Abstract
The family Malpighiaceae, particularly in the Neotropic, shows a similar floral morphology. Although floral attraction and rewards to pollinators are alike, stigmas and styles show more diversity. The stigmas were described covered with a thin and impermeable cuticle that needs to be ruptured by the mechanical action of the pollinators. However, this characteristic was only mentioned for a few species and the anatomy and ultrastructure of the stigmas were not explored. In this work, we analyze the morphology, anatomy, and ultrastructure of the stigma and style of Callaeum psilophyllum. Moreover, we identify the potential pollinators in order to evaluate how the disposition of the stigmas is related with their size and its role in the exposure of the receptive stigmatic surface. Our observations indicate that Centris flavifrons, C. fuscata, C. tarsata, and C. trigonoides are probably efficient pollinators of C. psilophyllum. The three stigmas are covered by a cuticle that remained intact in bagged flowers. The flowers exposed to visitors show the cuticle broken, more secretion in the intercellular spaces between sub-stigmatic cells and abundant electron-dense components inside vacuoles in stigmatic papillae. This indicates that the stigmas prepares in similar ways to receive pollen grains, but the pollinator action is required to break the cuticle, and once pollen tubes start growing, stigmatic and sub-stigmatic cells release more secretion by a granulocrine process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The floral morphology is assembled to fit with the pollinators physically, to provide the reward appropriately and guarantee the transference of pollen. Particularly, the structure of the stigmas and styles can be very diverse, although some of its functions as capture and germination of pollen, maintain hydration, and offer entry points to guide pollen tubes growth are common to all species, regardless to the form.
The structure of the stigma is generally correlated with taxonomic subdivision and reinforces the idea of coevolution between pollen and the structure of the stigma (Edlund et al. 2004). On this basis, it could be supposed that some morphological/anatomical patterns of styles/stigmas would be uniform among different members of a particular natural group of species, especially if they share pollinators and show the same floral syndrome. For example, the monophyletic and large genus Solanum, whose floral morphology (particularly the androecium) is uniform and all their species are pollinated by bees that buzz poricidal anthers (Knapp 2010). However, the Malpighiaceae are contrasting in this aspect. This family, particularly in the Neotropical genera, shows a relatively similar floral morphology especially in floral attraction and orientation and reward to pollinators, but the intrafloral position of sexual organs are more diverse. The gynoecium presents mostly 3 (2-4) carpels, and principally, the stigmas and styles show great diversity (Anderson 1979).
Most species of neotropical Malpighiaceae offer floral oil secreted from glands in the calyx named elaiophores, and Vogel (1974) established a new pollination system, the “oil flower syndrome.” In these species, the arrangement of stamens and stigmas in the center of the flower favors the contact of pollinators with both structures simultaneously during their visits (Sigrist and Sazima 2004). Females of numerous species of bees collect the floral oil for nest construction (mixed with other materials) and protection and/or jointed with pollen mass for larval food (Vinson et al. 1996). The effective pollinators of the Neotropical species of Malpighiaceae often hold the claw of the posterior petal with their mandibles and insert their front and middle legs between the petal claws to reach and gather oil from glands on the sepals. In this way, they contact the reproductive organs with the ventral surfaces of their bodies (Possobom and Machado 2017).
The pollination and reproductive biology of some species of the genera Banisteriopsis, Dicella, Heteropterys, Mascagnia, Stigmaphyllon, and Tetrapterys of the family Malpighiaceae, were studied in detail by Sigrist and Sazima (2004). These authors demonstrated that stigmas are covered with a thin and impermeable cuticle that prevents pollen from adhering, hydrating, or germinating. The stigmatic exudates are accumulated under a cuticle and released by rupture of this protective stigmatic cover, produced by the mechanical action of the pollinators. However, the anatomy and ultrastructure of the stigmas are not studied (Sigrist and Sazima 2004).
Likewise, the internal structure of the styles in species of Malpighiaceae is very little known. To mention a few examples, the studies on Byrsonima sericea (Guimarães et al. 2014) and species of Janusia, Mascagnia, and Tetrapterys (Souto and Oliveira 2013), indicate that styles are solid; and although this would probably be the ancestral condition for the family, the information is very scarce to generalize that hypothesis.
The structural features of the stigma and style and their physiological characteristics are related to the reproductive success of the plants. Few of these aspects were explored in some species of Malpighiaceae (Sigrist and Sazima 2004), and they are not described for Callaeum psilophyllum. The structure of the stigma and style and its relation with potential pollinators will contribute to the knowledge of the reproduction biology of the family.
Based on the proposal of Sigrist and Sazima (2004) about that the pollinators are necessary to break the stigmatic cuticle in Malpighiaceae, we studied the traits of the stigma and style of C. psilophyllum in open pollination (not bagged) vs. virgin flowers (bagged), in order to associate these data with the activity of specialized pollinators. Our aims are as follows: (1) to analyze the morphology, anatomy, and ultrastructure of the stigma and style, (2) to know the potential pollinators in a population located in the southernmost distributional area, (3) to evaluate how the disposition of the stigmas is related with the size of pollinators, and (4) to study the pollinator’s role in the exposure of the receptive stigmatic surface.
Materials and methods
Studied species and study site
Callaeum psilophyllum grows as climbing or prostrate woody vine in forest edges, riverbanks, and rocky slopes, with yellow flowers grouped in umbels or short racemes, with few flowers (Aliscioni and Torretta 2017). Field work was conducted during the period December 2013–February 2014 to locate a natural population of this species in the multipurpose “Martín García” Natural Reserve island, Buenos Aires Province, Argentina (34° 10′ S, 58° 14′ W).
Fresh flowers (n = 100) from five individuals of the natural population were collected in anthesis (flowers open pollination), then were fixed in FAA (formalin-acetic acid-alcohol mixture) for 48 h and stored in 70% alcohol.
To evaluate if the presence of pollinators was obligatory to break the stigmatic cuticle, some floral buds (n = 80) randomly chosen from four individuals were bagged with cloth bags to avoid contact with floral visitors (bagged flowers). During the anthesis, these virgin flowers were fixed in FAA to be observed and analyzed in the lab.
Floral visitors and potential pollinators
We observed and captured species of oil-collecting bees in flowers of C. psilophyllum, on 2–3 days per trips (a total of four visits), at different times of day (between 8.00 and 19.00 hours). Bee species that contacted reproductive structures while foraging were recorded as legitimate pollinators (i.e., discriminating pollinators from floral visitors). To achieve this, we conducted 10-min censuses on a known number of flowers (cumulative time = ca. 30–60 min) and we captured all floral visitors.
The captured insects were sacrificed in situ and preserved to be determined later. Taxonomic determination was carried out to specific level in the lab (Roig Alsina 2000; Torretta and Roig Alsina 2017). All captured specimens are preserved in the Entomological Collection of the General Botany Unit (FAUBA) at the Faculty of Agronomy, University of Buenos Aires. Also, vouchers of the vegetal specimen were deposited in herbarium BAA (Torretta 44, 45).
Relation of flowers of C. psilophyllum and potential pollinators
To estimate the association between the stigmatic surfaces in flowers of C. psilophyllum with different captured species of bees, some measures were taken. To perform that, digital photographs in frontal view of open pollination flower (n = 20) were taken after being visited. The photographs were used to measuring the distances between the three stigmas; imaginary lines were drawn from the center of each receptive area (the regions were the cuticles showed broken), joining the three stigmas (Fig. 1a).
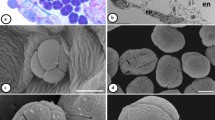
Relation between stigmas and potential pollinators. a Frontal view of the flower of Callaeum psilophyllum. a and b, posterior stigmas; c, anterior stigma; PP, posterior petal; C, claw. b Centris flavifrons; white triangle delimits posterior area procoxal and area postmetacoxal. a–b, posterior area procoxal; c, area postmetacoxal. Scale bars: a 3.5 mm, b 3 mm
Centris are medium-sized to very large (Michener 2007). The bee species captured were assigned to size groups depending on their intertegular spans (Cane 1987): medium ITS < 4 mm, large 4–6 mm, and very large > 6 mm). For the visitor bees, digital photographs in ventral view (n = 5 individuals per species) were taken, and we measure the ventral area delimiting a triangle included among posterior area procoxal and area postmetacoxal (Fig. 1b). The distances of flowers and bees were estimated using ImageJ software.
Morphological, anatomical, and ultrastructural observations
The fixed open pollinated (not bagged) and virgin (bagged) flowers were observed using stereomicroscope in the lab. We selected flowers in complete anthesis and without damage. We removed the gynoecia and processed these materials to obtain samples for bright field microscope and scanning electron microscopy (SEM). Samples were dehydrated in an ethanol series, transferred to xylene, embedded in paraffin (58 °C), and sectioned at a thickness of 6–7 μm on a rotary microtome (Leitz Wetzlar), using conventional methods (Zarlavsky 2014). Histological sections were stained with Safranin-Fast Green and mounted in Canada balsam (Zarlavsky 2014). Observations were made using a Motic bright field microscope. Photomicrographs and measurements were taken using Motic images plus 2.0.
To obtain samples for SEM, complete gynoecia were dehydrated and subjected to critical-point drying using liquid CO2. The material was then sputter-coated with gold and examined using a Philips XL 30 TMP microscope at an accelerating voltage of 80 kV.
To analyze ultrastructural differences between visited (not bagged) and virgin flowers (bagged), the gynoecia were prefixed in 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer for 3 h at room temperature, and then, we proceeded in a conventional manner for observations with transmission electronical microscope (TEM). The stigmas and the apical portion of the styles were washed in buffer, and then postfixed in 1.5% osmium tetroxide with the same buffer for 2 h, dehydrated in ethanol series, and embedded in Spurr resin. Ultrathin sections were obtained with glass knives, stained with uranyl acetate followed by lead citrate, and examined and photographed in a JEOL-JEM 1200 EX II TEM at 85.0 kV.
Results
Disposition of stigmas in flowers of C. psilophyllum
The stigmas of C. psilophyllum are separated and disposed in a triangle. One of the stigmas (the anterior) faces the center of the flower while the others (the posterior) are oriented upwards (Fig. 2a). The distance between the two posterior stigmas resulted 3.25 ± 0.76 mm (mean ± SD); distance between the right posterior stigma and the anterior stigma resulted 2.66 ± 0.63 mm and distance between the left posterior stigma and the anterior stigma) was 2.69 ± 0.72 mm (Table 1). In frontal view, the disposition of stigmas shows an inverted scalene triangle (Fig. 1a).
Pollinators and floral visitors
We captured four species of Centris (Apidae: Centridini), and one species Paratetrapedia (Apidae: Tapinotaspidini). During the field observations, 12 individuals of Centris trigonoides, 8 of C. tarsata (both medium-size species), 5 of C. fuscata (large species), and 4 of C. flavifrons (very large species) were captured. Also, five specimens of Paratetrapedia nigrispinis were captured, but they were not contacting stigmas neither stamens in the flowers.
Related to pollen deposition area in oil collecting bees, size triangle varies between the Centris species (Table 1). In ventral view, the pollen deposition area occupied an inverted scalene (medium-size species) or equilateral (large and very large species) triangles (Fig. 1b). Paratetrapedia nigrispinis were considered as illegitimate floral visitors and were not measured.
Morphological, anatomical, and ultrastructural observations
The stigmatic surface is concave and covered by a thick cuticle (Fig. 2a, b). Epicuticular crystalloids are observed as small irregular plates (Fig. 2b, c). In flowers exposed to visitors, the cuticle breaks to release the secretion (Figs. 2b and 3a). Pollen grains are observed immerse in the secretion between stigmatic papillae and sub-stigmatic cells (Fig. 3b).
Bright field microscope. a Longitudinal section of the stigma with broken cuticle (arrow). b Detail of the stigma, showing the cuticle (arrow), and a pollen grain (pg) immersed in the secretion (s). c Transverse section of the solid style. d Detail of the transmitting tissue (tt) and the furrow (arrow). Scale bars: a, c 125 μm; b, d 50 μm
The style is heart shaped in transverse section (Fig. 3c). It is solid and consists of an epidermis, a parenchyma, and a horseshoe-shaped transmitting tissue. The parenchyma occupies most of the style while the transmitting tissue is placed underneath the furrow (Fig. 3d).
Virgin flowers (bagged)
Stigmatic papillae present a thick cuticle and thick primary walls with fibrillar aspect. The cuticle consists of an amorphous outer region and a reticulate inner region. Cuticle projections between anticlinal walls of adjacent epidermal cells form cuticular pegs (Fig. 4a). Cavities of different sizes, some with low electrondense content, can be distinguished in the cell wall, between the cell wall and the cuticle, and in the cuticle (Fig. 4a). Similar low electrondense content is observed between the plasmalemma and the cell wall (Fig. 4a, b). A large central vacuole occupies most of the cell protoplast (Fig. 4a), and in the periphery of the cytoplasm many mitochondria, rough endoplasmic reticulum, a few dictyosomes, and many large plastids are present (Fig. 4a, b). These plastids contain starch and lipids observed as small high electrondense globules (Fig. 4b).
Transmission electron microscopy. Flowers not exposed to visitors. a Stigmatic papillae cytoplasm, cell wall with cavities (white arrows) and cuticle. b Detail of the cytoplasm of a stigmatic papilla, cavities in the cell wall (white arrows). c Longitudinal section of sub-stigmatic cells. d–f Detail of the cytoplasm of sub-stigmatic cells. d’ detail of a plastid showing grana (white arrow). c, cuticle; a, amorphous region; r, reticulate region; cp, cuticular peg; cw, cell wall; p, plastids; d, dictyosome; m, mitochondria; rer, rough endoplasmic reticulum; er, endoplasmic reticulum; v, vesicle; lg, lipid globule; n, nucleus; arrow heads, cavities; black arrows, plasmodesmata. Scale bars: a 2 μm, b–f 1 μm, d’ 0.5 μm
The wall of sub-stigmatic cells is thickened at the corners and small cavities are observed in them (Fig. 4c). Plasmodesmata connecting these cells are observed in tangential walls in a longitudinal section (Fig. 4c). Sub-stigmatic cells present a dense cytoplasm with a conspicuous nucleus, few lipid globules, rough endoplasmic reticulum, and several mitochondria (Fig. 4c, d). Many large plastids which contain starch grains and inner membranes that resemble grana are observed (Fig. 4c, d’). There are also abundant dictyosomes with numerous associated vesicles (Fig. 4d–f). Content with low electron density is observed between the plasmalemma and the cell wall (Fig. 4f).
Open pollinated flowers (not bagged)
The outer tangential wall of the stigmatic papillae in transverse section is thickened and covered by a conspicuous amorphous and reticulate cuticle (Fig. 5a). In these flowers, cavities of different sizes were also observed in the cuticle, between the cell wall and the cuticle, and in the cell wall (Fig. 5a, b). The cytoplasm of these cells is characterized by the presence of large vacuoles with electron-dense contents (Fig. 5a), dictyosomes, mitochondria, lipid globules, rough endoplasmic reticulum, and several plastids (Fig. 5c). These plastids are large and contain starch and lipids (Fig. 5c).
Transmission electron microscopy. Flowers exposed to visitors. a Stigmatic papillae cytoplasm, cell wall with cavities (white arrows) and cuticle. b, c Detail of the cytoplasm of a stigmatic papilla. d Transverse section of sub-stigmatic cells. e Transmitting tissue cells. c, cuticle; a, amorphous region; r, reticulate region; cp, cuticular peg; cw, cell wall; p, plastids; d, dictyosome; m, mitochondria; rer, rough endoplasmic reticulum; v, vesicle; lg, lipid globule; l, lipid; ml, middle lamella; S, secretion; asterisk, electrondense contents in vacuoles. Scale bars: a, c–e 2 μm, b 1 μm
A transverse section of the sub-stigmatic region shows isodiametric cells. Large intercellular spaces are filled with secretion (Fig. 5d). The primary wall is thinner than in stigmatic papillae but light wall ingrowths are also present (Fig. 5d). Distended middle lamella is observed in the corners of the transmitting tissue cells (Fig. 5e). Large plastids, some with starch, occupy most of the cytoplasm of these cells. Mitochondria and dictyosomes with associated vesicles are also observed (Fig. 5e). Content with low electron density is observed between the plasmalemma and the cell wall (Fig. 5e).
Discussion
The arrangement of the stigmas in a triangle as we observed in C. psilophyllum seems to be the most common condition in Malpighiaceae; among 12 species studied by Sigrist and Sazima (2004), 9 show this characteristic. In C. psilophyllum, the stamens accompanies this disposition; the three posterior stamens (out of ten) are shorter and with smaller anthers (Johnson 1986), coinciding with the position of claw of the posterior petal. Although no analysis of pollen viability was carried out here, we observed that these posterior anthers produce pollen grains.
The species of Centris observed in flowers of Callaeum psilophyllum are oil-collecting bees, and the behavior to remove the reward favors the contact of the ventral area of the bee with the stigmas, probably producing the rupture of the cuticle and the exposition of the stigmatic surface. The comparison between the ventral area of the bees and the triangle formed by the three stigmas shows that this coupling is possible because the values are very similar. This indicates that these Centris species are probably efficient pollinators of C. psilophyllum in the Martín García Island. However, large species of Centris (C. flavifrons and C. fuscata) can simultaneously contact the three stigmas in each visit, while in the medium-size bees (C. tarsata and C. trigonoides), the ventral area is lightly lesser than the stigmatic triangle. On the other hand, the genus Paratetrapedia includes some species that collect oil and pollen of Malpigihaceae. When these bees collect oil, they crawl outwardly around the flower and do not contact the reproductive sexual organs, but while they collect pollen, they can break the stigmatic cuticle and deposit small loads of pollen (Sigrist and Sazima 2004). Species of Paratetrapedia were suggested as potentially legitimate pollinators of small flowered genera of Malpighiaceae (Steiner 1985; Vogel 1990), but studied population of Callaeum psilophyllum presents flowers of 10–14 mm (Torretta, personal observation); therefore, P. nigrispinis would be rejected as a potential pollinator for this species of Malpighiaceae.
The presence of cuticle in the three stigmas of C. psilophyllum covering the receptive areas, probably avoids the possibility of spontaneously self-pollination. Moreover, the intact cuticle and the absence of pollen grains over stigmas observed in the bagged flowers, reinforce the idea that this species is dependent on pollinator.
The cuticles of the stigmatic receptive parts are adapted to the role played by the stigma in the interaction with pollen (Heslop-Harrison and Heslop-Harrison 1980; Li-Beisson et al. 2009; Zinkl et al. 1999; Javelle et al. 2011; Jessen et al. 2011; Borisjuk et al. 2014). There is a considerable variation in the structure of wet stigma cuticles (Heslop-Harrison and Heslop-Harrison 1982). In general, the dominant feature of plant cuticular membrane is an amorphous matrix that may present other components such as lamellae and fibrillae. Therefore, the cuticular matrix may be amorphous, lamellate, reticulate, or combined. Based on this, Holloway (1982) described six types of cuticular membranes. The stigmatic cuticle of Callaeum psilophyllum corresponds to type 3: outer region amorphous, inner region mainly reticulate. It is believed that the reticulate appearance occurs when polysaccharides mix with the cutin (Fich et al. 2016). This thick and stratified cuticle would require a more specific mechanism for its rupture, such as that exercised by the oil-collecting bees, which agrees with our observations for this species.
It is well known that dictyosomes are involved in the production of polysaccharides (Lüttge and Schnepf 1976; Meyberg 1988; Young et al. 2008; Paiva 2009; Mercadante-Simoes and Paiva 2013). Stigmatic and sub-stigmatic cells of C. psilophyllum present great amount of dictyosomes with associated vesicles in the periphery of the cytoplasm and fused with the plasma membrane. Starch is commonly present in the parenchyma surrounding the transmitting tissue and is degraded during pollen tube growth (Rosenfeldt and Galati 2000, 2009; Gotelli et al. 2012, 2017a). Starch is a source of energy for intensive metabolic cellular processes (Pacek and Stpiczynska 2007; Aliscioni et al. 2009). In C. psilophyllum, many large plastids with starch grains are observed in all tissues described, and plastids with grana are found in sub-stigmatic cells. Considering the stigma is green, these are probably chloroplasts. According to Fahn (1988), plastids with thylakoids and smooth endoplasmic reticulum are associated to the secretion of lipidic substances. Lipidic components seem to be essential for pollen tube penetration in the stigma and ulterior growth through the style (Lush et al. 1998; Wolters-Arts et al. 1998) while carbohydrates are a source of nutrients indispensable for pollen tube growth (Herrero and Dickinson 1979) or for the development of the ovary and ovules (Arbeloa and Herrero 1991).
Raghavan (1997) claims metabolically active cells have abundant ribosomes, mitochondria, endoplasmic reticulum, dictyosomes, and amyloplasts. A dense cytoplasm with those organelles and plasmodesmata are features of secretory cells (Gotelli et al. 2017b). These characteristics are present in stigmatic, sub-stigmatic, and transmittion tissue cells of C. psilophyllum. In this species, secretion is represented by the substances in the thickened cell wall and cuticle of stigmatic papilla, in the intercellular spaces of sub-stigmatic cells in flowers that were exposed to visitors, and in the distended middle lamella of the transmitting tissue. The ultrastructure of the transmitting tissue cells reported here is similar to the structure of other species of angiosperms in general (Johri and Rao 1984; Raghavan 1997; Pandey 1997).
The presence of wall ingrowths in cells with high metabolic activity, as the ones we describe for Callaeum psilophyllum, is a common trait of transfer cells (Gunning and Pate 1969; Pate and Gunning 1972). These localized wall expansions were also found in the stylar epithelial cells of species of diverse genera as Ornithogalum (Tilton and Horner 1980), Citrus (Ciampolini et al. 1981), and Discaria (Gotelli et al. 2012), and in the transmitting tissue cells of the style of Petunia (Herrero and Dickinson 1979), Oxalis (Rosenfeldt and Galati 2009), Colletia, Hovenia, Ziziphus, and Paliurus (Gotelli et al. 2017a). In two species of Oxalis (Rosenfeldt and Galati 2009) and in some species of Rhamnaceae (Gotelli et al. 2012, 2017a), the wall ingrowths have lower electron density than the primary wall as described in this work. According to Rosenfeldt and Galati (2009), these wall ingrowths could correspond to a secondary wall.
There are some hypotheses that explain how secretory products move from the cytoplasm towards the cell wall and out of it. Fahn (1979) described two modes of secretion: eccrine and granuloccrine. In the granulocrine secretion membrane-bound vesicles are involved while in the eccrine secretion, a molecular or ionic process is involved and substances are transported directly through the plasma membrane. Therefore, cells rich in endoplasmic reticulum, dictyosomes, and vesicles are often associated with granulocrine secretion (Fahn 1979, 1988, 2000; Durkee 1983; Arumugasamy et al. 1990). On the other hand, dictyosomes and endoplasmic reticulum are rare in eccrine secretion (Elias et al. 1975; Eriksson 1977; Nepi et al. 1996; Razem and Davis 1999; Stpiczynska 2003). According to the ultrastructure of the stigmatic, sub-stigmatic, and transmitting tissue cells of Callaeum psilophyllum, the secretion seems to be granulocrine.
These modes, however, do not describe the way accumulated products move through the cell wall and out of it. The cavities in the cell wall, as observed in stigmatic papillae of C. psilophyllum, may facilitate the transport of hydrophobic components across the hydrophilic cell wall (Kunst and Samuels 2003). It is an unusual character which was described for elaiophores of some members of Oncidiinae (Pacek and Stpiczynska 2007; Stpiczynska and Davies 2008; Aliscioni et al. 2009) and for glandular trichomes of Grindelia pulchella during and after the secretion process (Bartoli et al. 2011). Recently, Paiva (2016) proposed a more general and elaborated model of the cell cycle in which changes in the volume of the protoplast are responsible for releasing the products of secretory activity contained in the periplasmic space. He explains that the turgor pressure exerted by the expanding protoplast provides the force to the products accumulated needs to cross the cell wall. Repeated cycles of contraction and expansion are needed for this. In the initial state, vesicles derived from dictyosomes and the endoplasmic reticulum merge into provacuoles and then into larger vacuoles, within which the secretory product is temporarily accumulated. These vesicles can transport secretory products to the plasma membrane, fusing with it and releasing the products by a granulocrine process, as observed in C. psilophyllum. This accumulation of substances in the periplasmic space continually increases the pressure on the cytoplasm. As the process of secretion continues and more products accumulate in the protoplast, the pressure changes direction causing the accumulated substances in the periplasmic space to be pressed against the cell wall and forcing them to cross into intercellular spaces. The accumulation of secretion products inside subcuticular space can promote a pressure that permits secretion products to cross a cuticular barrier, in most cases by cuticle rupture, without a requirement of energy, even against a concentration gradient (Paiva 2009; Possobom et al. 2015). In Malpighiaceae, the cuticle ruptures by mechanical means, mostly by pollinator action, and the secretion accumulated under it is then released (Sigrist and Sazima 2004). In Callaeum psilophyllum, we observed three main ultrastructural differences between flowers exposed to visitors and flowers that were bagged: the rupture of the cuticle, more secretion in the intercellular space between sub-stigamtic cells and electron-dense components inside vacuoles in stigmatic papillae. It seems that the stigmas prepare in similar ways to receive pollen grains, the pollinator action is required to break the cuticle, and once pollen tubes start growing, stigmatic and sub-stigmatic cells release more secretion, probably following the cell cycle proposed by Paiva (2016).
The arrival of pollen to stigma is the beginning of the process of sexual reproduction of angiosperms. However, this does not ensure that the process is successful. In Callaeum psillophyllum, additional studies about the reproductive system (self-compatibility vs. self-incompatibility), pollinic viability, etc. are necessary to understand reproductive biology of this species.
Our results confirmed that the foraging activity of pollinators is necessary for the breakdown of the cuticle and that pollen grains to reach the stigmatic receptive surface. These data, together with those of Sigrist and Sazima (2004), for other species of Malpighiaceae, suggest that this mechanism would be generalized for the family.
References
Aliscioni SS, Torretta JP (2017) Malpighiaceae. In: Zuloaga FO, Belgrano MJ (eds) Flora Vascular de la República Argentina, Vol. 17. Buenos Aires, Estudio Sigma SRL, pp 163–205
Aliscioni SS, Torretta JP, Bello ME, Galati BG (2009) Elaiophores in Gomesa bifolia (Sims) M.W. Chase & N.H. Williams (Oncidiinae: Cymbidieae: Orchidaceae): structure and oil secretion. Ann Bot 104:1141–1149
Anderson WR (1979) Floral conservatism in neotropical Malpighiaceae. Biotropica 11:219–223
Arbeloa A, Herrero M (1991) Development of the ovular structures in peach [Prunus persica (L.) Batsch]. New Phytol 118:527–534
Arumugasamy K, Subramanian RB, Inamdar JA (1990) Cyathial nectaries of Euphorbia neriifolia L.: ultrastructure and secretion. Phytomorphology 40:281–288
Bartoli A, Galati B, Tortosa R (2011) Anatomical studies of the secretory structures: glandular trichomes and ducts, in Grindelia pulchella Dunal (Astereae, Asteraceae). Flora 206:1063–1068
Borisjuk N, Hrmova M, Lopato S (2014) Transcriptional regulation of cuticle biosynthesis. Biotechnol Adv 32:526–540
Cane JH (1987) Estimation of bee size using intertegular span (Apoidea). J Kansas Entomol Soc 60:145–147
Ciampolini F, Cresti M, Sarfatti G, Tiezzi A (1981) Ultrastructure of the stylar canal cells of Citrus limon (Rutaceae). Plant Syst Evol 138:263–274
Durkee LT (1983) The ultrastructure of floral and extrafloral nectaries. In: Bentley BL, Elias T (eds) The biology of nectarines. Columbia University Press, New York, pp 1–29
Edlund AF, Swanson R, Preuss D (2004) Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16:S84–S97. https://doi.org/10.1105/tpc.015800
Elias TE, Rozich WR, Newcombe L (1975) The foliar and floral nectaries of Turnera ulmifolia L. Am J Bot 62:570–576
Eriksson M (1977) The ultrastructure of the nectary of red clover (Trifolium pratense). J Apic Res 16:184–193
Fahn A (1979) Secretory tissues in plants. Academic Press, New York
Fahn A (1988) Secretory tissues in vascular plants. New Phytol 108:229–257
Fahn A (2000) Structure and function of secretory cells. Adv Bot Res 31:37–75
Fich EA, Segerson NA, Rose JKC (2016) The plant polyester cutin: biosynthesis, structure, and biological roles. Annu Rev Plant Biol 67:207–233
Gotelli MM, Galati BG, Medan D (2012) Structure of the stigma and style in Colletia and Discaria (Rhamnaceae: Colletieae). Plant Syst Evol 298:1635–1641
Gotelli MM, Galati BG, Zarlavsky G, Medan D (2017a) Structure of the style and pollen tube pathway in the Ziziphoid and Rhamnoid clades of Rhamnaceae. Protoplasma 255:501–515. https://doi.org/10.1007/s00709-017-1167-z
Gotelli M, Lattar E, Zini M, Galati B (2017b) Style morphology and pollen tube pathway. Plant Reproduction 30:155–170. https://doi.org/10.1007/s00497-017-0312-3
Guimarães ALA, Cruz SMS, Vieira ACM (2014) Structure of floral galls of Byrsonima sericea (Malpighiaceae) induced by Bruggmanniella byrsonimae (Cecidomyiidae, Diptera) and their effects on host plants. Plant Biol 16:467–475. https://doi.org/10.1111/plb.12060
Gunning BES, Pate JS (1969) Transfer cells: plant cells with wall ingrowths, specialized in relation to short distance transport of solutes, their occurrence, structure and development. Protoplasma 68:107–133
Herrero M, Dickinson HG (1979) Pollen–pistil incompatibility in Petunia hybrid, changes in the pistil following compatible and incompatible intraspecific crosses. J Cell Sci 36:1–18
Heslop-Harrison J, Heslop-Harrison Y (1980) The pollen-stigma interaction in the grasses. 1. Fine-structure and cytochemistry of the stigmas of Hordeum and Secale. Acta BotNeerl 29:261–276
Heslop-Harrison J, Heslop-Harrison Y (1982) The specialized cuticles of the receptive surfaces of angiosperm stigmas. In: Cutler DF, Alvin KL, Price CE (eds) The plant cuticle. Academic Press, London, pp 99–120
Holloway PJ (1982) Structure and histochemistry of plant cuticlar membranes: an overview. In: Cutler DF, Alvin KL, Price CE (eds) The plant cuticle. Academic Press, London, pp 1–32
Javelle M, Vernoud V, Rogowsky PM, Ingram GC (2011) Epidermis: the formation and functions of a fundamental plant tissue. New Phytol 189:17–39
Jessen D, Olbrich A, Knufer J, Kruger A, Hoppert M, Polle A et al (2011) Combined activity of LACS1 and LACS4 is required for proper pollen coat formation in Arabidopsis. Plant J 68:715–726
Johnson DM (1986) Revision of the neotropical genus Callaeum (Malpighiaceae). Syst Bot 11:335–353
Johri BM, Rao PS (1984) Experimental embryology. In Embryology of angiosperms. Springer, Berlin
Knapp S (2010) On ‘various contrivances’: pollination, phylogeny and flower form in the Solanaceae. Philos Trans R Soc Lond Ser B Biol Sci 365:449–460
Kunst L, Samuels AL (2003) Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 42:51–80
Li-Beisson Y, Pollard M, Sauveplane V, Pinot F, Ohlrogge J, Beisson F (2009) Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc Natl Acad Sci U S A 106:22008–22013
Lush WM, Grieser F, Wolters-Arts M (1998) Directional guidance of Nicotiana alata pollen tubes in vitro and on the stigma. Plant Physiol 118:733–741
Lüttge U, Schnepf E (1976) Elimination processes by glands. Organic substances. In: Lüttge U, Pitman MG (eds) Transport in plants II, encyclopedia of plant physiology, new series, vol 2B. Springer, New York, pp 244–277
Mercadante-Simoes MO, Paiva EAS (2013) Leaf colleters in Tontelea micrantha (Celastraceae, Salacioideae): ecological, morphological and structural aspects. C R Biol 336:400–406
Meyberg M (1988) Cytochemistry and ultrastructure of the mucilage secreting trichomes of Nymphoides peltata (Menyanthaceae). Ann Bot 62:537–547
Michener CD (2007) The bees of the world, 2nd edn. Johns Hopkins, Baltimore
Nepi M, Ciampolini F, Pacini E (1996) Development and ultrastructure of Cucurbita pepo nectaries of male flowers. Ann Bot 81:251–262
Pacek A, Stpiczynska M (2007) The structure of elaiophores in Oncidium cheirophorum Rchb. f. And Ornithocephalus kruegeri Rchb. f. (Orchidaceae). Acta Agrobot 60:9–14
Paiva EAS (2009) Occurrence, structure and functional aspects of the colleters of Copaifera langsdorffii Desf. (Fabaceae, Caesalpinioideae). C R Biol 332:1078–1084
Paiva EAS (2016) How do secretory products cross the plant cell wall to be released? A new hypothesis involving cyclic mechanical actions of the protoplast. Ann Bot 117:533–540. https://doi.org/10.1093/aob/mcw012
Pandey AK (1997) Introduction to the embryology of angiosperms. CBS Publishers and Distributors, Daryaganj
Pate JS, Gunning BES (1972) Transfer cells. Annu Rev Plant Physiol 23:173–196
Possobom CCF, Machado SR (2017) Elaiophores in three Neotropical Malpighiaceae species: a comparative study. Plant Syst Evol 303:1–18
Possobom CCF, Guimaraes E, Machado SR (2015) Structure and secretion mechanisms of floral glands in Diplopterys pubipetala (Malpighiaceae), a neotropical species. Flora 211:26–39
Raghavan V (1997) Molecular embryology of flowering plants. University Press, Cambridge, pp 33–35
Razem FA, Davis AR (1999) Anatomical and ultrastructural changes of the floral nectary of Pisum sativum L. during flower development. Protoplasma 206:57–72
Roig Alsina A (2000) Claves para las especies argentinas de Centris (Hymenoptera, Apidae), con descripción de nuevas especies y notas sobre distribución. Rev Mus Argentino Cienc Nat ns 2:171–193
Rosenfeldt S, Galati BG (2000) Stigma and style morphology in Ceiba insignis (Bombacaceae). Phytomorphology 50:69–74
Rosenfeldt S, Galati BG (2009) The structure of the stigma and the style of Oxalis spp. (Oxalidaceae). J Torrey Bot Soc 136:33–45
Sigrist MR, Sazima M (2004) Pollination and reproductive biology of twelve species of neotropical Malpighiaceae: stigma morphology and its implications for the breeding system. Ann Bot 94:33–41. https://doi.org/10.1093/aob/mch108
Souto LS, Oliveira DMT (2013) Evaluation of the floral vasculature of the Janusia, Mascagnia and Tetrapterys species as a tool to explain the decrease of floral organs in Malpighiaceae. Flora 208:351–359. https://doi.org/10.1016/j.flora.2013.05.002
Steiner KE (1985) Functional dioecism in the Malpighiaceae: the breeding system of Spachea membranacea Cuatr. Am J Bot 72:1537–1543
Stpiczynska M (2003) Nectar resorption in the spur of Platanthera chlorantha custer (Rchb.) Orchidaceae- structural and microautoradiographic study. Plant Syst Evol 238:119–126
Stpiczynska M, Davies K (2008) Elaiophore structure and oil secretion in flowers of Oncidium trulliferum Lindl. And Ornithophora radicans (Rchb.f.) Garay & Pabst (Oncidiinae: Orchidaceae). Ann Bot 101:375–384
Tilton VR, Horner HT Jr (1980) Stigma, style, and obturator of Ornithogalum caudatum (Liliaceae) and their function in reproductive process. Am J Bot 67:1113–1131
Torretta JP, Roig Alsina A (2017) Las abejas colectoras de aceite del género Paratetrapedia Moure (Hymenoptera, Apidae, Tapinotaspidini) en la Argentina. Rev Mus Argentino Cienc Nat ns 19:131–140
Vinson SB, Frankie GW, Williams HJ (1996) Chemical ecology of bees of the genus Centris (Hymenoptera: Apidae). Fla Entomol 79:109–129
Vogel S (1974) Ölblumen und Glsammelnde Bienen. Tropische und subtropische Pflanzenwelt, Nr. 7. F. Steiner, Wiesbaden 267 pp
Vogel S (1990) History of the Malpighiaceae in the light of pollination ecology. Mem New York Bot Gard 55:130–142
Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen-tube growth. Nature 392:818–821
Young RE, McFarlane HE, Hahn MG, Western TL, Haughn GW, Samuels AL (2008) Analysis of the Golgi apparatus in Arabidopsis seed coat cells during polarized secretion of pectin-rich mucilage. Plant Cell 20:1623–1638
Zarlavsky GE (2014) Histología Vegetal: técnicas simples y complejas. Sociedad Argentina de Botánica, Buenos Aires
Zinkl GM, Zwiebel B, Grier DG, Preuss D (1999) Pollen-stigma adhesion in Arabidopsis: a species-specific interaction mediated by hydrophobic molecules in the pollen exine. Development 126:5431–5440
Acknowledgements
We thank to G. Zarvlasky for technical assistance and the Dirección de Áreas Naturales Protegidas, Organismo Provincial para el Desarrollo Sostenible, of the Provincia de Buenos Aires (Multiple-uses Natural Reserve Martín García) for permissions to conduct this study.
Funding
This work was funded by a research grant from Agencia Nacional de Promoción Científica y Tecnológica, grant number PICT 2013-1867 to S. Aliscioni, Consejo Nacional de Investigaciones Científicas y Técnicas, grant number PIP 11220110100312, and Universidad de Buenos Aires, grant number UBACyT 20020130200203BA to J. P. Torretta. Sandra Aliscioni, Marina Gotelli, and Juan Pablo Torretta are affiliated with Consejo Nacional de Investigaciones Científicas y Técnicas, and Universidad de Buenos Aires, Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Aliscioni, S.S., Gotelli, M. & Torretta, J.P. Structure of the stigma and style of Callaeum psilophyllum (Malpighiaceae) and its relation with potential pollinators. Protoplasma 255, 1433–1442 (2018). https://doi.org/10.1007/s00709-018-1245-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-018-1245-x