Summary
Background
Genetic analysis of choroidal melanoma is frequently used to estimate the risk of metastatic spread of the tumor. Obtaining a biopsy for genetic analysis, however, can be difficult and sometimes unsuccessful. We evaluated the feasibility and accuracy of genetic testing using array comparative genomic hybridization (CGH) after radiotherapy, from tumor samples obtained by endoresection or after secondary enucleation.
Material and methods
Fifteen choroidal melanoma samples obtained after radiotherapy (Ruthenium-106 plaque brachytherapy or Gamma-Knife radiosurgery) were analyzed by array CGH to detect chromosomal aberrations (monosomy 3 and trisomy 8q), and the results were compared with pre-irradiation findings in five cases.
Results
Array CGH was successfully performed in all 15 cases. Time from radiotherapy to obtaining the sample for cytogenetic testing was between 14 and 879 days. Results of post-radiotherapy genetic analysis did not differ from pre-radiotherapy findings.
Conclusion
Post-radiation CGH appears to be a promising option for prognostic testing if a first biopsy before radiotherapy failed or was not performed. It could be useful to avoid an additional surgical procedure before radiotherapy if vitrectomy or endoresection is planned after radiotherapy.
Zusammenfassung
Hintergrund
Die genetische Untersuchung von Aderhautmelanomen ist eine zunehmend häufiger eingesetzte Methode, um das Risiko der Metastasenentwicklung bei PatientInnen mit Aderhautmelanomen einzuschätzen. Die Gewinnung einer Gewebeprobe zur Durchführung der Untersuchung ist jedoch manchmal schwierig und nicht in allen Fällen erfolgreich. Wir untersuchten die Durchführbarkeit und Genauigkeit der genetischen Untersuchung von Aderhautmelanomen mittels array comparative genomic hybridization (CGH) nach Strahlentherapie, an mittels Endoresektion oder nach Enukleation gewonnenem Tumormaterial.
Material und Methode
Fünfzehn Gewebeproben von strahlentherapeutisch behandelten Aderhautmelanomen wurden mittels array-CGH auf Veränderungen an den Chromosomen 3 und 8 untersucht (Monosomie 3, Trisomie 8q). Die Ergebnisse wurden mit den in fünf Fällen vorliegenden Resultaten der genetischen Untersuchung vor Bestrahlung verglichen.
Resultate
Die array CGH konnte in allen 15 Fällen nach Bestrahlung erfolgreich durchgeführt werden. Die Zeitspanne von der Bestrahlung bis zur genetischen Untersuchung lag zwischen 14 und 879 Tagen. Die Resultate der genetischen Untersuchung nach Bestrahlung unterschieden sich nicht von den Ergebnissen der in 5 Fällen vorliegenden Ergebnissen vor der Bestrahlung.
Schlußfolgerung
Die array CGH von Aderhautmelanomen nach Strahlentherapie erscheint eine vielversprechende Option zur prognostischen Unteruchung in den Fällen zu sein, in denen eine Biopsie vor Bestrahlung nicht durchgeführt wurde oder nicht erfolgreich war. Im Falle einer geplanten Endoresektion nach Bestrahlung, könnte auf einen zusätzlichen Eingiff zu Biopsie vor der Bestrahlung verzichtet werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The eye is the most common location for non-cutaneous primary melanoma [1]. A total of 85 % of all ocular melanomas arise from the uvea, and uveal melanoma is the most common primary intraocular tumor in adults [2]. In contrast to melanoma of the skin, the frequency of uveal melanoma has not increased over the past decades [3]. Mortality is mainly due to metastasis to the liver, which occurs in up to 45 % of patients within 10 years after treatment [4]. Local treatment for intraocular melanoma, usually enucleation or radiotherapy, has a high success rate and achieves excellent tumor control, but fails to improve survival [3]. Clinical and histopathologic risk factors for the development of metastatic disease have been identified and evaluated for their prognostic significance [5]. Classical risk factors include a large tumor size, ciliary body involvement, epitheloid cell type, and extravascular matrix patterns. Over the past decade, cytogenetic studies of choroidal melanoma identified characteristic non-random chromosomal abnormalities in choroidal melanoma cells, affecting chromosomes 1, 3, 6, and 8 in up to 50 % of melanoma patients [6, 7]. Specific cytogenetic abnormalities, such as loss of one copy of chromosome 3 and amplification of the long arm of chromosome 8, have been shown to be associated with unfavorable prognosis, and are superior to traditional prognostic markers in predicting metastatic spread [8, 9]. Over the past years, cytogenetic analysis of choroidal melanoma slowly developed from a research tool into a routine clinical test [10]. Selected centers have been using genetic testing in clinical routine for more than a decade now [11]. For analysis, only a small sample of tumor tissue has to be obtained. However, the proportion of patients treated with enucleation has decreased over the past decades, and an increasing number of patients are treated with globe-preserving intent, mainly by radiotherapy [3]. Tumor tissue, therefore, has to be acquired by taking biopsies from the melanoma via a transvitreal or transscleral approach before treatment [12–14]. On the other hand, an increasing number of patients undergo vitrectomy and/or endoresection of the tumor after radiotherapy [15–17]. Cytogenetic analysis of tumor material obtained by endoresection after radiation would be a convenient method to acquire a sufficiently large tumor sample for genetic analysis. However, there is no literature available regarding the validity of commonly used genetic tests for uveal melanoma after radiotherapy. In this report, we present results of post-radiotherapy genetic testing using array comparative genomic hybridization (CGH) and compare the results with pre-radiation findings in a small series of patients with choroidal melanoma.
Materials and methods
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 [5]. Approval of the local ethics committee was obtained to review all cases of patients with choroidal melanoma, who had pre- and/or post-radiation cytogenetic testing. All patients gave their written informed consent to evaluate the results from genetic analysis for research before the test was performed.
Biopsy technique
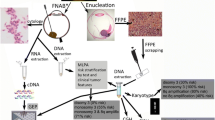
Since 2007, we have been offering cytogenetic testing to all our patients with ocular melanoma, following an approach similar to the one described by Damato et al. [10]. Diagnosis of choroidal melanoma was made after a comprehensive ophthalmologic examination, using indirect ophthalmoscopy and ultrasonography in all cases. Fluorescein and indocyanin green angiographies were done if necessary. All patients were sent to an oncologist or a specialist for internal medicine to rule out distant metastases. Systemic evaluation before radiotherapy included ultrasonography of the liver, magnetic resonance imaging (MRI) of the head/orbit and abdomen, and blood test, including liver parameters. To obtain tumor samples during plaque brachytherapy, we used a short 27-gauge needle connected to a 5-ml syringe via a short plastic tube (4 cm). After removal of the dummy plaque and immediately before suturing the plaque to the globe, the surgeon (Werner Wackernagel) dried the sclera with a cotton tip and then perforated the sclera in a tangential direction, to make the wound self-sealing. When entering the tumor, the needle direction was changed to almost perpendicular to the scleral surface, and suction was applied via the syringe to aspirate tumor cells. After withdrawal of the needle, a cotton tip was pressed onto the sclera to prevent bleeding. The cells were flushed from the needle tip into the syringe by aspirating balanced salt solution (BSS) and were immediately sent for genetic analysis. Before Gamma-Knife radiosurgery, specimens were obtained by 23-gauge transvitreal biopsy. The instruments were entered into the eye in typical and standardized manner [18–20]. The vitreous cutter was inserted into the melanoma, and tumor tissue was aspirated for 10–20 s. The instruments were withdrawn, and the biopsy material was aspirated from the tube into a 5-ml syringe. The trocars of the 23-gauge vitrectomy system prevented direct contact between the vitreous cutter and the sclera at the entry sites. To prevent post-operative hypotony, sclerotomies were sutured in case they did not appear to be completely self-sealing. Samples for post-radiotherapy testing were obtained after secondary enucleation or during endoresection. After enucleation, the globe was cut along the meridian opposite the tumor base. A small sample (2 × 2 × 2 mm3) was cut from the tumor apex, put into BSS, and sent for cytogenetic testing. Endoresection was performed by one surgeon (Andreas Wedrich) using a standard 3-port 20-gauge vitrectomy system without systemic hypotension [21]. Tumor material was aspirated into a 10-ml syringe and sent for cytogenetic testing immediately after surgery.
DNA isolation and amplification
DNA was extracted by the means of the Qiagen Mini Kit (Qiagen, Vienna, Austria) according to the manufacturer’s instructions. In cases where not enough tumor material was available, cells were applied onto a polyethylene terephthalate (PET) membrane-covered microscope slide (Zeiss, Austria). Isolation of the cells of interest was carried out using a laser microdissection and pressure catapulting system (LMPC; P.A.L.M., Zeiss, Austria). The cells were selected and directly catapulted into the cap of a 200-µl Eppendorf tube containing 10 µl of digestion mix.
Whole-genome amplification of the DNA was performed using the GenomePlex Single Cell Whole Genome Amplification Kit (#WGA4; Sigma-Aldrich, Germany). After purification using the GenElute PCR Clean-up Kit (#NA1020; Sigma-Aldrich, UK), DNA concentration was determined by a Nanodrop spectrophotometer. Amplified DNA was stored at ‒ 20 °C.
Array CGH
Array CGH was carried out using a commercially available whole-genome oligonucleotide microarray platform (Human Genome CGH 44B and 60K Microarray Kit, Agilent Technologies, Santa Clara, CA). As a reference DNA, commercially available male DNA was used (Promega, Madison, WI, USA), and in case of amplified test DNA, amplified reference DNA was used. Samples were labeled with the Bioprime Array CGH Genomic Labeling System (Invitrogen, Carlsberg, CA, USA) according to the manufacturer’s instructions. Briefly, 250–500 ng of test DNA and reference DNA were differentially labeled with dCTP-Cy5 or dCTP-Cy3 (GE Healthcare Corp., Piscataway, NJ, USA). Further steps were performed according to the manufacturer’s protocol (version 6.0; http://www.agilent.com). Slides were scanned using Agilent’s microarray scanner G2505B (Agilent Technologies), and images were analyzed using Feature Extraction and DNA Workbench software 5.0.14.
Results
In total, 15 patients had post-radiotherapy genetic analysis, either after Ruthenium-106 plaque brachytherapy (5 patients) or after Gamma-Knife radiotherapy (10 patients). In ten cases, only post-radiotherapy testing was available; 5 patients had genetic analysis done before and after radiotherapy.
Radiation dose was 100 Gy to the tumor apex for brachytherapy and 30 Gy [50 % isodose encompassing the PTV (planning target volume)] for Gamma-Knife radiosurgery in all cases.
In total, median time between radiotherapy and post-radiation cytogenetic testing for all 15 patients was 154 (range: 14–879) days . Five patients showed concurrent monosomy 3 and gains of chromosome 8, two showed monosomy 3 only, four showed only gains of chromosome 8, and four showed no changes of chromosome 3 or 8. The median observation time after radiotherapy for the eight patients without monosomy 3 was 911 (range: 62–2,685) days, and as expected, none of these patients developed liver metastases. The median follow-up time for the seven patients with monosomy 3 was 1501 (range: 851–2161) days. One of those seven patients developed liver metastasis 14 months after initial treatment.
Pre- and post-radiotherapy testing: before radiotherapy, two patients had transscleral fine-needle aspiration biopsy (before Ru-106 brachytherapy), and in three patients, biopsy of the melanoma was obtained by 23-gauge transvitreal biopsy (before Gamma-Knife radiosurgery). In four of those melanomas, we observed both loss of chromosome 3 and gain of the long arm of chromosome 8. One melanoma had only a gain of 8q (Table. 1). Median time between radiotherapy and post-radiation genetic analysis in those five cases was 76 (range: 34–526) days.
The comparison between pre- and post-treatment results revealed unchanged status of chromosomes 3 and 8 in all cases (Table. 2, Fig. 1). However, in two cases (case 1 and case 5), the breakpoints identified on chromosome 8q were slightly different before and after radiotherapy.
Post-radiotherapy testing only: 10 patients who did not have pre-operative genetic testing underwent endoresection (6 cases) or enucleation (1 case) after Gamma-Knife radiosurgery, or endoresection after brachytherapy (3 cases), and asked for cytogenetic analysis of the irradiated tumor. Array CGH analysis was successfully performed on the post-radiation material between 14 and 879 days after radiotherapy (median: 347 days) and allowed to establish the copy number status of chromosomes 3, 8, and other chromosomes. Of those 10 melanomas, 3 showed monosomy 3, 4 showed gains of chromosome 8, 1 melanoma showed both changes, and in 4 cases, chromosomal status was normal.
Conclusion
Cytogenetic testing of uveal melanoma has advanced from a research tool to a prognostic test used in daily clinical routine [10]. Until now, genetic testing has been performed and published on specimens obtained from enucleated eyes or on biopsies taken before radiotherapy only [12, 22, 23]. Our results demonstrate the feasibility of cytogenetic testing after radiotherapy and show that results of array CGH are not altered by radiotherapy.
There are several possible indications for post-radiation cytogenetic testing. First, post-radiation testing offers a chance for prognostic genetic analysis if a first attempt before radiotherapy was unsuccessful. Unsuccessful analysis has been reported in up to 25 % of patients for fluorescence in situ hybridization on fine-needle aspiration biopsies [23]. A reliable salvage procedure to obtain genetic profile might become increasingly important when patients with high-risk melanoma—and only those—are to be included into adjuvant treatment trials. Second, if endoresection is planned, or the need for additional intraocular surgery is foreseeable, one could avoid an additional surgical procedure before radiotherapy and the possible complications resulting from tumor biopsy before radiotherapy. Endoresection has become increasingly common after radiotherapy of large uveal melanomas [15, 17]. During surgery, large tumor samples can be obtained without an additional surgical procedure and without jeopardizing visual outcome [24]. Third, post-radiation biopsy might help to avoid the hypothetical risk of spreading tumor cells into the blood stream or seeding cells in the needle tract [25]. There is no evidence supporting a hypothetical spreading of melanoma cells into systemic circulation by tumor biopsy, and a correlation between treatment modality and the amount of circulating tumor cells could not be established [26]. On the other hand, most centers still try to avoid endoresection without previous irradiation of the tumor [15, 27]. Similarly, seeding of tumor cells at the sclerotomy site seems to be a rare event. However, a few cases of extraocular extension after biopsy have been reported recently [28]. Post-radiation biopsy might help to further reduce the risk of spreading tumor cells, and thus increase the acceptance of prognostic genetic testing.
Time after radiotherapy did not appear to be a limiting factor for genetic analysis by array CGH in our case series. Even patients who had their melanoma treated several years ago, when cytogenetic analysis was not routinely performed, could perhaps be offered this prognostic test. Vital-appearing tumor cells have been found in uveal melanomas years after brachytherapy, without clinical evidence of tumor recurrence [29]. As CGH is based on copy number variations, it is unlikely to be altered after radiotherapy. Whether gene expression profiling—another method for estimating prognosis in uveal melanoma—is influenced by preceding radiotherapy remains to be established [30, 31].
In summary, post-radiation array CGH for genetic analysis of uveal melanoma seems to be an attractive option to obtain important prognostic information if pre-radiation biopsy was not performed or failed and genetic status is required.
References
Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110:956–61.
Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma. a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–78.
Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–5.
Singh AD, Topham A. Survival rates with uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110:962–5.
Mudhar HS, Parsons MA, Sisley K, et al. A critical appraisal of the prognostic and predictive factors for uveal malignant melanoma. Histopathology. 2004;45:1–12.
Prescher G, Bornfeld N, Becher R. Nonrandom chromosomal abnormalities in primary uveal melanoma. J Natl Cancer Inst. 1990;82:1765–9.
Sisley K, Rennie IG, Cottam DW, et al. Cytogenetic findings in six posterior uveal melanomas: involvement of chromosomes 3, 6, and 8. Genes, chromosomes & cancer. 1990;2:205–9.
Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–5.
Sisley K, Rennie IG, Parsons MA, et al. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes, chromosomes & cancer. 1997;19:22–8.
Damato B, Coupland SE. Translating uveal melanoma cytogenetics into clinical care. Arch Ophthalmol. 2009;127:423–9.
Damato B, Duke C, Coupland SE, et al. Cytogenetics of uveal melanoma: a 7-year clinical experience. Ophthalmology. 2007;114:1925–31.
Midena E, Bonaldi L, Parrozzani R et al In vivo detection of monosomy 3 in eyes with medium-sized uveal melanoma using transscleral fine needle aspiration biopsy. European journal of ophthalmology. 2006;16:422–5.
Young TA, Burgess BL, Rao NP, et al. Transscleral fine-needle aspiration biopsy of macular choroidal melanoma. Am J Ophthalmol. 2008;145:297–302.
Wackernagel W, Schmutzer M, Mayer CF, et al. Biopsy of intraocular tumors in clinically uncertain diagnosis. Spektrum der Augenheilkunde. 2005;19:171–5.
Bechrakis NE, Foerster MH. Neoadjuvant proton beam radiotherapy combined with subsequent endoresection of choroidal melanomas. Int Ophthalmol Clin. 2006;46:95–107.
Singh AD, Triozzi PL. Endoresection for choroidal melanoma: palliative or curative intent? Br J Ophthalmol. 2008;92:1015–6.
Bechrakis NE, Blatsios G, Schmid E, et al. Surgical resection techniques of large uveal melanomas. Spektrum Der Augenheilkunde. 2010;24:17–22.
Herwig M, Eter N. 23-gauge versus 20-gauge vitrectomy: analysis of 110 consecutive cases undergoing epiretinal membrane peeling and macular hole repair. Spektrum Der Augenheilkunde. 2012;26:172–4.
Bezatis A, Laufenbock C, Zehetner C, Kieselbach G, Kralinger M, et al. Macular hole surgery: anatomical and functional results. Spektrum Der Augenheilkunde. 2011;25:302–5.
Tarmann L, Wedrich A, Hass A, et al. Limited vitrectomy with intravitreal bevacizumab, rt-PA and gas for submacular hemorrhage due to age-related macular degeneration. Spektrum Der Augenheilkunde. 2012;26:197–201.
Mayer CF, Langmann G, Wackernagel W, et al. Globe preservation and visual function after endoresection and Gamma-Knife radiosurgery for uveal melanomas. Spektrum der Augenheilkunde. 2009;23:347–52.
Shields CL, Ganguly A, Materin MA, et al. Chromosome 3 analysis of uveal melanoma using fine-needle aspiration biopsy at the time of plaque radiotherapy in 140 consecutive cases. Transactions of the American Ophthalmological Society. 2007;105:43–52; discussion–3.
Young TA, Rao NP, Glasgow BJ, et al. Fluorescent in situ hybridization for monosomy 3 via 30-gauge fine-needle aspiration biopsy of choroidal melanoma in vivo. Ophthalmology. 2007;114:142–6.
Foster WJ, Harbour JW, Holekamp NM, et al. Pars plana vitrectomy in eyes containing a treated posterior uveal melanoma. Am J Ophthalmol. 2003;136:471–6.
Glasgow BJ, Brown HH, Zargoza AM, et al. Quantitation of tumor seeding from fine needle aspiration of ocular melanomas. Am J Ophthalmol. 1988;105:538–46.
Suesskind D, Ulmer A, Schiebel U, et al. Circulating melanoma cells in peripheral blood of patients with uveal melanoma before and after different therapies and association with prognostic parameters: a pilot study. Acta ophthalmologica. 2011;89:17–24.
Garcia-Arumi J, Zapata MA, Balaguer O, et al. Endoresection in high posterior choroidal melanomas: long-term outcome. Br J Ophthalmol. 2008;92:1040–5.
Schefler AC, Gologorsky D, Marr BP et al Extraocular extension of uveal melanoma after fine-needle aspiration, vitrectomy, and open biopsy. JAMA ophthalmology. 2013;131:1220–4.
Pe’er J, Stefani FH, Seregard S, et al. Cell proliferation activity in posterior uveal melanoma after Ru-106 brachytherapy: an EORTC ocular oncology group study. Br J Ophthalmol. 2001;85:1208–12.
Tschentscher F, Husing J, Holter T et al Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer research. 2003;63:2578–84.
Onken MD, Worley LA, Ehlers JP et al Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer research. 2004;64:7205–9.
Acknowledgments
We thank Anna Obenauf, PhD, from Department of Human Genetics, Medical University Graz, for the support in genetic analysis of the samples.
Conflict of interest
Werner Wackernagel, Lisa Tarmann, Christoph Mayer, Gerald Langmann, and Andreas Wedrich declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Data were presented in part at the 51st meeting of the Austrian Ophthalmological Society, ARVO Science Day, May 14th, 2010, Zell am See, Austria.
Rights and permissions
About this article
Cite this article
Wackernagel, W., Tarmann, L., Mayer, C. et al. Genetic analysis of uveal melanoma by array comparative genomic hybridization before and after radiotherapy. Spektrum Augenheilkd. 27, 286–291 (2013). https://doi.org/10.1007/s00717-013-0195-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00717-013-0195-0