, we should point out two important results: first, specialized basis sets, such as N07 family or EPR family, enable much better agreement with the experimental data than the conventional basis sets on far less computational prices even in this case of very small hfs coupling. Second, we point out that, for some reasons, PBE0 functional systematically gives better agreement of calculated 13C isotropic hfs constant with the experimental one in this particular case, while B3LYP functional, in turn, underestimates it, though both approaches yield results which are within the experimental error limits and agree well with the experimental value. We conclude that combination of PBE0/N07D or PBE0/EPR-II can be recommended as a cheap and precise way for computation of small hfs couplings in nitroxide biradicals. A more detailed comparison of the experimental results obtained in different solvents with calculated ones taking into account various polarities, we intend to do in the next paper.
4 Conclusion
A nitroxide biradical R6-13C≡C-p-C6H4–C≡13C-R6, B3, where R6 = 1-oxyl-2,2,6,6-tetramethyl–1,2,3,6-tetrahydropyridine nitroxide, has been investigated by X-band EPR and electron-nuclear double resonance (ENDOR) spectroscopy. Spin density distribution and hyperfine splitting (hfs) constant value on 13C atoms in the bridge in biradical B3 were calculated using B3LYP and PBE0 functionals and several different basis sets including N07 family. These results were compared with the experimental value of the hfs constant on 13C atoms measured from ENDOR spectra of B3. A few recommendations concerning the calculation of electron spin density distribution and the isotropic hfs constant values in nitroxide biradicals are given. Results presented in this paper confirm the fact that intramolecular electron spin exchange in B3 biradical is realized by the indirect mechanism.
References
M. Gomberg, J. Am. Chem. Soc. 22, 757 (1900)
R.G. Hicks (ed.), Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds (Wiley, West Sussex, 2010)
Spin Labeling. Theory and Applications, ed. by L.J. Berliner (Academic Press, New York, 1976)
A.N. Kuznetsov, The Method of Spin Probes (Nauka, Moscow, 1976)
G.I. Likhtenshtein, Spin Labeling Methods in Molecular Biology (Wiley, New York, 1976)
A.M. Wasserman, A.L. Kovarsky, Spin Labels and Probes in Physical Chemistry of Polymers (Nauka, Moscow, 1986)
Imidazoline Nitroxides. Synthesis, Properties, Applications, vol. 1, 2, ed. by L.B. Volodarsky (CRC Press, Boca Raton, 1988)
S.S. Eaton, G.R. Eaton, Electron Paramagnet. Reson. 19, 318 (2004)
G. Likhtenshtein, J. Yamauchi, S. Nakatsuji, A.I. Smirnov, R. Tamura, Nitroxides: Applications in Chemistry, Biomedicine, and Materials Science (Wiley-VCH, New York, 2008)
K. Moebius, A. Savitsky, High-Field EPR Spectroscopy on Proteins and Their Model Systems (RSC Publishing, London, 2009)
H.P. Nguyen, A.M. Popova, K. Hideg, P.Z. Qin, BMC Biophys. 8, 6 (2015)
C. Altenbach, C.J. López, K. Hideg, W.L. Hubbell, Methods Enzymol. 564, 59 (2015)
S.M. Winter, S. Hill, R.T. Oakley, J. Am. Chem. Soc. 137, 3720 (2015)
A. Rajca, Y. Wang, M. Boska, J.T. Paletta, A. Olankitwanit, M.A. Swanson, D.G. Mitchell, S.S. Eaton, G.R. Eaton, S. Rajca, J. Am. Chem. Soc. 134, 15724 (2012)
T. Janoschka, N. Martin, U. Martin, C. Friebe, S. Morgenstern, H. Hiller, M.D. Hager, U.S. Schubert, Nature 527, 78 (2015)
M. Rafiee, K.C. Miles, S.S. Stahl, J. Am. Chem. Soc. 137, 14751 (2015)
M.Y. Zaremski, Polymer Sci. Ser. C 57, 65 (2015)
F. Mentink-Vigier, U. Akbey, H. Oschkinat, S. Vega, A.J. Feintuch, Magn. Reson. 258, 102 (2015)
C. Sauvée, M. Rosai, G. Casano, F. Aussennc, T.R. Weber, R. Ouari, P. Tordo, Angew. Chem. Int. Ed. 52, 10858 (2013)
A.I. Kokorin, V.N. Khrustalev, O.I. Gromov, Appl. Magn. Reson. 46, 1429 (2015)
E.B. Böde, D. Margraf, J. Plackmeyer, G. Dürner, T.F. Prisner, O. Schiemann, J. Am. Chem. Soc. 129, 6736 (2007)
E.G. Rozantsev, Free Nitroxyl Radicals (Plenum Press, New York, 1970)
A.B. Shapiro, M.G. Goldfield, E.G. Rozantsev, Tetrahedron Lett. 24, 2183 (1973)
V.V. Pavlikov, A.B. Shapiro, E.G. Rozantsev, Izv. AN SSSR Ser. Khim. 1, 128 (1980)
A.I. Kokorin, V.V. Pavlikov, A.B. Shapiro, Proc. Acad. Sci. USSR Dokl. Phys. Chem. 253, 525 (1980)
S. Torii, T. Hase, M. Kuroboshi, C. Amatore, A. Jutand, H. Kawafuchi, Tetrahedron Lett. 38(42), 7391 (1997)
A.I. Kokorin, Appl. Magn. Reson. 26, 253 (2004)
A.I. Kokorin, V.A. Tran, K. Rasmussen, G. Grampp. Appl. Magn. Reson. 30, 35 (2006)
V.A. Tran, A.I. Kokorin, G. Grampp, K. Rasmussen, Appl. Magn. Reson. 35, 389 (2009)
A.I. Kokorin, E.N. Golubeva, B.Y. Mladenova, V.A. Tran, T. Kálai, K. Hideg, G. Grampp, Appl. Magn. Reson. 44, 1041 (2013)
O.I. Gromov, E.N. Golubeva, V.N. Khrustalev, T. Kálai, K. Hideg, A.I. Kokorin, Appl. Magn. Reson. 45, 981 (2014)
V.N. Parmon, A.I. Kokorin, G.M. Zhidomirov, Stable Biradicals (Nauka, Moscow, 1980)
V.N. Parmon, G.M. Zhidomirov, Mol. Phys. 27, 367 (1974)
S.H. Glarum, J.H. Marshall, J. Chem. Phys. 47, 1374 (1967)
H. Lemaire, J. Chim. Phys. 64, 559 (1967)
T. Kálai, J. Jekő, Z. Berente, K. Hideg, Synthesis, 439 (2006)
J.A. Riddick, W.B. Bunger, K.T. Sakano, in: Techniques of Chemistry. Vol. II: Organic Solvents, Physical Chemistry and Methods of Purification (Wiley, New York, 1986)
Yu.N. Molin, K.M. Salikhov, K.I. Zamaraev, Spin Exchange (Springer, Berlin, 1980)
A.I. Kokorin, V.N. Parmon, A.A. Shubin, Atlas of Anisotropic EPR Spectra of Nitroxide Biradicals (Nauka, Moscow, 1984)
Ya.S. Lebedev, O.Ya. Grinberg, A.A. Dubinsky, O.G. Poluektov, in: Bioactive Spin Labels, ed. by R.I. Zhdanov (Springer, Berlin, 1992), pp. 228–254
W.B. Mims, Proc. R. Soc. Lond. 283, 452 (1965)
F. Neese, The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2, 73 (2012)
V. Barone, in: Recent Advances in Density Functional Methods, Part I, ed. by D.P. Chong (World Scientific Publishing Co., Singapore, 1996)
T.H. Dunning Jr., J. Chem. Phys. 90, 1007 (1989)
V. Barone, P. Cimino, E. Stendardo, J. Chem. Theory Comput. 4, 751 (2008)
S. Sinnecker, A. Rajendran, A. Klamt, M. Diedenhofen, F. Neese, J. Phys. Chem. A 110, 2235 (2006)
V. Sadovnichy, A. Tikhonravov, Vl. Voevodin, V. Opanasenko, Lomonosov, Supercomputing at Moscow State University, in: Contemporary High Performance Computing: from Petascale Toward Exascale (Chapman & Hall/CRC Computational Science, CRC Press, Boca Raton, 2013), pp. 283–307
Acknowledgments
This research was partly supported by Hungarian National, Research, Development and Innovation Office (OTKA104956). The study was also supported by the Supercomputing Center of M. V. Lomonosov Moscow State University [47]. AIK and OIG are grateful to Dr. E. N. Golubeva (Chemistry Department, M.V. Lomonosov Moscow State University) for helpful discussions, and to Dr. A. A. Shubin (G.K. Boreskov Institute of Catalysis, Siberian Branch of Russian Academy of Sciences), who kindly provided us with his program package.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kokorin, A.I., Zaripov, R.B., Gromov, O.I. et al. Spin Density Distribution in a Nitroxide Biradical Containing 13C-Enriched Acetylene Groups in the Bridge: DFT Calculations and EPR Investigation. Appl Magn Reson 47, 1057–1067 (2016). https://doi.org/10.1007/s00723-016-0813-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-016-0813-5