Abstract
Two nitroxide biradicals of similar composition: R5-C≡C-Ph-Ph-C≡C-R5 (B3a) and R6-C≡C-Ph-Ph-C≡C-R6 (B3b), where Ph = p-C6H4, and R6 is 2,6,6-tetramethyl-5,6-dihydropyridin-1(2H)-yloxyl, and R5 is 2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-1-yloxyl nitroxide rings, have been studied by electron paramagnetic resonance (EPR) spectroscopy. Variations of the intramolecular electron spin exchange in the biradicals, dissolved in toluene, as a function of temperature were characterized by changes in the isotropic 14N hyperfine splitting (hfs) constant a, values of the exchange integral |J|, and compared with the data obtained by density functional theory calculations (DFT). Thermodynamic parameters of the conformational rearrangements were calculated. Geometries of nitroxide biradicals in PES local minima and transition states in the triplet state were calculated at UDFT/B3LYP level with split-valence basis set cc-PVTZ. Probable differences in biradicals behavior are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
During the last 50 years, stable nitroxide biradicals became routine subjects of numerous investigations. Many hundreds of them have been synthesized, studied and utilized in different applications as spin probes, as polarizing agents for dynamic nuclear polarization, etc. [1–10], and in “References” therein. Among these hundreds of various structures, there is a special group of chemically rigid biradicals, which contain several acetylene groups in the bridge connecting two paramagnetic nitroxide rings [1, 3, 5, 11–20]. In such biradicals, intramolecular spin exchange is realized by the indirect mechanism, i.e., by the spin coupling via the chain of atoms and bonds [3, 5]. Temperature dependence of |J| in liquid solutions of such biradicals is not so much as in the case of flexible biradicals and only slightly depends on the solvent nature [21–25]. It was recently shown [19, 20, 26] that in such biradicals as R6-(C≡C)2-R6 (B1) and R6-C≡C-Ph-C≡C-R6 (B2), where Ph = p-C6H4, and R6 is 2,2,6,6-tetramethyl-5,6-dihydropyridin-1(2H)-yloxyl nitroxide ring, which look like chemically rigid structures with high degree of conjugation between two unpaired electrons locating in paramagnetic >N–O groups [11–15, 17] are not really rigid. These biradicals are characterized by a very slight dependence of the exchange integral |J| on temperature, similar in different molecular solvents. Values of |J| can be easily measured from their electron paramagnetic resonance (EPR) spectra with high accuracy, and biradicals structure can be determined with high precision by quantum-chemical calculations based on density functional theory (DFT).
For biradicals B1 and B2 expected to be rigid because of conjugation of double bonds in the nitroxide ring with triple bonds in the bridge, we showed using the UDFT/B3LYP/cc-pVDZ calculations that the rotation barriers for B1 and B2 biradicals do not exceed a value of 1.0 and ~8 kJ mol−1 respectively, therefore, the internal rotation around the main axis in both biradicals is practically free at ambient temperatures. It was also published that for a short acetylene biradical, R6-C≡C-R6, the barrier of the internal rotation is also very low and equal to 4.0 kJ/mol. It is of scientific interest to proceed with such DFT calculations and to compare them with EPR measurements for similar biradicals of bigger size.
In this paper we report our experimental and theoretical results about the structure and EPR spectra peculiarities of two nitroxide biradicals with similar structures with two acetylene and two para-phenylene groups in the spacer. R–C≡C-Ph-Ph-C≡C-R, in which R depicts five or six-membered nitroxide ring.
2 Experimental
2.1 Synthesis of the Biradicals
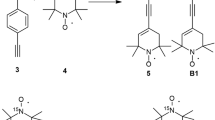
In our work, biradicals B3a and B3b were synthesized by the procedure which is shown in Fig. 1 and described below in details from compounds 2a [27] and 2b [28], which were prepared according to published procedures. 4,4′-diethinylbifenyl was purchased from TCI, other reagents were purchased from Aldrich or Alfa Aesar. Anhydrous THF was used and Et3N was distilled from CaH2 prior to use. Melting points were determined with a Boetius micro melting point apparatus and are uncorrected. Elemental analyses (C, H, N, S) were performed on Fisons EA 1110 CHNS elemental analyzer. Mass spectra were recorded on a Thermoquest Automass Multi. Flash column chromatography was performed on Merck Kieselgel 60 (0.040–0.063 mm). Qualitative TLC was carried out on commercially available plates (20 × 20 × 0.02 cm) coated with Merck Kieselgel GF254.
To a degassed solution of 2a or 2b (2.0 mmol) in THF (20 mL) and Et3N (2.5 mL), CuI (20 mg, 0.1 mmol) and Pd (PPh3)4 (50 mg, 0.04 mmol) was added under N2 and after stirring for 15 min. compound 1 (202 mg, 1.0 mmol) in THF (5 mL) was added dropwise. After stirring the mixture overnight at room temperature, it was filtered through Celite, the solvent was evaporated and the residue was dissolved in CH2Cl2 (15 mL), washed with brine (10 mL), the organic phase was separated, dried (MgSO4), activated MnO2 (86 mg, 1.0 mmol) was added and O2 was bubbled through 15 min. The mixture was set aside for 6 h, then filtered, evaporated and the residue was purified by flash column chromatography (hexane/CH2Cl2) to yield compound B3a or B3b.
3,3′-([1,1′-Biphenyl]-4,4′-diylbis(ethyn-2,1-diyl))bis(2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-1-yloxyl) Biradical (B3a): 215 mg (45 %), pale yellow solid, mp 228–230 °C, R f: 0.54 (hexane/EtOAc, 2:1). MS (70 eV): m/z = 478 (M+, 20), 448 (8), 418 (100), 403 (8). Anal calcd for C32H34N2O2: C, 80.30; H,7.16; N, 5.85; found: C, 80.21; H 7.02; N, 5.74.
4,4′-([1,1′-Biphenyl]-4,4′-diylbis(ethyn-2,1-diyl))bis(2,2,6,6-tetramethyl-5,6-dihydropyridin-1(2H)-yloxyl) Biradical (B3b): 116 mg (23 %), deep yellow solid, mp 230–233 °C, R f: 0.60 (hexane/EtOAc, 2:1). MS (70 eV): m/z = 506 (M+, 32), 491 (45), 476 (58), 461 (85), 446 (75), 165 (100). Anal calcd for C34H38N2O2: C, 80.60; H,7.56; N, 5.53; found: C, 80.42; H 7.46; N, 5.48.
2.2 EPR Measurements
Toluene was selected as a solvent and was carefully purified according to literature procedure [29]. Solutions were prepared, bubbled with nitrogen for 20–25 min. 0.5 ml of a solution was taken into a thin capillary and degassed by frieze-pump circle to remove oxygen, and sealed off under vacuum. Radical concentrations were sufficiently low (≤4 × 10−4 mol l−1) to eliminate intermolecular exchange broadening of EPR lines [30].
EPR spectra were recorded at X-band on a Bruker EMX-8 spectrometer with a modulation frequency of 100 kHz. Temperatures were controlled with accuracy ±0.5 °C at temperatures between 298 and 368 K by means of a JEOL JNM-VT-30 temperature control system. EPR parameters: the hyperfine splitting, hfs, constant on nitrogen 14N atom, a, and a value of the exchange integral |J/a|, which is sensitive to any changes of the spin density distribution of the unpaired electron in the system. The exchange integral values |J/a| were calculated in accordance with [3, 31]. EPR spectra of biradicals were simulated with the computer program package created by Dr. A. A. Shubin (Boreskov Institute of Catalysis, Siberian Branch, Russian Academy of Sciences) and described in detail in Ref. [31]. For our calculations, we used spin-Hamiltonian parameters of similar nitroxide radicals collected in [32].
In liquid solutions with rather low viscosity, the spin Hamiltonian Ĥ comprises the isotropic hyperfine, the Zeeman interactions and the exchange coupling. In the case when both radical fragments are identical and each carries only one nucleus with a nonzero nuclear spin I the following equation is valid [33, 34]:
The spin Hamiltonian is here written in frequency units; superscripts 1 and 2 denote different radical fragments; S (k) are electron spin operators; S (k)z and I (m)z are projections of the electron and nuclear spins to the Z-axis, respectively; g is the isotropic g-factor of the radical fragments; β e is the Bohr magneton; H 0 is the external magnetic field; a ≈ 3·108 rad/s denotes the 14N isotropic hyperfine splitting (hfs) constant, and J is the exchange integral. In low-viscous solvents, the tensor of the dipole–dipole coupling is averaged to zero by fast rotation of the biradical molecule [32, 33]. For any individual conformation, the one value of |J| should correctly describe the position and integral intensities of all lines in the EPR spectrum. Values of |J| are usually measured in units of the hfs constant a, i.e., as |J/a|, with an accuracy of ±2 to 3 %.
For the two-conformational model [3, 33, 34], the Arrhenius plot of |J/a| should be linear for any biradical, and this allows determining the differences in enthalpies, ΔH, and entropies, ΔS, of these two conformations:
The experimental observation of such linear plot may confirm the validity of the two-conformational model for a biradical.
2.3 Calculation Details
Calculations were performed with ORCA 3.0.3 program package [35]. Geometries of nitroxide biradicals in PES local minima and transition states in the triplet state were calculated at UDFT/B3LYP level with split-valence basis set cc-PVTZ [36]. Stationary point achievement was verified by calculation of normal mode frequencies. Open-shell singlet state geometries were calculated using Broken Symmetry approximation [37, 38] at the same level of theory and were found to match triplet state equilibrium geometries. Distances RNO–NO were taken as distances between centers of N–O bonds. Zero field splitting values D were also calculated at UDFT/B3LYP/cc-PVTZ level neglecting a spin–orbit part. Spin–spin part was calculated on the basis of unrestricted natural orbitals [39].
3 Results and Discussion
Typical EPR spectra of biradicals B3a and B3b at 338 K are shown in Fig. 2. The comparison of simulated (open circles) and experimental (solid lines) spectra shows that they are in a good agreement, positions of all “exchange” lines in the magnetic field practically coincide (Fig. 2); therefore, the exchange integral values are measured with high precision.
One can see from Fig. 2 that amplitudes of the central and high-field simulated main “radical” lines are bigger than those of experimental ones. This is caused by the “stick-shape” of both molecules, that is realized in the anisotropic rotation of both biradicals performed in the experimental spectrum, while spectra simulations have been done for the case of the isotropic rotation [31]. Positions of all lines for both spectra coincide. Measured from spectra simulation values of the exchange integral |J| at 338 K are equal to 7.6 ± 0.3 G (a = 14.32 G) and 4.35 ± 0.2 G (a = 15.0 G) for B3a and B3b, respectively. Temperature changes of the hfs constant a′ = da/dT are equal to −7.2·10−4 and 3.4·10−3 G·K−1 for B3b and B3a, which are comparable with data published in [5].
Temperature dependences of |J/a| values for both B3a and B3b biradicals dissolved in toluene are shown in Fig. 3. One can see slight increase of |J/a| with the increase of temperature in the case of B3a, while in the case of B3b this dependence has the opposite tendency similar to that one reported for biradical B2 (see Fig. 3) in Ref. [19] and explained in Ref. [30] for the biradical B2: the barrier of rotation, E a, of piperidine rings in biradicals with acetylene groups in the bridge is rather low as results from the DFT calculations: it is not more than 8 kJ mol−1 for B3. Librations of the piperidine planes relatively the p-C6H4 plane by up to 5° have a value of E a less than 0.1 kJ mol−1, i.e., negligible. These results correlate well with the experimentally measured values of enthalpy, ΔH, which are equal to −0.5 kJ mol−1 and close to −1.0 kJ mol−1 in the case of B3 dissolved in toluene and ethanol, respectively. It should be also mentioned that such small values of E a for biradical B3 results in rather free intramolecular rotation of the nitroxide rings even at low temperatures, i.e., the EPR spectra and measured values of |J/a| do not characterize individual conformations of B3 but an averaged pattern with very fast transitions between several rotamers, and the measured value of |J/a| is averaged by all these conformations.
Formal enthalpies of conformational transitions in both biradicals B3a and B3b were calculated from linearized |J/a| dependencies: ΔH B3a = 0.8 ± 0.2 kJ mol−1, and ΔH B3b = −3.0 ± 0.2 kJ mol−1. Though in the present case DFT calculation predict identical energies of all possible conformations of B3a and B3b biradicals (Fig. 4). Further, B3b biradical has 8 energy equivalent conformations with the angle between SOMOs varying from 0°, this conformer corresponds to the maximal J value, to ~60° at conformers with much lower |J| values. It makes a simple two-conformational model inapplicable in our case.
B3a and B3b biradicals with two acetylene and two p-phenylene groups in the bridge exhibit fast inversions of R6′ ring (in the case of B3b biradical only) and internal rotations around the axis passing through the main molecular axis of the bridge (Fig. 5) even at rather low temperatures, averaging the |J/a| values. Low barriers E a allow “mixing”, i.e., averaging the conformers spectral parameters at high temperatures effectively as have been described in Refs. [19, 20].
Slight decrease of |J/a| values with the increase of temperature for B3b biradical cannot be explained by the population of distinct conformations with lower |J|, but rather a higher accessibility of larger deviations from equilibrium geometries leading to lowering of the exchange coupling. In the case of B3a biradical, there is an inverse dependence (Fig. 3): slight increase of |J/a| with temperature. The idea that changes shown in Fig. 3 are caused by temperature changes of the hfs constant a are also not correct: indeed, it was shown in [5], that a values decrease with temperature in the case of all studied piperidine-type radicals and biradicals except O=R6 radical [5], but a increases with temperature for five-membered pyrroline- and pyrrolidine-type nitroxides [5, 40]. Therefore, |J/a| value dependences vs. temperature are similar to those of a. We assume that such differences between B3a and B3b should be connected with structural peculiarities of these biradicals, transitions between their conformers, which are manifested in very small values of the activation energy E a and of enthalpy ΔH. We intend to start such complex quantum chemical calculations of these systems in the future.
Important spectroscopic and structural parameters: values of the dipolar coupling constant D (a zero-field splitting) and a distance R NO–NO between centers of >N–O groups were determined by DFT calculations (Table 1). This distance in the case of B3b is about 8 % (1.6–1.7 Å) longer than for both cis- and trans-conformations of B3a.
Studying different nitroxide biradicals with several acetylene and p-phenylene groups in the bridge connecting nitroxide rings [14], it was proposed that in such long stick-shape biradicals the intramolecular spin exchange can not be realized by the direct mechanism (straight overlapping of the wave functions of the unpaired electrons localized on >N–O groups), and can be performed by the indirect mechanism: via spin density coupling through the bridge of atoms and bonds. EPR spectra of such biradicals are weakly temperature-dependent, and |J/a| values measured for the set of them allowed authors to suggest that the efficiency of spin density delocalization of unpaired electrons should be characterized with the use of the “coefficient of attenuation” γ = |J i/J k| for the biradicals with (|J k/a|) and without (|J i/a|) a certain group or atom in the bridge. |J k/a| and |J i/a| values should both be measured at the same temperature and in the same solvent [5]. This “attenuation coefficient” γ k is a characteristic parameter of the functional group or atom and does not depend on other groups forming the bridge.
Knowledge of all γ k coefficients for all groups in the bridge allows estimating the spin density ρ s localized on the carbon atom in the fourth position of the piperidine or in the third position of the five-membered ring connected with the nearest (first) atom of the biradical bridge, or the exchange integral value |J RR| for a biradical R6–R6 or a hypothetic biradical R5–R5:
where γ i, γ j, γ k are the attenuation coefficients for i-, j- or k-atom or a group in the bridge between two R6 or R5 fragments in a real biradical with its |J Birad|. Using this procedure, in the case of B3b (|J 298/a| = 0.29) and B2 (|J 298/a| = 2.2 [19]), one can calculate γ Ph = 7.6, which is close to the value γ Ph = 6.6 obtaining from the pair B2 and B1. This deviation is likely to result from the fact that in B3b biradical phenyl rings are not coplanar, hence, conjugation in the whole system is weaker, than in the case of B2 biradical. This means that the electron spin exchange integral |J| decreases approximately seven-fold passing through p-C6H4 group. This is in a good agreement with the result published in [3]. Using this value of γ Ph and a value of γ C≡C = 2.2 ± 0.15 [5], one can calculate the following value: |J R6R6/a B3b| = |J B3b/a B3b|·γ 2Ph ·γ 2C≡C = 81, or |J R6R6| ≈ 1220 G ≫ a B3b ≈ 15.0 G for biradical B3b. This value is in a good correlation with |J R6R6| ≥ 0.12 cm−1 ≈ 1285 G obtained in Ref. [20]. The same value estimated in [5] was equal to |J R6R6| = 1050 G. Similarly, for B3a, the value of |J R5R5| ≈ 2220 G (|J 298/a| = 0.53, a ≈ 14.3 G). The bigger value of |J R5R5| comparing to |J R6R6| is understandable because the C3 atom in a five-membered ring is located much closer to the paramagnetic >N–O group than the C4 atom in the six-membered ring.
4 Conclusions
Nature of the exchange coupling in two nitroxide biradicals R6-C≡C-Ph–Ph-C≡C-R6 (B3b) and R5-C≡C-Ph-Ph-C≡C-R5 (B3a) is discussed basing on the results obtained by DFT calculations and X-band EPR data. The attenuation coefficients of Ph group found in the work are in a good agreement with previously observed ones. Temperature dependences of the exchange couplings are explained well by the results of DFT calculations with low activation energy barriers of internal motions (rotations or librations) responsible for the averaging of the exchange coupling. Energies of transition states of these motions are found to be substantially small allowing practically free internal rotation around the main molecular axis of the biradical.
References
E.G. Rozantsev, Free Nitroxyl Radicals (Plenum Press, New York, 1970)
Spin Labeling. Theory and Applications, ed. by L.J. Berliner (Academic Press, New York, 1976)
V.N. Parmon, A.I. Kokorin, G.M. Zhidomirov, Stable Biradicals (Nauka, Moscow, 1980)
L.B. Volodarsky (ed.), Imidazoline Nitroxides. Synthesis, Properties, Applications, vol. 1, 2 (CRC Press, Boca Raton, FL, 1988)
A.I. Kokorin, Appl. Magn. Reson. 26, 253 (2004)
A.M. Wasserman, A.L. Kovarsky, Spin Labels and Probes in Physical Chemistry of Polymers (Nauka, Moscow, 1986)
S.S. Eaton, G.R. Eaton, Electron Paramagn. Reson. 19, 318 (2004)
K. Moebius, A. Savitsky, High-Field EPR Spectroscopy on Proteins and their Model Systems (RSC Publishing, London, 2009)
C. Sauvée, M. Rosai, G. Casano, F. Aussennc, T.R. Weber, R. Ouari, P. Tordo, Angew. Chemie Int. Ed. 52, 10858 (2013)
F. Mentink-Vigier, U. Akbey, H. Oschkinat, S. Vega, A.J. Feintuch, J. Magn. Reson. 258, 102 (2015)
A.B. Shapiro, M.G. Goldfield, E.G. Rozantsev, Tetrahedron Lett. 24, 2183 (1973)
V.V. Pavlikov, A.B. Shapiro, E.G. Rozantsev, Izv. AN SSSR, Ser. Khim., 128(1) (1980)
V.V. Pavlikov, V.V. Muraviev, A.B. Shapiro, Izv. AN SSSR, Ser. Khim., 1200(5) (1980)
A.I. Kokorin, V.V. Pavlikov, A.B. Shapiro, Proc. Acad. Sci. USSR, Doklady Phys. Chem. 253, 147 (1980)
A.B. Shapiro, V.N. Parmon, V.V. Pavlikov, V.I. Rubtsov, E.G. Rozantsev, Izv. AN SSSR, Ser. Khim., 449(2) (1980)
E.G. Rozantsev, O.A. Ozhogina, R.R. Rakhimov, A.I. Prokof’ev, Mol. Phys. 76, 1009 (1992)
S. Torii, T. Hase, M. Kuroboshi, C. Amatore, A. Jutand, H. Kawafuchi, Tetrahedron Lett. 38, 7391 (1997)
A.I. Kokorin, V.A. Tran, K. Rasmussen, G. Grampp, Appl. Magn. Reson. 30, 35 (2006)
A.I. Kokorin, E.N. Golubeva, B. Mladenova, V.A. Tran, T. Kalai, K. Hideg, G. Grampp, Appl. Magn. Reson. 44, 1041 (2013)
O.I. Gromov, E.N. Golubeva, V.N. Khrustalev, T. Kálai, K. Hideg, A.I. Kokorin, Appl. Magn. Reson. 45, 981 (2014)
V.A. Tran, K. Rasmussen, G. Grampp, A.I. Kokorin, Appl. Magn. Reson. 32, 395 (2007)
V.A. Tran, A.I. Kokorin, G. Grampp, K. Rasmussen, Appl. Magn. Reson. 35, 389 (2009)
A.I. Kokorin, V.A. Tran, G.A. Vorobieva, Appl. Magn. Reson. 37, 473 (2010)
G. Grampp, K. Rasmussen, A.I. Kokorin, Appl. Magn. Reson. 26, 245 (2004)
E.G. Ionita, G.A. Vorobieva, V. Chechik, A.I. Kokorin, Appl. Magn. Reson. 46, 251 (2015)
O.I. Gromov, E.N. Golubeva, V.N. Khrustalev, E.N. Degtyarev, A.A Dubinsky, A.I. Kokorin, in Atmosphere, Ionosphere, Safety, ed. by I.V. Karpov (IK BFU, Kaliningrad, 2014), pp. 148–153
T. Kálai, B. Bognár, J. Jekő, K. Hideg, Synthesis (2006), p. 2573
T. Kálai, J. Jekő, Z. Berente, K. Hideg, Synthesis (2006), p. 439
J.A. Riddick, W.B. Bunger, K.T. Sakano, in Techniques of Chemistry. Vol. II: Organic Solvents, Physical Chemistry and Methods of Purification (Wiley, New York, 1986)
YuN Molin, K.M. Salikhov, K.I. Zamaraev, Spin Exchange (Springer, Berlin, 1980)
A.I. Kokorin, V.N. Parmon, A.A. Shubin, Atlas of Anisotropic EPR Spectra of Nitroxide Biradicals (Nauka, Moscow, 1984)
Ya.S. Lebedev, O.Ya. Grinberg, A.A. Dubinsky, O.G. Poluektov, in Bioactive Spin Labels, ed. by R.I. Zhdanov (Springer, Berlin, 1992), pp. 228–254
S.H. Glarum, J.H. Marshall, J. Chem. Phys. 47, 1374 (1967)
H. Lemaire, J. Chim. Phys. 64, 559 (1967)
F. Neese, The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2, 73 (2012)
T.H. Dunning Jr., J. Chem. Phys. 90, 1007–1023 (1989)
L. Noodleman, J. Chem. Phys. 74, 5737–5743 (1981)
L. Noodleman, E.R. Davidson, Chem. Phys. 109, 131–143 (1986)
F. Neese, J. Chem. Phys. 127, 164112 (2007)
A. Weber, O. Schiemann, B. Bode, T.F. Prisner, J. Magn. Reson. 157, 277–285 (2002)
V. Sadovnichy, A. Tikhonravov, Vl. Voevodin, V. Opanasenko Lomonosov, Supercomputing at Moscow State University, in Contemporary High Performance Computing: From Petascale toward Exascale (Chapman & Hall/CRC Computational Science, CRC Press, Boca Raton, USA, 2013), pp. 283–307
Acknowledgments
This work was financially supported by National Research Development and Innovation Office, Hungary (OTKA104956). Authors are grateful to Prof. E. N. Golubeva (M. V. Lomonosov Moscow State University, Chemistry Department) for useful comments and to Dr. A. A. Shubin (Boreskov Institute of Catalysis, Siberian Branch, Russian Academy of Sciences, Novosibirsk) who provided us with his computer program package of EPR spectra simulation. DFT calculations were performed using resources of the Supercomputing Center of M. V. Lomonosov Moscow State University [41].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kokorin, A.I., Gromov, O.I., Kálai, T. et al. Peculiarities of Spin Exchange in Nitroxide Biradicals Containing Two para-Phenylene Groups in the Bridge: EPR Investigation and DFT Calculations. Appl Magn Reson 47, 1283–1293 (2016). https://doi.org/10.1007/s00723-016-0826-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-016-0826-0