Abstract
Chitinases are pathogenesis-related proteins, which play an important role in plant growth regulation, defense mechanism, and stress tolerance. Embryogenic cultures from Vitis vinifera cv. Tempranillo exposed to in vitro stress exhibited the expression of an extracellular class IV endochitinase VvChit-IV. Phylogenetic and conserved motif analyses provided insights into the evolutionary relationships of chitinases. A computation-based investigation showed conserved domains and illustrated a chitin-binding site for chitin cleavage with a catalytic domain of glycoside hydrolase. Interestingly, gene expression pattern showed a differential expression of VvChit-IV associated with embryonic stress response to in vitro conditions. In response to in vitro stress, transcript level of VvChit-IV increased in embryogenic calli and cell suspensions and peaked at 1.5 and 3 folds, respectively, when compared to an internal reference gene. Evidence of tissue culture stress-induced endochitinase was reported here for the first time indicating that in vitro stress could mitigate elicitor application to induce chitinase expression and can stimulate an immune response against abiotic constraints. Data showed that up-regulation of VvChit-IV was associated with a substantial increase of H2O2 and proline without significant change in malondialdehyde content suggesting that the H2O2 signaling network might trigger a priming effect to boost the defense response against environmental stress. Endochitinase activation in plant stress mitigation was thus highlighted to improve tolerance through attenuation of oxidative stress. This study revealed that the grapevine endochitinase is promising for enhancing coping-oriented adaptation and abiotic stress tolerance, which gives new insights into its feasibility for use in cross-tolerance and crop improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vitro tissue culture is a basic tool for cell manipulation, plant regeneration, gene transfer, and plant biotechnology in general. Tissue culture being an unnatural process, the plant tissues are exposed to stress combinations that they may not have encountered in nature in their long evolution (Cassells et al. 2002; de Klerk 2007). Since tissue culture is perceived as a stress by the cells and plantlets, they respond by activating a number of systemic defense mechanisms and developmental responses including changes in physiological behavior and/or morphogenetic potential. In particular, cell suspensions are most affected by the following constraints: mechanical injury, wounding, osmotic shock (partly due to high sucrose concentration, high salt, and unbalanced mineral composition of the basal medium), hormonal imbalance, nitrogen, ethylene toxicity (de Klerk 2007; Desjardins et al. 2009), and competition in tissue culture (Yildiz 2011). First, plant cells are exposed to oxidative stress resulting from severe wounding at the excision site of the explant associated with the activation of cell dedifferentiation and expression of totipotency (Cassells et al. 2002). Experimental evidence for the occurrence of free radical processes and their deleterious changes during in vitro manipulations was gathered (Benson et al. 1997). Investigating these responses in plant tissue culture under aseptic and controlled conditions offers various opportunities for researchers to study plant responses against environmental stresses (Sen 2012). It is a remarkable plasticity of the plant genome that cells deploy to survive this confined environment, adjust their cellular metabolism, and respond to novel in vitro stresses through epigenetic modifications that have repercussions on changes in the proteome and metabolome. Thus, to overcome stressful conditions imposed by tissue culture, cell suspensions synthesize some stress-responsive proteins including pathogenesis-related (PR) proteins involved in hypersensitive response and systemic acquired resistance against infection or environmental stresses (Enoki and Suzuki 2016). These PR proteins are considered promising tools for engineering plants with multiple stress tolerance (Ali et al. 2018; Ben-Amar 2021).
Chitinases are among the most well-known PR proteins produced as a response of biotic or abiotic stress signaling associated with the plant defense mechanism and are used to actively improve plant resistance against pathogens. Chitinases (E.C. 3.2.1) hydrolyze the β-(1,4)-linked-N-acetyl-glucosamine (GlcNAc) units, commonly known as chitin, a structural component found in several animals and fungi (Grover 2012). Different subgroups of chitinase genes were identified in plants (Kesari et al. 2015). Typically, plant chitinases are endo-type chitinases which randomly hydrolyze the internal acetyl glucosaminidic linkages of chitin/chitosan harboring chitinase cleavage sites and producing small lipo-chito-oligosaccharides (LCOs) (Singh 2014). These LCOs, enclosing a small number of monosaccharide residues linked to chitin, likely act as endogenous signal molecules during environmental stress or pathogen infection response.
A few studies suggested that the expected substrates of plant chitinases may be arabinogalactan proteins (AGPs), lipochito-oligosaccharides (LCOs), N-acetyl-chito-oligosaccharides, and other GlcNAc-containing glycoproteins or glycolipids (Van Hengel et al. 2001). Grapevine embryogenic cells cultivated under in vitro liquid system were applied to produce endochitinases targeting the AGPs located in the cell wall matrix and might generate signal molecules of LCOs responsible for induction and maintenance of early-stage somatic embryogenesis as well as involved in the promotion of cell proliferation (Ben-Amar et al. 2007; Ben-Amar and Reustle 2013).
The exact function of the endochitinase remains unknown. Nonetheless, it appears to be a part of a phylogenetically conserved pathway as endochitinase isolated from sugarbeet stimulates somatic embryogenesis of Picea abies cell cultures (Egertsdotter and Von Arnold 1998). Plant chitinase proteins are commonly divided into six classes (I to VI). The classes III and V belong to the glycoside hydrolase GH18 family, whereas classes I, II, IV, and VI belong to the GH19 family (Patil et al. 2000). Most of the grapevine chitinases are class IV chitinases that fit into PR-3 family (Enoki and Suzuki 2016) among the GH sub-family characterized by a catalytic GH19 domain with an α-Helix-rich/lysozyme-like domain (Oyeleye and Normi 2018) and consisting of a signal peptide of 25 amino acids.
Genome-wide identification and expression analysis of chitinases from grapevine Vitis vinifera (Žiarovská et al. 2020) and Ammopiptanthus nanus (Cao et al. 2019) highlighting their antimicrobial activity were recently achieved. Most reports focused extensively on the chitinase production upon fungal infection along with isolation and expression of cloned chitinase genes in transgenic plants which provide further evidence for their involvement in defense mechanism against biotic stresses (Cao et al. 2019; Jabeen et al. 2015; Kuba et al. 2018). However, few studies showed that plant chitinase and chitinase-like genes play a key role not only in defense-related process, but also in abiotic stress mitigation. For instance, involvement of chitinases in the tolerance against abiotic stresses was reported in tobacco and maize (Liu et al. 2020). Interestingly, an accumulation of chitinases under environmental stress conditions was observed when a chitinase gene was strongly expressed in response to salt, drought, and osmotic stress in Arabidopsis (Hong and Hwang 2006; Seo et al. 2008). In addition, the expression of the class II chitinase was up-regulated in bromegrass and highbush blueberry exposed to cold treatment providing an antifreeze activity (Kikuchi and Masuda 2009; Nakamura et al. 2008), while heat shock treatment enhanced chitinase gene expression and promoted salicylic acid pathway in melon (Widiastuti et al. 2013). Similarly, heavy metal stress has been shown to induce accumulation of plant chitinases and therefore significantly increased abiotic stress resistance (Békésiova et al. 2008). So far, chitinases among other secreted extracellular proteins gained great attention and are considered important target key genes for crop improvement (Grover 2012) and used in various biotechnological applications (Ben-Amar 2021).
Although grapevine chitinases have been described earlier (Busam et al. 1997; Robinson et al. 1997; Van Hengel et al. 2001), evidence of their implication in abiotic stress tolerance has not been reported previously. In this study, grapevine chitinase genes were surveyed, and comparative analysis was conducted based on bioinformatics’ tool, amino acid sequence alignment from GenBank database, and subsequent phylogenetic analysis. Identification of an in vitro stress-inducible grapevine class IV endochitinase VvChit-IV in embryogenic cell suspension was achieved, and transcription profile was assessed during stress exposure on cultured cell lines.
Materiel and methods
Establishment of embryogenic cultures and cell suspensions
The Spanish grapevine cultivar Vitis vinifera cv. Tempranillo was used in this study. Embryogenic material-derived anther culture was induced as described previously (Ben-Amar et al. 2007) and kindly provided by the Group of Dr. Reustle (AgroScience.GmbH, AlPlanta-Institute for Plant Research, Germany). Yellow friable pro-embryogenic masses (PEM) thus developed were maintained on Petri dishes solid media for multiplication and then transferred into liquid culture using the conditioned medium procedure to establish cell suspension as mentioned before (Ben-Amar et al. 2007). Embryogenic callus (EC) and embryogenic cell suspension (ECS) were taken as in vitro stressed material, whereas unstressed anther tissue (A) was used as control.
Multiple sequence alignment and phylogenetic and computational analysis
Bioinformatic tools were used to associate several ortholog proteins of class IV chitinases for comparison from NCBI database (http://www.ncbi.nlm.nih.gov). Multiple alignments for homology search were performed using ClustalW algorithm (Thompson et al. 1994), and subsequent phylogenetic study was done using MEGA-X software (Kumar et al. 2018). A total of 52 chitinases were phylogenetically analyzed, and the evolutionary history was inferred using the neighbor-joining method (Saitou and Nei 1987). The tree is drawn to scale, and the evolutionary distances were computed using the Poisson correction model (Zuckerkandl and Pauling 1965) and are in the units of the number of amino acid substitutions per site. Among these 52 sequences, multiple alignments of 12 representative amino acid chitinase sequences were performed, and in silico analysis was done using NCBI BLAST by selecting the Conserved Domain Database (CDD) tool https://www.ncbi.nlm.nih.gov/cdd for the prediction of conserved domains (Lu et al. 2019). The three-dimensional structure prediction of the target endochitinase as well as protein features has been determined using the SwissModel, an automated protein modeling server developed by the Swiss Institute of Bioinformatics (Waterhouse et al. 2018). The online tool STRING was used for the identification of interacting protein analyses (Szklarczyk et al. 2018).
Cloning of VvChit-IV
Based on the multiple sequence alignment of different endochitinases, conserved domains were identified and grapevine endochitinase primers were designed using Primer BLAST software (NCBI). Primers used in the present study are listed in Table 1. The open reading frame (ORF) of chitinase gene VvChit-IV was amplified from the cDNA of V. vinifera cv. Tempranillo with specific set of primers (Table 1). PCR reaction was performed containing 5 μL of 5 × PCR buffer, 0.2 μL of each primer (10 μM), 0.4 μL dNTP (10 mM), 0.2 μL of Phusion®High-Fidelity Taq polymerase (New England Biolabs), and 1 μL of cDNA (100 ng/μL) in a total volume of 25 μL. The reaction conditions were as follows: an initial denaturation at 98 °C for 2 min followed by 32 cycles of 98 °C for 15 s, 55 °C for 30 s, and 72 °C for 45 s, and then a final extension at 72 °C for 4 min and subsequent storage at 4 °C.
The VvChit-IV cDNA PCR product was subjected to ligation overnight at 4 °C in pGEM-easy vector (Promega, USA) with T4DNA-ligase. A 50 μL aliquot of competent cells was transformed with the ligation product by heat shock and spread on LB medium supplemented with ampicillin 100 mg/L with 30 μL of X-Gal per Petri dish. After overnight culture at 37 °C, selected white colonies were subjected to bacterial DNA extraction using QIAprep Spin Miniprep kit (Qiagen, Germany) followed by control digestion with Hind III to bring out the inserted cDNA fragment. For this, 2 μL of plasmid DNA was mixed to 1 μL RNase, 1 μL buffer, and 0.2 μL of HindIII endonuclease in a final volume of 5 μL. The mix was incubated 2 h at 37 °C for complete digestion. Following agarose gel electrophoresis, the insert band was cut from the gel and cleaned up using QIAquick Gel Extraction kit (Qiagen, Germany) before sequencing and blast annotation to confirm the presence of VvChit-IV gene.
Total RNA extraction and gene expression analysis
Total RNA from anther tissue, callus, and cell suspension was extracted using Trizol® reagent (Invitrogen™, CA, USA) following the manufacturer’s instructions. The quantity and purity of each RNA sample was estimated using a NanoDrop device (ND-1000 Spectrophotometer, Wilmington). RNA was treated with RNase-free DNase I (Qiagen, Germany) before being transcribed into cDNA to remove DNA contamination. The cDNA was synthesized using the SuperScript-III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Invitrogen™, CA, USA) according to the manufacturer’s guidelines. PCR amplifications were carried out in GeneAmp9700® Thermal Cycler (Applied Biosystems, CA, USA) using 10 μL of RT-Mix buffer (2 ×), 0.4 μL of each of primer (10 μM), and 0.4 μL of SuperScript-III One-Step RT-PCR Taq DNA Polymerase (Invitrogen™, CA,USA) with 1 μL of template RNA (500 ng/μL) in a final volume of 20 μL. The reaction conditions were as follows: reverse transcription at 50 °C for 30 min, denaturation at 94 °C for 2 min followed by 32 cycles of 94 °C for 15 s, 55 °C for 30 s, and 68 °C for 45 s, and final extension at 68 °C for 4 min. At the end of the PCR cycles, the amplified product was run on a 1.2% agarose gel with a 1 Kb DNA ladder (Fermentas, Germany). The amplification cycles were adjusted for monitoring differential gene transcripts of endochitinase in anther, callus, and cell suspension. The experiments were repeated twice independently. The expression levels of VvChit-IV were normalized against an internal reference gene, Vvef1 (elongation factor 1). Semi-quantitative analysis intended for the quantification of mRNA levels was performed through digital image densitometry analysis of electrophoretic gel using ImageJ software.
Determination of hydrogen peroxide, malondialdehyde, and proline contents
Hydrogen peroxide (H2O2) assay was performed following the protocols described by Patterson et al. (1984) and Velikova et al. (2000). Briefly, fresh tissue (100 mg) was frozen in liquid nitrogen and ground to powder together with 1 mL 0.1% (w/v) trichloroacetic acid (TCA). The mixture was centrifuged at 10 000 × g for 20 min at 4 °C, and the supernatant was treated with potassium iodide (1 M) in the presence of phosphate buffer (10 mM, pH 7.0). Absorbance at 390 nm was determined spectrophotometrically (Ultrasepec 2000, Pharmacia Biotech, Sweden). The content of H2O2 was calculated by comparison with a standard calibration curve, previously plotted by using different concentrations of H2O2.
Lipid peroxidation was determined based on the thiobarbituric acid (TBA) reaction by measuring the amount of malondialdehyde (MDA) following the method of Hodges et al. (1999). MDA was extracted from plant material in 0.1% TCA as described above. After centrifugation, the supernatant was added to 0.1% (w/v) TBA in 20% (w/v) TCA and incubated in boiling water for 30 min. The samples were then centrifuged at 10 000 × for 5 min, before reading the absorbance at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The amount of MDA-TBA red complex was calculated from the extinction coefficient of 155 mM−1 cm−1 and expressed as nmol MDA/g fresh weight.
Proline content was determined after homogenization of ground plant material in 3% aqueous sulfosalicylic acid on ice and quantitatively measured by ninhydrin-based colorimetric assay at 520 nm using toluene as reference, as described by Bates et al. (1973) and Abraham et al. (2010). The proline concentration was determined using a standard curve and calculated on fresh weight basis (expressed as μmol/g fresh weight).
Statistical analysis
Experiments were performed in three replicates. All experiments described here were repeated independently twice. Data were expressed as mean value ± SD and were compared by one-way analysis of variance (ANOVA). Significant differences between means for all treatments were assessed by Fisher’s test at 0.05. Histogram followed by different letters is significantly different at P ≤ 0.05. All analyses were performed using XL-STAT software.
Results and discussion
Phylogenetic relationships of chitinase gene family
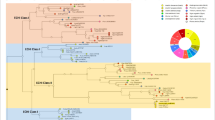
We previously reported the role of extracellular proteins derived from conditioned medium of grapevine embryogenic cell cultures and their roles on the establishment and stress mitigation of cell suspensions (Ben-Amar et al. 2007; Ben-Amar and Reustle 2013) suggesting the involvement of an endochitinase in catalyzing the cleavage of AGPs in small oligosaccharides. To study this chitinase, we focus here on its isolation as well as to explore its expression profile under in vitro stress conditions. In order to gain insight into the relationship among the stress-responsive endochitinase identified in this work and others from members of the chitinase family reported in the literature, molecular evolutionary phylogeny was performed. A total of 52 amino acid sequences of ortholog endochitinase genes were downloaded from the NCBI database and aligned to identify the conserved domain architecture. Based on this alignment, the obtained phylogenetic tree (Fig. 1) showed that chitinase sequences were clustered into nine subgroups (A to I) according to the divergence relationship. The V. vinifera class IV chitinase is shown with a star symbol as the identified acidic extracellular protein secreted into the culture medium of embryogenic cell suspension. The most grapevine endochitinases are clustered in the group A, with two endochitinases as extracellular protein EP3 clustered in the group E. Among these endochitinases, multiple alignments of 12 amino acid sequences from the GenBank database were analyzed by ClustalW (Fig. 2). Based on multiple alignments, a set of 12 chitinase protein sequences studied, with accession number, amino acid length, chitin-binding domain, and catalytic domain localization, are listed in Table 2. The amino acid sequence homologous to the active site of chitinases was found in all the chitinases used for analysis.
Phylogenetic tree of endochitinase family genes in grapevine and other plant species. The evolutionary history was inferred using the neighbor-joining method. The optimal tree shown is divided in 9 groups. Red circles are grapevine endochitinases, and the star mark indicates the VvChit-IV identified in cell suspension. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. This analysis involved 52 amino acid sequences. All positions containing gaps and missing data were eliminated (complete deletion option). There were a total of 230 positions in the final dataset. Evolutionary analyses were conducted in MEGA X
Multiple alignments of V. vinifera class IV endochitinase with others orthologs from related plant species in glycoside hydrolase GH19 family. Sequence alignment was performed using ClustalW. Shaded amino acid sequences are 70–100% homologous. The red line above the sequence represents the enzymatic active catalytic domain of the GH19 family (amino acid sequence highly conserved through evolution). The cysteine-rich chitin-binding domain (CBD) is illustrated in orange line (amino acid residues 30–60), followed by the hinge region in blue line, rich in proline/glycine (amino acid residues 60–75), and green line represents the gap regions
Molecular identification of grapevine endochitinase and protein sequence analysis
Since tissue culture is perceived as a stress by the plant cells, which respond by activating a number of systemic defense mechanisms and developmental responses, unstressed anther tissue material (A), embryogenic callus (EC), and embryogenic cell suspension (ECS) as illustrated in Fig. 3a were harvested to extract RNA. Reverse transcription-PCR amplification (RT-PCR) with designed VvChit-IV primers resulted in a cDNA product of the grapevine endochitinase ORF region with a length of 795 bp (Fig. 3b) that encoded a protein of 264 amino acids. cDNA cloning of VvChit-IV was carried out by inserting the PCR product corresponding to the ORF of VvChit-IV in the pGEM-T easy vector (Fig. 3c). This plasmid was used to transform competent cells, and following transformation, the putative transformed colonies were used for plasmid DNA extraction. Subsequent control digestion by HindIII takes out the insert to check the positive clones which will be selected for sequencing. Blast annotation of insert sequence was achieved on confirmed positive clones with a grapevine chitinase.
Molecular identification and cloning of grapevine VvChit-IV endochitinase. a Plant material used including anther (A), embryogenic callus (EC), and embryogenic cell suspension (ECS). b Grapevine endochitinase cDNA ORF (795 bp) amplified by RT-PCR from total RNA of anther material (A), callus (EC), and cell suspension (ECS). c Cloning of VvChit-IV ORF cDNA in pGEM-easy vector and control digestion with Hind III that confirmed the presence of the insert in transformed competent cells
Domain architecture, putative function, and protein interaction
Furthermore, two conserved domains have been identified in the grapevine endochitinase: chitin-binding 1 and glycoside hydrolase family 19. These domains are located at position 25–49 aa and 67–264 aa, respectively (Table 3). The predicted gene ontology of VvChit-IV protein revealed two categories, mainly including antifungal activity including hypersensitive/defense response and cell wall catabolic processes related to chitin degradation. The homologs sharing the same domain architecture of the target gene showed similar functions associated to fungal defense response as described in recent studies (Ali et al. 2020).
To elucidate the putative function of the class IV chitinase, the domain architecture of the VvChit-IV protein was illustrated in Fig. 4a. The typical domains of chitinases including signal peptide, chitin-binding site, and catalytic domain of the family 19 of glycoside hydrolases (GH19) were presented. All these chitinases have a lysozyme-like domain in their structure that could exhibit a lysozyme activity as proposed by Su et al. (2015). Unlike in chitinases class I and class V that are alkaline, all of class IV endochitinases as well as class III are acidic. Most of the endochitinases contain a cysteine-rich chitin-binding domain (CBD) and signal peptides at the N-terminal end, indicating that most of them are secreted to apoplast (Cao et al. 2019) and involved in extracellular matrix remodeling through the hydrolysis of various arabinogalactan protein present in the plant cell wall.
Grapevine endochitinase prediction, domain architecture, and protein features. a Domain architecture of grapevine endochitinase including catalytic domain GH19 family, chitin-binding domain, signal peptide, and hinge region, assembled using online tool Conserved Domain Database from NCBI. b Prediction of 3-D endochitinase protein model and associated protein features using Swiss-model software
Additionally, the three-dimensional (3D) structure of the grapevine class IV endochitinase containing 264 aa, along with other associated structural features, was predicted according to the amino acid sequence modeling algorithm (Fig. 4b). Based on previously studied chitinase genes, the putative biological function of grapevine class IV chitinase was investigated within the protein interaction network. Thus, for understanding the possible role of the target chitinase gene, the protein interaction map was drawn (Fig. 5). The STRING network revealed that the VvChit-IV protein has closely interacted with a set of plant defense (PR) proteins including the essential beta-hexosaminidase, beta-1,3-glucanase, and polygalacturonase inhibitor. All these proteins represent important factors of plant resistance against microbial pathogens to delay the invasion of the plant tissues and thus play a crucial role in the plant defense mechanism. Our findings suggest that the target gene (VvChit-IV) may also play a key position in the plant’s response to biotic stress. Interestingly, the present study will elucidate that the VvChit-IV gene is also deeply involved in environmental stress tolerance, like the case of in vitro stress. All together suggest that the V. vinifera class IV endochitinase has a key role in the apoplast and the cell wall matrix in general, which is mainly considered the first barrier in touch with any kind of stress exposure. Endochitinase-mediated changes in plant cell matrix architecture play a determinant function in the perception of stress signal and management of response mechanisms towards inducing an array of defense strategies through a well-connected signaling network at biotic and abiotic stresses. Thus, up-regulation of endochitinase clearly reflects a priming effect that could enhance host immune system against various pathogens and environmental stresses.
STRING map illustrating the protein interaction network of the grapevine class IV chitinase. The black encircled protein symbolized the class IV chitinase from V. vinifera (264 aa) that was used as a query sequence to predict the protein–protein interaction using the STRING software. The protein encircled with a colored dotted line indicates interactive defense-related proteins including Beta-hexosaminidase (black line), polygalacturonase inhibitor (blue line), and beta-1,3glucanase (red line). Note: the legend below represents the predicted functional partners
Up-regulation of VvChit-IV transcript in embryogenic cell cultures during exposure to environmental in vitro stress
To compare the VvChit-IV transcript levels between studied samples differing in stress exposure, a semi-quantitative RT-PCR was performed against the internal reference gene elongation factor Vvef1 of V. vinifera to analyze the tissue-specific expression pattern of chitinase. Data showed that expression levels of the endochitinase gene were clearly different among samples (Fig. 6a). For instance, VvChit-IV was up-regulated 1.5-fold in embryogenic callus (EC) and about threefold in embryogenic cell suspension (ECS) in liquid culture relative to the reference gene Vvef1, whereas unstressed anther material (A) taken as a control showed a very slight expression (0.1-fold of Vvef1 transcript) that could be qualified as insignificant (Fig. 6a). When compared with anther material considered here as control, stressed EC and ECS exhibited an up-regulated VvChit-IV transcript of 15 to 30 times, respectively. This fact indicated a stress-inducible expression of the VvChit-IV in EC and ECS, which is corroborated by previous works stating an increase of chitinase expression during pathogen infection (Busam et al. 1997; Su et al. 2015) or exposure to abiotic stress (Cao et al. 2019). They also noted an up-regulated chitinase expression following elicitor application like salicylic acid (SA), abscisic acid (ABA), and jasmonic acid (JA) (Busam et al. 1997; Su et al. 2015) or accumulation of ethylene (Watanabe et al. 1999) considered the primary defense signal compounds for systemic acquired resistance and induced systemic resistance. In fact, cell suspension cultures are already well-known to produce a significant amount of ethylene that may act as an elicitor of endochitinase production within in vitro cultured cell system. This most likely explains the ethylene-inducible expression of VvChit-IV in this study. Ethylene and JA-related gene expression has been reported to be up-regulated and cause ethylene or JA accumulation under biotic and abiotic stress (Su et al. 2015; Wasternack 2007; Watanabe et al. 1999). Accordingly, by pre-exposure of the cell lines to ethylene among other in vitro stress components present in liquid culture, a priming effect takes place to enable these PEMs to be more tolerant to further stress events. This suggests priming to be a promising strategy for plants to cope with abiotic stresses under global climate change scenarios. This biodynamic environment was documented to lead an increase in enzymatic activities of endochitinase (EC 3.2.1.14), exochitinase (β-N-acetylhexosaminidase, EC 3.2.1.52, chitin 1,4-β-chitobiosidase), and β-1,3-glucanase (EC 3.2.1.39), which are mostly correlated with plant responses against stress and associated with induced plant resistance (Botelho et al. 2015).
Gene expression pattern of grapevine VvChit-IV endochitinase and related biochemical analyses. a Relative expression of VvChit-IV transcript level using semi-quantitative RT-PCR in unstressed anther material (A) and in vitro stressed material: callus (EC) and cell suspension (ECS) compared with Vvef1 reference gene. b Hydrogen peroxide (H2O2) content. c MDA content. d Proline content
Grapevine endochitinase alleviated oxidative damage in embryogenic cultures and conferred tolerance to in vitro stress
Commonly, in vitro stress like other environmental constraints induces an oxidative stress through overproduction of reactive oxygen species (ROS). To study whether endochitinase expression improves tolerance to ROS generated under in vitro culture, we estimated the level of oxidative stress by quantifying hydrogen peroxide content. In the same way, the level of lipid peroxidation associated with oxidative damage to membranes and cellular structures resulting from abiotic stress was expressed by the malondialdehyde (MDA) content. In this study, data revealed that EC and ECS, in which endochitinase transcripts are up-regulated (Fig. 6a), exhibited a substantial increase of H2O2 level (increase by 143% and 170%, respectively, in EC and ECS), while MDA content does not show significant change when compared to unstressed anther material (Fig. 6b,c). Interestingly, H2O2 increase in embryogenic callus and cell suspensions does not affect membrane peroxidation by keeping the MDA level unchanged (Fig. 6c), suggesting that H2O2 signaling could activate a defense mechanism and trigger a priming effect to enhance stress tolerance. This effect is most likely related to free amino acid accumulation as revealed by changes in proline content widely used as indicator of osmotic stress and associated with osmoregulation and ROS scavenging-mediated stress protection. Data showed a significant accumulation of proline in EC and ECS (with an increase of 200% and 236%, respectively), compared to unstressed material (Fig. 6d) revealing that in vitro cultured cells are really exposed to a stressful environment. Proline production increased with increasing H2O2 levels hinting at its antioxidant role in detoxifying ROS and its function as osmolyte to alleviate osmotic stress along with stabilizing membranes and maintaining the MDA at lower level. Although H2O2 levels are known to dramatically increase on exposure to environmental stress factors (Sen 2012), pre-treatment with H2O2 can produce a small oxidative burst that activates a redox-dependent signaling network, allowing antioxidants and latent defense components to accumulate. This results in a primed state and an enhanced stress response (Gonzalez-Bosch 2018). Earlier studies noted that H2O2-mediated priming was found to modulate abiotic stress tolerance as indicated by a lower MDA content (Hossain et al. 2015; Si et al. 2018) and thus could be suggested as a link in endochitinase-mediated stress tolerance.
Based on previous reports (Ali et al. 2020; Zhu et al. 2016) and the current study, we suggest a working model for the function of grapevine endochitinase (Fig. 7). In vitro-induced stress is characterized by various elicitors (like H2O2, ethylene, sugars, proline) and the role of their signaling responses in the mitigation of environmental stress that trigger a set of defense-related genes. Among them, the chitinase family is one of the most important PR proteins generated by plant cells to mainly promote chitin degradation, LCO generation from the AGP catabolism, activating an early immune response that alleviate biotic/abiotic stresses and boost cell proliferation of PEMs in embryogenic culture systems simultaneously. The addition of H2O2 to the cultivation medium also promoted the specific chitinase productivity of Wasabia japonica cell suspension (Akimoto et al. 2000). Ali et al. (2020) provides evidence that the chitinase gene from pepper positively regulates the hypersensitive and defense response to fungal infection, accompanied by an induction of H2O2. Altogether, this suggests that the endochitinase was most likely responsible to attenuate the oxidative stress and ROS-related cellular injuries probably by enhancing antioxidant defense response through a signaling network where H2O2 and proline emerge among prominent components in chitinase-mediated stress tolerance. Besides hydrogen peroxide, other reports also proposed the involvement of ethylene produced and accumulated in vitro, within the culture vessels in sufficient amounts, in the control of plant morphogenesis particularly in cell suspensions (Desjardins et al. 2009). These stress-mediators regulate different signaling pathways involved in stress tolerance (Slesak et al. 2007; Zhu et al. 2016) and together could activate chitinase gene expression and other induced PR proteins in response to environmental stresses.
Flow chart illustrating the model proposed for the grapevine endochitinase-mediated stress tolerance. Upon in vitro stress in cell cultures, the chitinase gene Vv.Chit-IV is expressed to trigger defense responses in embryogenic callus (EC) and embryogenic cell suspension (ECS) through a set of signaling molecules and oxidative stress. H2O2, proline, and lipochito-oligosaccharides (LCO) produced by arabinogalactan protein (AGPs) catabolism were the main signal molecules involved in mitigation strategies against in vitro stress. Involvement of LCO and AGPs in stress signaling under in vitro conditions was achieved according our previous works (Ben-Amar et al. 2007; Ben-Amar and Reustle 2013). Ethylene was also suggested to have a crucial role in alleviating environmental stress (Arraes et al. 2015; Desjardins et al. 2009) and involved with other potential signaling pathways in endochitinase-mediated stress tolerance
Conclusion
The presence of chitinases in plants suggests that plant cells have acquired an antifungal defense system. In vitro stress could mitigate pathogen infection by stimulating PR-protein production and particularly chitinase synthesis that boost up the plant immune system against stress. This study provided evidence that embryogenic cell suspension, a highly in vitro stressed material, showed increased endochitinase expression when compared to callus-derived PEM and unstressed anther tissue, suggesting a chemical priming that emerged as an important tool for mitigating the effects of abiotic stress. These findings have great potential to overcome environmental constraints in biotech cell systems and engineered plants exposed to stressful conditions. Data from multiple alignments of amino acid sequences of several class IV plant endochitinases and their assembling in a phylogenetic tree showed conserved catalytic domain of GH19 family suggesting importance across multiple species in cross-tolerance connecting abiotic and biotic stresses required for cell defense mechanism. The fast-growing field of bioinformatics and the availability of more computational tools and databases predicting protein structure and function could help to streamline engineering of chitinases towards a dual-track application in multiple abiotic stress mitigation as well as a potential antifungal treatment in sustainable agriculture.
References
Abraham E, Hourton-Cabassa C, Erdei L, Szabados L (2010) Methods for determination of proline in plants. Methods Mol Biol 639:317–331. https://doi.org/10.1007/978-1-60761-702-0_20
Akimoto C, Aoyagi H, Dicosmo F, Tanaka H (2000) Synergistic effect of active oxygen species and alginate on chitinase production by Wasabia japonica cells and its application. J Biosci Bioeng 89(2):131–137. https://doi.org/10.1016/s1389-1723(00)88726-5
Ali M, Li QH, Zou T, Wei AM, Gombojav G, Lu G, Gong ZH (2020) Chitinase gene positively regulates hypersensitive and defense responses of pepper to Collectotrishum acutatum infection. Int J Mol Sci 21:6624. https://doi.org/10.3390/ijms21186624
Ali S, Ganai BA, Kamili AN, Bhat AA, Mir ZA, Bhat JA, Tyagi A, Islam ST, Mushtaq M, Yadav P, Rawat S, Grover A (2018) Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol Res 212:29–37. https://doi.org/10.1016/j.micres.2018.04.008
Arraes FBM, Beneventi MA, Lisei de Sa ME, Paixao JFR, Albuquerque EVS, Marin SRR, Purgatto E, Nepomuceno AL, Grossi-de Sa MR (2015) Implications of ethylene biosynthesis and signaling in soybean drought stress tolerance. BMC Plant Biol 15:213. https://doi.org/10.1186/s12870-015-0597-z
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Békésiova B, Hraska S, Libantova J, Moravcikova J, Matusikova I (2008) Heavy-metal stress induced accumulation of chitinase isoforms in plants. Mol Biol Rep 35:579–588. https://doi.org/10.1007/s11033-007-9127-x
Ben-Amar (2021) Seretome-derived cultured cell system: overview towards extracellular protein characterization and biotechnological applications. J Basic Appl Sci 17:13–24. https://doi.org/10.29169/1927-5129.2021.17.02
Ben-Amar A, Cobanov P, Boonrod K, Bouzid S, Ghorbel A, Krczal G, Reustle G (2007) Efficient procedure for grape embryogenic suspensions establishment and plant regeneration: role of conditioned medium in cell proliferation. Plant Cell Rep 26:1439–1447. https://doi.org/10.1007/s00299-007-0341-8
Ben-Amar A, Reustle G (2013) Extracellular proteins induced plant cell proliferation. In: Zhang C, Zeng X (eds) Cell proliferation: processes, regulation and disorders. Nova Biomedical, New York, pp 65–80
Benson EE, Magill WJ, Bremner DH (1997) Free radical processes in plant tissue cultures: implications for plant biotechnology programmes. Phyton, special issue "Free Radicals" 37:31–38
Botelho RV, Roberti R, Tessarin P, Garcia-Mina JM, Rombolà AD (2015) Physiological responses of grapevine to biodynamic management. In: Renewable Agriculture and Food System 31:5. https://doi.org/10.1017/S1742170515000320
Busam G, Kassemeyer HH, Matern U (1997) Differential expression of chitinases in Vitis vinifera L. responding to systemic acquired resistance activators or fungal challenge. Plant Physiol 115(3):1029–1038. https://doi.org/10.1104/pp.115.3.1029
Cao S, Wang Y, Li Z, Shi W, Gao F, Zhou Y, Zhang G, Feng J (2019) Genome-wide identification and expression analyses of the chitinases under cold and osmotic stress in Ammopiptanthus nanus. Genes 10:472. https://doi.org/10.3390/genes10060472
Cassells AC, Joyce SM, O’Herlihy EA, Perez-Sanz MJ, Walsh C (2002) Stress and quality in in vitro culture. Acta Hort. https://doi.org/10.17660/ActaHortic.2003.625.16
de Klerk GJ (2007) Stress in plants cultured in vitro. Propagation of Ornemental Plants 7(3):129–137
Desjardins Y, Dubuc J, Badr A (2009) In vitro culture of plants: a stressful activity! Acta Hort 812:29–50. https://doi.org/10.17660/ActaHortic.2009.812.1
Egertsdotter U, von Arnold S (1998) Importance of arabinogalactan proteins for development of somatic embryos of Norway spruce (Picea abies). Physiol Plant 93:334–345. https://doi.org/10.1111/j.1399-3054.1995.tb02237.x
Enoki S, Suzuki S (2016) Pathogenesis-related proteins in grape. In: Morata A, Loira I (eds.), Grape and Wine Biotechnology. Intech Open, Rijeka, Croatia, pp 43–58.
Gonzalez-Bosch C (2018) Priming plant resistance by activation of redox-sensitive genes. Free Radical Biol Med 122:171–180. https://doi.org/10.1016/j.freeradbiomed.2017.12.028
Grover A (2012) Plant chitinases: genetic diversity and physiological roles. Crit Rev Plant Sci 31:57–73. https://doi.org/10.1080/07352689.2011.616043
Hodges DM, Delong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611. https://doi.org/10.1007/s00425-017-2699-3
Hong JK, Hwang BK (2006) Promoter activation of pepper class II basic chitinase gene, CAChi2, and enhanced bacterial disease resistance and osmotic stress tolerance in the CAChi2 overexpressing Arabidopsis. Planta 223:433–448. https://doi.org/10.1007/s00425-005-0099-6
Hossain MA, Bhattacharjee S, Armin SM, Qian P, Xin W, Li HY, Burritt DJ, Fujita M, Tran LSP (2015) Hydrogene peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420. https://doi.org/10.3389/fpls.2015.00420
Jabeen N, Chaudhary Z, Gulfraz M, Rashid H, Mirza B (2015) Expression of rice chitinase gene in genetically engineered tomato confers enhanced resistance to Fusarium wilt and early blight. Plant Pathol J 31(3):252–258. https://doi.org/10.5423/PPJ.OA.03.2015.0026
Kesari P, Patil DN, Kumar P, Tomar S, Sharma AK (2015) Structural and functional evolution of chitinase-like proteins from plants. Proteomics 15:1693–1705. https://doi.org/10.1002/pmic.201400421
Kikuchi T, Masuda K (2009) Class II chitinase accumulated in the bark tissue involves with the cold hardiness of shoot stems in highbush blueberry (Vaccinium corymbosum L.), and characterization of plant chitinase with a novel domain combination from lycophyte Selaginella doederleinii. Biosci Biotechnol Biochem 82:1742–1752. https://doi.org/10.1016/j.scienta.2008.11.007
Kuba Y, Takashima T, Uechi K, Taira T (2018) Purification, cDNA cloning and characterization of plant chitinase with a novel domain combination from lycophyte Selaginella doederleinii. Biosci Biotech Biochem 82(10):1742–1752. https://doi.org/10.1080/09168451.2018.1491285
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Liu X, Yu Y, Liu Q, Deng S, Jin X, Yin Y, Guo J, Li N, Liu Y, Han S, Wang C, Hao D (2020) A Na2CO3-responsive chitinase gene from Leymus chinensis improve pathogen resistance and saline-alkali stress tolerance in transgenic tobacco and maize. Front Plant Sci 11:504. https://doi.org/10.3389/fpls.2020.00504
Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS et al (2019) CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res 48:D265–D268. https://doi.org/10.1093/nar/gkz991
Nakamura T, Ishikawa M, Nakatani H, Oda A (2008) Characterization of cold-responsive extracellular chitinase in bromegrass cell cultures and its relationship to antifreeze activity. Plant Physiol 147:391–401. https://doi.org/10.1104/pp.106.081497
Oyeleye A, Normi YM (2018) Chitinase: diversity, limitations and trends in engineering for suitable applications. Biosci Rep 38:BSR2018032300. 10.1042/BSR20180323
Patil RS, Ghormade V, Deshpande MV (2000) Chitinolytic enzymes: an exploration. Enzyme Microb Tech 26:473–483. https://doi.org/10.1016/s0141-0229(00)00134-4
Patterson BD, Macrae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492. https://doi.org/10.1016/0003-2697(84)90039-3
Robinson SP, Jacobs AK, Dry IB (1997) A class IV chitinase is highly expressed in grape berries during ripening. Plant Physiol 114(3):771–778. https://doi.org/10.1104/pp.114.3.771
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Sen A (2012) Oxidative stress studies in plant tissue culture. Intech Open, Rijeka, Croatia, pp 59–87. https://doi.org/10.5772/48292
Seo PJ, Lee AK, Xiang FN, Park CM (2008) Molecular and functional profiling of Arabidopsis pathogenesis-related genes: insights into their roles in salt response of seed germination. Plant Cell Physiol 49:334–344. https://doi.org/10.1093/pcp/pcn011
Si T, Wang X, Zhao C, Huang M, Cai J, Zhaou Q, Dai T, Jiang D (2018) The role of hydrogen peroxide in mediating the mechanical wounding-induced freezing tolerance in wheat. Front Plant Sci 9:327. https://doi.org/10.3389/fpls.2018.00327
Singh A (2014) Isolation and expression of a chitinase family protein AT4G01700 from Arabidopsis thaliana. Austr J Biotechnol Bioeng 1(4):7. https://doi.org/10.7150/thno.7811
Slesak I, Libik M, Karpinska B, Kapinski S, Miszalski Z (2007) The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochem Pol 54(1):39–50. https://doi.org/10.18388/abp.2007_3267
Su Y, Xu L, Wang S, Wang Z, Yang Y, Chen Y, Que Y (2015) Identification, phylogeny, and transcript of chitinase family genes in sugarcane. Sci Rep 5:10708. https://doi.org/10.1038/srep10708
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P et al (2018) STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47:D607–D613. https://doi.org/10.1093/nar/gky1131
Thompson J, Higgins D, Gibson T (1994) CLUSTAL W: improving the sensitivity of processive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nuc Acid Res 22(22):4673–4680. https://doi.org/10.1093/nar/22.22.4673
Van Hengel A, Tadesse Z, Immerzeel P, Schols H, Van Kammen A, De Vries SC (2001) N-Acetylglucosamine and glucosamine-containing Arabinogalactan proteins control somatic embryogenesis. Plant Physiol 125(4):1881–1890. https://doi.org/10.1104/pp.125.4.1880
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot-London 100:681–697. https://doi.org/10.1093/aob/mcm079
Watanabe A, Nong VH, Zhang D, Arahira M, Yeboah NA, Udaka K, Fukazawa C (1999) Molecular cloning and ethylene-inducible expression of Chib1 chitinase from soybean Glycine max L. Biosci Biotechnol Biochem 63:251–256. https://doi.org/10.1271/bbb.63.251
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, De Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modeling of protein structures and complexes. Nuc Acid Res 46(W1):W296–W303. https://doi.org/10.1093/nar/gky427
Widiastuti A, Yoshino M, Hasegawa M, Nitta Y, Sato T (2013) Heat shock-induced resistance increases chitinase-1 gene expression and stimulates salicylic acid production in melon (Cucumis melo L.). Physiol Mol Plant Pathol 82:51–55. https://doi.org/10.1016/j.pmpp.2013.08.003
Yildiz M (2011) Evaluation of the effect of in vitro stress and competition in tissue culture response of flax. Biol Plant 55(3):541–544. https://doi.org/10.1007/s10535-011-0121-8
Zhu T, Deng X, Zhou X, Zhu L, Zou L, Li P, Zhang D, Lin H (2016) Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci Rep 6:35392. https://doi.org/10.1038/srep35392
Žiarovská J, Zamiešková L, Bilcíková J, Fialková V, Sabo J, Kunová S, Kacániová M (2020) Expression of specific class I chitinase mRNA levels in different grape varieties and their antimicrobial activity. Agronomy 10:1176. https://doi.org/10.3390/agronomy10081176
Zuckerkandl E, Pauling L (1965) Evolutionary divergence and convergence in proteins. In: Bryson V, Vogel HJ (eds) Evolving Genes and Proteins. Academic Press, New York, pp 97–166
Acknowledgements
We are grateful to Dr. Goetz Reustle and Dr. Pascal Cobanov (AlPlanta-Institute for Plant Research, Germany) for kindly providing embryogenic material and for helpful discussion.
Funding
The work was supported by the Tunisian Ministry of Higher Education and Scientific Research. AB received a financial support for scholarship in Germany.
Author information
Authors and Affiliations
Contributions
AB conceived and designed the study, conducted the experiment, and wrote the manuscript. DA performed biochemical assays and statistical analysis. AB, DA, and AM contributed in reviewing, editing, and approving the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ben-Amar, A., Allel, D. & Mliki, A. Up-regulation of a stress-responsive endochitinase VvChit-IV in grapevine cell cultures improves in vitro stress tolerance. Protoplasma 259, 1189–1203 (2022). https://doi.org/10.1007/s00709-021-01733-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01733-y