Abstract
Drought is the main factor that significantly affects plant growth and has devastating effects on crop production of jute. NAC (NAM, ATAF, and CUC2) transcription factors (TFs) are a large gene family in plants that have been shown to play many important roles in regulating developmental processes and abiotic stress resistance. In this study, a NAC transcription factor, CcNAC1, was cloned and characterized its function in jute. RT-qPCR analysis showed that CcNAC1 expression peaks after 8 h of drought stress. CcNAC1 overexpression and knockdown plants were created by Agrobacterium-mediated genetic transformation. PCR and southern hybridization results indicate that the CcNAC1 gene was integrated into the genome of jute. Overexpression of the CcNAC1 gene sped up the plant growth, promoted early flowering, and increased drought tolerance compared to the control plants. 3-Ketoacyl-CoA synthase (KCS) gene expression level increased significantly in the CcNAC1-overexpression plants and decreased in knockdown plants, which showed that CcNAC1 transcription factor regulated KCS gene expression. Yeast-2-Hybrid (Y2H) assays validated the physical interaction between CcNAC1 and KCS. The results provide relatively comprehensive information on the molecular mechanisms of CcNAC1 gene underlying the regulation of plant growth and drought stress resistance, and indicate that CcNAC1 acts as a positive regulator in drought tolerance in jute (Corchorus capsularis L.).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jute (Corchorus capsularis L.) is a plant that is conventionally used for the production of natural fibers, paper, chemical fertilizers, building materials, and medicine. Fibers from jute have good glossiness, high drapability, good hygroscopicity, high water dispersibility, good biodegradability, and other beneficial characteristics. The advantages of jute over other plants have led to it being widely applied in various fields.
Drought is among the most important abiotic factors that affect the growth and development of jute. Studies on drought tolerance of jute have mainly focused on its physiological and biochemical aspects (Li et al. 2013; Yao et al. 2013). The water content of jute plants is approximately 70–91% and varies greatly among different tissues, organs, growth states, and reproductive stages (Su and Dai 2017; Mitra and Basu 1974; Ayodele and Fawusi 1990). During its reproductive period, jute shows the greatest water consumption, the highest sensitivity to drought stress, and the largest leaf area (Li et al. 2013). In another study of jute, Li et al. revealed that the plant-height ratio and stem diameter ratio between the seedling stage and rapid growth stage decreased as the time and severity of drought stress increased (Li et al. 2013). Moreover, drought stress increased the ratio of electric conductivity, the content of chlorophyll, proline, soluble protein, soluble carbohydrate, and malondialdehyde in the leaf between the seedling, and rapid growth stage of jute.
NAC transcription factors are a family of plant-specific transcription factors. Appeared to be widespread in plants such as Arabidopsis, rice, wheat, soya bean, and cotton (Meng et al. 2009; Puranik et al. 2012; Mao et al. 2012; Wu et al. 2012). Studies have shown that NAC transcription factors regulate plant growth and development and have important functions in biotic and abiotic stress responses (Peng et al. 2009; Jeong et al. 2013). Several studies reported that a subfamily of NAC transcription factors played a pivotal role in various abiotic stresses including salinity, drought, and low temperature (Yuan et al. 2019; Puranik et al. 2012; Hao et al. 2011; Hu et al. 2008). A cotton NAC transcription factor GhirNAC2 plays positive roles in drought tolerance via regulating ABA biosynthesis. The rice NAC TFs play vital roles in diverse aspects of plant growth, development, and stress responses (Puranik et al. 2012). The involvement of the NAC TFs in rice abiotic stress responses has been extensively explored, and some rice NAC genes have been reported to function in abiotic stress tolerance (Chen et al. 2014). Several studies have reported that OsNAC2 has important functions in rice (Jiang et al. 2018). In a promoter activation–tagging mutant, an increase in OsNAC2 expression results in an increased tiller angle in rice (Mao et al. 2007). In addition, OsNAC2 is associated with plant height (Chen et al. 2015), the promotion of leaf senescence (Mao et al. 2017), and increased yields (Jiang et al. 2018), highlighting the important roles this gene plays in rice growth and development. Some NAC TFs can improve abiotic stress tolerance (Hu et al. 2006; Saada et al. 2013; Chen et al. 2014; Hong et al. 2016; Huang et al. 2016), with some even increasing yields under abiotic stress conditions (Jeong et al. 2010; Redillas et al. 2012). Recent results showed a novel stress-responsive grapevine (Vitis vinifera L.) NAC transcription factor (VvNAC17) increases sensitivity to abscisic acid and enhances salinity, freezing, and drought tolerance in transgenic Arabidopsis (Ju et al. 2019). Overexpression of a microRNA-targeted NAC transcription factor improves drought and salt tolerance in rice via ABA-mediated pathways (Jiang et al. 2019). Additionally, transgenic rice lines overexpressing some of the stress-related NAC TFs exhibited significant improvement of abiotic stress tolerance under severe stress conditions without any adverse effect on yield or even with yield increase (Jeong et al. 2013), providing a promising potential for application of these stress-related NAC TFs in improvement of abiotic stress tolerance in crops (Hu and Xiong 2013).
3-Ketoacyl-CoA synthase (KCS) is involved in very long-chain fatty acid synthesis in vegetative tissues and also plays a role in wax biosynthesis. Accumulation of cuticular waxes is one contributor to drought resistance for coping with drought or water-deficit conditions in plants. It has been reported that MYB96 TFs play a role in drought resistance; it regulates cuticular wax biosynthesis by binding directly to the promoters of KCS gene that constitute a rate-limiting step in cuticular wax biosynthesis (Pil et al. 2011). The KCS1 synthase gene related to drought stress has been analyzed in cotton (Meng et al. 2009) and ramie (An et al. 2015), which is involved in both the decarbonylation and acyl-reduction wax synthesis pathways (Todd and Jaworski 1999). NAC TFs and KCS gene expression are induced by drought stress in our previous studies (Zhang et al. 2018); however, there have been no reports on the relationship between NAC transcription factors and 3-ketoacyl-CoA synthase in jute.
Here, we reported the CcNAC1 gene and identified the gene function in jute. CcNAC1 gene expression peaks after 8 h of drought stress analyzed by RT-qPCR. CcNAC1-overexpression transgenic jute increased drought tolerance, sped up the plant growth, and promoted early flowering, but the CcNAC1 gene knockdown plants were drought-sensitive. We also found that the expression levels of the KCS gene were increased in CcNAC1-overexpression lines compared to the control plants. Y2H assays validated the physical interaction between CcNAC1 and KCS. The study provides new insights that may enable the development of high-yielding jute varieties with increased drought tolerance using genetic engineering.
Materials and methods
Experimental materials and PEG6000 treatment
The seeds of “Huangma 179” were provided by Professor Jianmin Qi of Fujian Agricultural and Forestry University; they were bred from the hybrid offspring of Meifeng 2 × Minma 5; the variety has early maturation, high yield, and drought tolerance. The seeds were planted on MS solid medium and cultured in a light incubator (32 °C/24 °C, 16 h light/8 h dark). The leaves of the plant were used for DNA and RNA extraction. After 30 days, the plantlets were planted in pots. Vegetable soil (contains humus, straw fiber, perlite, and bioorganic matter) and sandy soil (agglomerate particle from mountain slopes facing the sun in Shangrao area) were mixed (3:1), when plants with similar height and growth vigor were subjected to controlled trials under drought stress. Each pot was fully irrigated. The soil water content of each pot was maintained at an identical level. After irrigation was stopped, 15-day seedlings were treated by 20% polyethylene glycol (PEG6000) hypertonic solution simulating drought stress for 24 h for RT-qPCR analysis, and the plant nutrient solution (PEG6000-free)–treated plants as control lines. There were 3 biological replicates in this experiment.
Isolation of NAC transcription factor (CcNAC1) and analysis of expression pattern under drought stress
We used Significance Analysis of Microarray (SAM) method for selecting NAC transcription factor (CcNAC1) gene from the transcriptome database on drought stress of jute. The CcNAC1 gene was isolated by the following method: Total RNA was extracted from jute leaves using Trizol reagent (5417–12), and cDNA was synthesized using a reverse transcription kit (R235, Shanghai Biology Company). Full-length of CcNAC1 cDNA was amplified using the following primers: F1, 5′-ATGGGAGTCCCGGAAACAGACCCCT-3′ and R1, 5′-TTATTGCCTAATCCCAAACCCACCC-3′ (primers were designed by the primer premier 5.0 software). The PCR reactions and cloning were performed as previously described (Zhang et al. 2014). The reaction system contained 1 μL cDNA, 1 μL upstream and downstream primers (10 μmol L−1), 2.5 μL 10 × buffer, 2 μL dNTP, 0.2 μL Taq polymerase (10 U/μL), and brought to 25 μL with ddH2O. PCR conditions were as follows: denaturation at 94 °C for 30 s, followed by 35 cycles at 58 °C for 40 s, and extension at 72 °C for 2 min; the final extension was carried out at 72 °C for 10 min. The resulting CcNAC1 sequence was used to perform a homology search of the NCBI BLAST database. When the plants are in rapid growing period (about 50 days after seed germination), a 20% polyethylene glycol (PEG6000) hypertonic solution was used to treat plants for 24 h to simulate drought stress. Leaves were collected at 0 h, 2 h, 4 h, 6 h, 8 h, 12 h, 16 h, 20 h, and 24 h after drought stress for analysis of the expression level CcNAC1 gene. RT-qPCR was performed using SYBR Green qPCR Master Mix (Takara) on a iQ5™ multicolor real-time PCR detection system (Bio-Rad) according to the method described by Livak and Schmittgen (Livak and Schmittgen 2001). The following primers were used for qRT-PCR: QF, 5′-TCGGCTATGACTGGGCACAACAGA-3 and QR, 5′-TTGAAGCTGGGACATACGGAAC-3′. The RT-qPCR thermal cycle was as follows: denaturation at 95 °C for 8 min, followed by 40 cycles at 95 °C for 15 s, and 56 °C for 40 s, the actin 7 (ACT7) gene of jute was selected as the endogenous control (Hossain et al. 2019). There were 3 biological replicates in the PEG6000 drought stress experiment.
The sequence analysis of NAC transcription factor (CcNAC1)
The cDNA sequences of CcNAC1 were used as queries in BLASTX searches against NCBI (https://www.ncbi.nlm.nih.gov/). The open reading frame (ORF) and amino acid sequences were analyzed by DNAMAN 6.0, and a phylogenetic tree was constructed using the MEGA 6 software. The conserved domains were predicted by Pfam 26.0 (http://pfam.xfam.org/) and the Conserved Domains program in NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Transformation vector construction and validation of transgenic plants
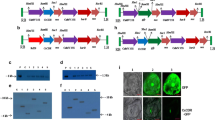
The pCAMBIA1301 plasmid (Fig. 1a) contains the jute CcNAC1 gene driven by the (CaMV) 35S promoter and a hygromycin resistance marker gene (HYG). The TCK303 plasmid (Fig. 1b) contains a rice intron, CcNAC1 gene fragments inserted into vector in opposite directions between the rice intron, and the same HYG selectable marker as plasmid pCAMBIA1301.
The construction of the overexpression and RNAi interference vectors was performed as Zhang described. The open reading frame of CcNAC1 gene was amplified with the sense primer (5′-GAAGATC ATGGGAGTCCCGGAAACAGAC-3′), and antisense primer (5′-GGGTAACC TTATTGCCTAATCCCAAAC-3′) (restriction endonuclease BglII and BstEII sites are noted in underlined letters). The amplified PCR product was digested with BglII and BSTEII, and inserted into the pCAMBIA1301 binary vector containing the hygromycin phosphotransferase (hpt) gene, under the control of a CaMV35S promoter. Sequence analysis confirmed proper insertion of CcNAC1 gene into the vector (Zhang et al. 2014). Stable transgenic jute plants were obtained by Agrobacterium-mediated gene transfer following a method described previously. Agrobacterium tumefaciens EHA105 carrying the binary vector pCAMBIA1301 that contains the selectable marker nptII gene and screenable marker GUS gene were grown on a YEB medium (0.5% beef extract, 0.4% yeast extract, 0.1% peptone, 0.04% MgSO4, 7H2O; pH = 7.4) containing kanamycin 50 μg/mL as the selective agent at 230 rpm in a shaker for 18 h at 28 °C. The bacterial concentration (OD = 0.5) was determined using a spectrophotometer at a wavelength of 600 nm. The prepared explants (cotyledonary nodes of jute) were transferred into Agrobacterium bacterial liquid. The infection time was 10 min, post-infection; the explants were co-cultured on MS medium without a regulator at 28 °C in the dark for 2 days (Bharadwaj et al. 2011; Zhang et al. 2014). Total DNA was isolated from putative transgenic plants of T0 generation, and the hygromycin phosphotransferase (selection marker) gene was detected using PCR with the following primers: 5′-TAGCTAGCATGCAGTTGCAG-3′ and 5′-AGTCGTCGACGATGCTAGAGTA-3′. We then used the PCR-validated transgenic plants for Southern blot analysis. According to the transformation vector plasmid pCAMBIA1301, the chosen three single restriction sites, respectively, are BSTEII, BglII, and NcoI; 5–10 g genomic DNA was digested overnight, after electrophoresis, transfer film, print, pre hybridization, and hybridization. Hygromycin phosphotransferase gene probe was labeled with the alkaline phosphatase labeling kit (ROCHE Company). The primers for southern hybridization are R: 5′-CATACTTGAGACCAGTGTTT-3′, F: 5′-CCGACCTTAACTAGCTCAAT-3′; the results were collected use the gel imaging system Fluor Chem SP 50-mm f11.4 lens (Zhang et al. 2014).
The drought resistance evaluation and the growth rate measurement of CcNAC1-overexpression and control plants
The seeds of CcNAC1 overexpression and control plants were planted in the germinating box. Two treatments were set, 20% PEG6000 drought stress and distilled water treatment; 10 mL of 20% PEG6000 was added to each germination box in the drought stress treatment group, and 10 mL of distilled water was added to each germination box as control group. The seeds were cultured in a light incubator (32 °C/24 °C, 16 h light/8 h dark) for germination. The number of seed germination was recorded once a day; the record lasted for 10 days; there are 3 replicates per treatment. The germination rate (%) = the number of all germinated seeds/all the tested seeds × 100%. The standard of drought resistance of seed germination was estimated according to Lan’s method in Table 1 (Lan 1998).
The plantlets were planted in pots. Vegetable soil (contains humus, straw fiber, perlite, and bioorganic matter) and sandy soil (agglomerate particle from mountain slopes facing the sun in Shangrao area) were mixed (3:1), After 15 days of growth under normal conditions, the plants were treated with 20% PEG for 2 h for observing the phenotypes of CcNAC1-overexpression and control plants. After 40 days of growth under normal conditions of CcNAC1-overexpression and control plants, the whole aboveground and root parts of the plants were measured.
Yeast two-hybrid assays
To examine the protein-protein interactions between NAC and KCS, the coding regions of NAC and KCS were cloned from jute cDNA for use in yeast two-hybrid (Y2H) assays. Y2H experiments were performed using the pGBKT7-KNAC, pGADT7-KCS, and pGBKT7 (empty vector control) constructs transformed into the yeast strain MaV203. The subsequent yeast mating and selection were performed as previously described (Sanz et al. 2018).
Results
Isolation of full-length transcription factor (CcNAC1) cDNA and determination of the expression patterns under drought stress
The NAC-like transcription factor SiNAC110 in foxtail millet (Setaria italica L.) confers tolerance to drought and high salt stress through an ABA-independent signaling pathway (Xie et al. 2017); six NAC-like transcription factors were discovered from the transcriptome of jute; we selected the one with the most expression level after drought stress treatment for analyzing its function. The CcNAC1 gene is predicted to encode a 348–amino acid (Supplementary material), 38.65-kDa protein. Alignment analyses (Fig. 2) demonstrated that CcNAC1 shares 84% similarity with Hibiscus syriacus NAC domain–containing protein 72, which belongs to the NAC family in hibiscus (Hibiscus syriacus). The N-terminal of NAC transcription factor contains a highly conserved DNA-binding domain, consisting of about 160 amino acid residues, and the C-terminal is a highly variable transcriptional activation or inhibition domain. The N-terminal of NAC transcription factor regulates the expression of genes by recognizing that NACRS, CGTA, CGTC, and CACG are core sites of NAC transcription factor binding. CcNAC1 transcription factor with a cDNA length of 1044 bp was isolated from jute (Fig. 3a and Supplementary material). Expression of the CcNAC1 transcription factor gene peaks at 8 h of drought stress (Fig. 3b) and then gradually decreases, but there were no changes observed in the CK (the control plants) lines. (Fig. 3b).
Isolation and expression of the transcription factor CcNAC1 and molecular detection of the transgenic plants. a Isolation of transcription factor CcNAC1, M: DNA marker, 1 and 2: CNAC1 transcription factor. b The relative expression level of CcNAC1 gene in leaves under drought stress and CK lines. c PCR screening of CcNAC1-overexpression and knockdown plants, M: DNA marker: 1–4, CcNAC1-overexpression plants; 5–8, CcNAC1 knockdown plants; 9, Negative control. d Southern blot identification of CcNAC1-overexpression and CcNAC1 knockdown plants. 1–5, samples; 6, negative control; M, DNA marker
PCR and Southern blots identification of CcNAC1-overexpression and knockdown plants
In order to determine the function of the CcNAC1 transcription factor, we analyzed CcNAC1-overexpression and RNAi knockdown lines of jute. We used gene-specific primers to amplify a fragment of the HPT marker gene (approximately 795 bp) in plants with CcNAC1-overexpression and CcNAC1 knockdown plants, but no amplified bands were found in negative control plants (Fig. 3c). We then used the HPT gene fragment as a probe for Southern blots to validate plants positive for the transgene construct. Genomic DNA was digested with HindIII, and the abovementioned HPT gene probe was used for Southern hybridization. The PCR result showed that a single copy of the HPT gene was inserted into the genome of all positive plants (Fig. 3d).
The physiological characteristics and drought tolerance of CcNAC1 gene transgenic and control lines
There are many transcription factors that regulate plant growth and development; the NAC gene superfamily transcription factors were discovered in the plant kingdom recently. Previous studies have shown that NAC TF proteins have important functions in the growth and development of many plants. Under 20% PEG stress, the seed germination rate and growth rate after germination of CcNAC1 gene transgenic plants and the control plants were significantly different (Table 2, Table 3, Fig. 4a). Compared with the control lines, the germination rate of CcNAC1 gene transgenic plants was significantly higher than that of the control group (Table 3), and the germination rate of transgenic lines was increased by 34.1%, 36.8%, and 27.6%. According to the evaluation standard of drought resistance during seed germination, the drought resistance of the control lines was close to moderate resistance, while that of CcNAC1 gene transgenic plants was close to strong resistance (Table 3). Our results show that the overexpression of the CcNAC1 transcription factor increased the growth rates (Table 2, Fig. 4a) and drought tolerance (Table 3, Fig. 4b) and promoted early flowering of jute (Fig. 4c), compared to that of the control plants. These results indicated that CcNAC1 transcription factor was involved in plant growth and drought stress response.
Physiological characteristics and drought tolerance of CcNAC1-overexpression and control plants. a The growth rate of CcNAC1-overexpression and control plants. Left: CcNAC1-overexpression plants, Right: The control plants. b The drought tolerance of CcNAC1-overexpression and control plants after drought stress with 20% PEG6000 for 2 h. Upper: CcNAC1-overexpression plants; bottom: the control plants. c The comparison flowering of CcNAC1-overexpression and control plants. Left: CcNAC1-overexpression plants; right: the control plants
CcNAC1 directly regulates expression of KCS gene
To assess the relationship between the KCS gene and the CcNAC1 transcription factor and further investigate the possible mechanisms by which CcNAC1 regulates the expression of related gene, we evaluated KCS gene expression level in the WT, CcNAC1-overexpressing and CcNAC1-RNAi knockdown lines by RT-qPCR. Total RNA was extracted from these three lines. The expression of the drought-induced gene KCS (encoding 3-ketoacyl-CoA synthase) was more than 2-fold higher in the CcNAC1-overexpressing plants (Fig. 5a), while the expression of KCS gene was significantly decreased in CcNAC1-RNAi knockdown lines compared with the WT (Fig. 5a). To further verify the protein-protein interaction data, Y2H assays was conducted. After resolving the auto-activation issue by using auto-activation detection, a strong protein–protein interaction between CcNAC1 transcription factor and KCS protein was detected (Fig. 5b and Fig. 5c). These results suggest that the CcNAC1 transcription factor regulated KCS gene expression in a manner involved in drought tolerance in jute.
The relative expression of KCS and physical interaction with CNAC1 in yeast. a Expression level of KCS gene in WT (control lines), CNAC1-knockdown lines and CNAC1-overexpression lines. Error bars represent SD of three biological replicates. ** P < 0.01 (t test). b Display of the auto-activation of CNAC1 and KCS in Y2H assays. c Colonies were gown on Leu, Trp, His, and Ura (left panel) and with X-Gal (right panel). Negative controls contained empty bait and prey vector
Discussion
NACs (NAM (No apical meristem), ATAF1 (Arabidopsis transcription activation factor 1), ATAF2, and CUC2 (cup-shaped cotyledon 2)) are important, plant-specific transcription factors involved in growth and physiological processes in plants, including senescence, positive regulation of cell death, and salt stress response (Buchanan Wollaston et al. 2010). NAC transcription factors not only regulate plant growth and development but also have important functions in biotic and abiotic stress responses. In this study, we identified CcNAC1 as a positive regulatory factor of drought stress tolerance, as the CcNAC1-overexpressing plants exhibited an increased tolerance to drought. Previous studies have shown that the expression levels of SNAC1 (stress-responsive NAC1) and OsNAC6 are induced by drought stress, and that transgenic rice plants overexpressing these genes also display increased drought tolerance (Hu et al. 2006; Lee et al. 2017). Taken together, these results and our work point to similar roles for the NAC transcription factors in abiotic stress responses. Several other plant NAC TFs can increase yields under abiotic stress conditions (Shang et al. 2020; Jeong et al. 2010, 2013). In rice, OsNAC10 overexpression increased plants yields under stress conditions (Jeong et al. 2013); overexpressing OsNAC5 plants enhanced drought tolerance and increased grain yields under drought stress; overexpressing OsNAC2 plants have an ideotype architecture, with thick stems, well-developed root systems, and high yields (Jeong et al. 2013). Our results also revealed that overexpressing CcNAC1 enhances plants growth and promotes early flowering; this is partly because of NAC TFs’ direct regulation of other genes involved in the growth and development.
Ketoacyl synthases (KCSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA, or with malonyl-ACP to form 3-ketoacyl-ACP (Tresch et al. 2012). KCS is involved in very long-chain fatty acid synthesis in vegetative tissues and also plays a role in wax biosynthesis, can limit non-stomatal water loss, and reduce damage due to UV radiation. Epidermal wax also protects against bacterial and fungal pathogens and water stress as well as reduces plant transpiration rate and the risk of irreversible light suppression (Chen et al. 2011). To address water deficits, there is increasing research on the important roles of epidermal wax in plants. KCS is involved in very long-chain fatty acid synthesis in vegetative tissues and also plays a role in wax biosynthesis. In our study, the expression of the KCS (encoding 3-ketoacyl-CoA synthase) gene was more than 2-fold higher in the CcNAC1-overexpressing plants. These findings suggest that CcNAC1 regulates the expression of KCS biosynthesis gene to increase the wax content, thereby increasing drought tolerance. It was previously reported that OsNAC2 directly promotes the expression of OsNCED3 to increase the ABA content (Mao et al. 2017). Via analysis of KCS mutants in Arabidopsis, KCS (KCS6/CER6) was found to be an important enzyme for cuticular wax biosynthesis (Todd and Jaworski 1999). The expression levels of the KCS20 and KCS2/DAISY genes were found to increase 2-fold and 65-fold, respectively, after drought stress (Lee et al. 2009).
The NAC domain transcription factor VASCULAR-RELATED NAC-DOMAIN7 (VND7) acts as a master regulator of xylem vessel differentiation in Arabidopsis thaliana. A yeast two-hybrid screen (Y2H) identified cDNAs encoding a NAC domain protein, VND-INTERACTING1 (VNI2), which interacts with VND7. VNI2 acts as a transcriptional repressor and physically interacts with VND proteins involved in xylem cell specification (Yamaguchi et al. 2010). OsNAC5 directly interacts with the promoter of OsLEA3 to regulate salt tolerance (Jeong et al. 2013). Here, we illustrated that CcNAC1 interacts with the KCS in the Yeast-2-Hybrid system; this showed that CcNAC1 directly interacts with KCS to regulate drought tolerance.
NAC TF proteins have important functions in the growth and development of jute. Our findings indicate that the CcNAC1 gene is a potential positive regulator of the drought stress response. Overexpressing CcNAC1 enhances plant growth (Table 2, Fig. 4a), promotes early flowering (Fig. 4c), and increases drought tolerance (Table 3, Fig. 4b). CcNAC1 transcription factor regulates KCS gene expression level by binding to the KCS protein involved in drought response. Our study, along with others, indicates that NAC TF has several important functions in the growth and development of rice (Jiang et al. 2018). These findings provide new sight for understanding the regulatory mechanism of CcNAC1 transcription factor and enable the production of new drought-tolerant jute varieties.
References
An X, Chen J, Zhang J, Liao Y, Dai L, Wang B, Liu L, Peng D (2015) Transcriptome profiling and identification of transcription factors in ramie (Boehmeria nivea L. gaud) in response to PEG treatment, using Illumina paired-end sequencing technology. Int J Mol Sci 16:3493–3511
Ayodele VI, Fawusi MOA (1990) Studies on drought susceptibility of Corchorus olitorius L.: II. Effects of moisture stress at different physiological stages on vegetative growth and seed yield of C. olitorius cv. ‘Oniyaya’. Biotronics 19:33–37
Bharadwaj P, Beena MR, Sinha MK, Kirti PB (2011) In vitro regeneration and optimization of conditions for Agrobacterium mediated transformation in jute, Corchorus capsularis. J Plant Biochem Biotechnol 20:39–46
Buchanan Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Issizaki J, Leaver CJ (2010) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42:567–585
Chen Y, Kelly EE, Masluk RP, Nelson C, Reilly P (2011) Structural classification and properties of ketoacyl synthases. Protein Sci 20:1659–1667
Chen X, Wang YF, Lv B, Li J, Luo LQ, Lu SC, Zhang X, Ma H, Ming F (2014) The NAC family transcription factor OsNAP confers abiotic stress response through the ABA pathway. Plant Cell Physiol 55:604–619
Chen X, Lu SC, Wang YF, Zhang X, Lv B, Luo LQ, Xi DD, Chen JB, Ma H, Ming F (2015) OsNAC2 encoding a NAC transcription factor that affects plant height through mediating the gibberellic acid pathway in rice. Plant J 82:302–314
Hao YJ, Wei W, Song QX, Chen HW, Zhang YQ (2011) Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J 68:302–313
Hong Y, Zhang H, Huang L, Li D, Song F (2016) Overexpression of a stress responsive NAC transcription factor gene ONAC022 improves drought and salt tolerance in Rice. Front Plant Sci 7:4
Hossain MS, Ahmed R, Haque MS, Alam MM, Islam MS (2019) Identification and validation of reference genes for real-time quantitative RT-PCR analysis in jute. BMC Mol Biol 20:13. https://doi.org/10.1186/s12867-019-0130-2
Hu H, Xiong L (2013) Genetic engineering and breeding of drought-resistant crops. Annu Rev Plant Biol 65:715–741
Hu HH, Dai MQ, Yao JL, Xiao BZ, Li XH, Zhang QF, Xiong LZ (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci U S A 103:12987–12992
Hu HH, You J, Fang YJ, Zhu XY, Qi ZY et al (2008) Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol Biol 67:169–181
Huang L, Hong YB, Zhang HJ, Li DY, Song MF (2016) Rice NAC transcription factor ONAC095 plays opposite roles in drought and cold stress tolerance. BMC Plant Biol 16:203
Jeong JS, Kim YS, Baek KH, Jung H, Ha SH, Choi YD, Kim MY, Reuzeau C, Kim JK (2010) Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol 153:185–197
Jeong JS, Kim YS, Redillas MC, Jang G, Jung H, Bang SW, Choi YD, Ha SH, Reuzeau C, Kim JK (2013) OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol J 11:101–114
Jiang DG, Chen WT, Dong JF, Li J, Yang F, Wu ZC, Zhou H, Wang WS, Zhuang CX (2018) Overexpression of OsmiR164b-resistant OsNAC2 improves plant architecture and grain yield in rice. J Exp Bot 69:1533–1543
Jiang DG, Zhou LY, Chen WT, Ye NH, Xia JX, Zhuang CX (2019) Overexpression of a microRNA-targeted NAC transcription factor improves drought and salt tolerance in rice via ABA-mediated pathways. Rice 12:76. https://doi.org/10.1186/s12284-019-0334-6
Ju YL, Yue XF, Min Z, Wang XH, Fang YL, Zhang JX (2019) VvNAC17, a novel stress-responsive grapevine (Vitis vinifera L.) NAC transcription factor, increases sensitivity to abscisic acid and enhances salinity, freezing, and drought tolerance in transgenic Arabidopsis. Plant Physiol Biochem 146:98–111
Lan JS (1998) Comparison of evaluating methods for agronomic drought resistance in crops. Acta Agriculturae Boreali-occidentalis Sinica 7:85–87
Lee SB, Jung SJ, Go YS, Kim HU, Cho HJ, Park OK, Suh MC (2009) Two Arabidopsis 3-ketoacyl CoA synthase genes, KCS20 and KCS2/DAISY, are functionally redundant in cuticular wax and root suberin biosynthesis, but differentially controlled by osmotic stress. Plant J 60:462–475
Lee DK, Chung PJ, Jeong JS, Jang G, Bang SW, Jung H, Kim YS, Ha SH, Choi YD, Kim JK (2017) The rice OsNAC6 transcription factor orchestrates multiple molecular mechanisms involving root structural adaptions and nicotianamine biosynthesis for drought tolerance. Plant Biotechnol J 15:754–764
Li D, Yang J, Dou JH, Wu LY, Chen P, Zhou Q, Mo LY, He B, Fan ZL, Zhou RY (2013) Physiological response and drought-resistance evaluation of jute seedling under drought stress. Southwest China J Agric Sci 26:125–130
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Mao CZ, Ding WN, Wu YN, Yu J, He XW, Shou HX, Wu P (2007) Overexpression of a NAC-domain protein promotes shoot branching in rice. New Phytol 176:288–298
Mao X, Zhang H, Qian X, Li A, Zhao G, Jing R (2012) TaNAC2, a NAC-typewheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. J Exp Bot 63:2933–2946
Mao CJ, Lu SC, Lv B, Zhang B, Shen JB, He JM, Luo LQ, Xi DD, Chen X, Ming F (2017) A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiol 174:1747–1763
Meng CM, Cai CP, Zhang TZ, Guo WZ (2009) Characterization of six novelNAC genes and their responses to abiotic stresses in Gossypium hirsutum L. Plant Sci 176:352–359
Mitra GC, Basu NC (1974) Studies on the size and distribution of stomata in jute (Corchorus olitorius and Corchorus capsularis) and its bearing on resistance to drought. Acta Agron Budap 2013:192–199
Peng H, Cheng HY, Chen C, Yu XW, Yang JN, Gao WR, Shi QH, Zhang H, Li JG, Ma H (2009) A NAC transcription factor gene of Chickpea (Cicer arietinum), CarNAC3, is involved in drought stress response and various developmental processes. J Plant Physiol 166:1934–1945
Pil JS, Saet BL, Mi CS, Mi JP, Young SG, Chung MP (2011) The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 23:1138–1152
Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17:369–381
Redillas MC, Jeong JS, Kim YS, Jung H, Bang SW, Choi YD, Ha SW, Reuzeau C, Kim J (2012) The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol J 10:792–805
Saada AS, Li X, Li HP, Huang T, Gao CS, Guo MW, Cheng W, Zhao GY, Liao YC (2013) A rice stress-responsive NAC gene enhances tolerance of transgenic wheat to drought and salt stresses. Plant Sci 203–204:33–40
Sanz P, Viana R, Garcia-Gimeno MA (2018) AMPK protein interaction analyses by yeast two-hybrid. Methods Mol Biol 1732:143–157
Shang XG, Yu YJ, Zhu LJ, Liu HQ, Chai QC, Guo WZ (2020) A cotton NAC transcription factor GhirNAC2 plays positive roles in drought tolerance via regulating ABA biosynthesis. Plant Sci 296:110498
Su JG, Dai ZG (2017) Germplasm resources and main characters of hemp crops in China. Chinese agricultural press
Todd J, Jaworski J (1999) KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J 17:119–130
Tresch S, Heilmann M, Christiansen N, Looser R, Grossmann K (2012) Inhibition of saturatedvery-long-chain fatty acid biosynthesis by mefluidide and perfluidone, selective inhibitors of 3-ketoacyl-CoA synthases. Phytochemistry 76:162–171
Wu A, Allu AD, Garapati P, Siddiqui H, Dortay H, Zanor MI, Asensi-Fabado MA, Munné-Bosch S, Antonio C, Tohge T, Fernie AR, Kaufmann K, Xue GP, Mueller-Roeber B, Balazadeh S (2012) JUNGBRUNNEN1, a reactive oxygen species–responsive NAC transcriptionfactor, regulates longevity in Arabidopsis. Plant Cell 24:482–506
Xie LN, Chen M, Min DH, Feng L, Xu ZS, Zhou YB, Xu DB, Li LC, Ma YZ, Zhng XH (2017) The NAC-like transcription factor, SiNAC110, in foxtail millet (Setaria italica, L.) confers tolerance to drought and high salt stress through an ABA independent signaling pathway. J Integr Agric 16:559–571
Yamaguchi M, Ohtani M, Mitsuda N, Kubo M, Ohmetakagi M, Fukuda H, Demura T (2010) VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell 22:1249–1263
Yao YF, Hong JJ, Zeng RQ, Yang YX (2013) Drought resistance of various jutes (Corchorus L.) in seedling under PEG stress. Fujian J Agric Sci 28:457–562
Yuan X, Wang H, Cai JT, Bi Y, Li DY, Song FM (2019) Rice NAC transcription factor ONAC066 functions as a positive regulator of drought and oxidative stress response. BMC Plant Biol 19:278. https://doi.org/10.1186/s12870-019-1883-y
Zhang GY, Zhang YJ, Xu JT, Niu XP, Qi JM, Tao AF, Zhang LW, Fang PP, Lin LH, Su JG (2014) The CCoAOMT1 gene from jute (Corchorus capsularis l.) is involved in lignin biosynthesis in Arabidopsis thaliana. Gene 546:398–402
Zhang GY, Shan SL, Deng JL, Deng HG, Huang SQ, Li DF (2018) The segregation and functional identification of jute β-ketoyl-CoA synthase gene (KCS). Mol Plant Breed 20:6718–6722
Acknowledgments
We would like to deeply thank Jiantang Xu for their constructive guidance and discussions, and Jie Chen for valuable proofreading and language-polishing services.
Funding
This work was supported by grants from the National Science Foundation of China (grant no. 31860396) in the design of the study and data collection and the Key Laboratory of Fiber Biology of the Chinese Academy of Agricultural Sciences (grant number 201602) and the Science and Technology Project of the Education Department of Jiangxi Province (grant nos. GJJ161047 and GJJ180890) for the analysis and interpretation of data and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
GY, SS, and DF conceived the research plan, analyzed the data, and wrote the manuscript. ZC, JL, and YW analyzed the function of NAC1. JM and SL did the qPCR and vector construction. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Néstor Carrillo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, G., Huang, S., Zhang, C. et al. Overexpression of CcNAC1 gene promotes early flowering and enhances drought tolerance of jute (Corchorus capsularis L.). Protoplasma 258, 337–345 (2021). https://doi.org/10.1007/s00709-020-01569-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01569-y