Abstract
The application of Se to plants growing under Cd contamination may become an alternative strategy to minimize Cd damage. However, there is no specific information available regarding whether Se can affect the anatomical structure and photosynthetic rates of plants under Cd stress. To address questions related to Se-protective responses under Cd stress, we evaluated the structural and ultrastructural aspects, photosynthetic rates and growth of tomato cv. Micro-Tom plants. Plants were exposed to 0.5 mM CdCl2 and further supplemented with 1.0 μM of selenite or selenate. The overall results revealed different trends according to the Se source and Cd application. Both Se sources improved growth, photosynthesis, leaf characteristics and middle lamella thickness between mesophyll cells. In contrast, Cd caused decreases in photosynthesis and growth and damage to the ultrastructure of the chloroplast. The number of mitochondria, peroxisomes, starch grains and plastogloboli and the disorganization of the thylakoids and the middle lamella in plants increased in the presence of Cd or Cd + Se. Se plays an important role in plant cultivation under normal conditions. This finding was corroborated by the identification of specific structural changes in Se-treated plants, which could benefit plant development. However, a reversal of Cd stress effects was not observed in the presence of Se.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium is considered to be a nonessential nutrient to plants because it is not fundamental for plant structure or metabolism. However, its foliar or soil solution application at low concentrations provides numerous benefits to plants. Recent studies indicate that Se application to plants improves plant growth (Ashraf et al. 2018; Yin et al. 2019) and photosynthetic attributes (Haghighi et al. 2016) and decreases Cd uptake in tomato plants (Abd Allah et al. 2016) and Cd translocation from the root to shoot in rice (Wan et al. 2016). Moreover, Se seems to play an important role in plant tolerance during biotic and abiotic stress (Hawrylak-Nowak et al. 2018). This is especially the case when plants are exposed to heavy metals, and Se has been shown to alleviate the negative effects of Cd-induced stress. For instance, Se application increased stress-responsive protein production (Sun et al. 2016), regulated ROS scavenger metabolism (Castillo-Godina et al. 2016) and improved flowering (Shekari et al. 2019) and antioxidant defense systems (Alyemeni et al. 2018).
Cadmium (Cd) is one of the most toxic heavy metals to living organisms. Due to its increasing and widespread use in industry, Cd is becoming hazardous on a worldwide scale (Alves et al. 2016; Edelstein and Ben-Hur 2018). Plants can easily absorb this heavy metal, which leads to uncontrolled oxidation, disrupting cell balance and causing electrolyte leakage (Jarvis et al. 1976; Gratão et al. 2015). Even at low concentrations, Cd can increase the content of reactive oxygen species (ROS), which affects cell and tissue structure (Alves et al. 2017; Shanying et al. 2017; Zhu et al. 2018). Moreover, Cd causes structural damage to root cells, leading to a smaller nucleus and a ruptured nuclear envelope (Ali et al. 2014). Cd-induced damage to aerial plant parts includes a decrease in the size of intercellular air spaces and modifications to chloroplast structure (Djebali et al. 2005). Yue et al. (2018) demonstrated disorders in the lamellar structure of the chloroplast grana and the thylakoid membranes, with an increase in the size of the interlamellar spaces. In addition, Cd significantly reduced the quantity and size of starch grains in the chloroplasts of wheat, inhibiting starch accumulation (Yue et al. 2018). These results suggest that Cd can drastically affect the photosynthetic capacity of plants, thus affecting plant survival and productivity. Additionally, Gratão et al. (2009) showed that tomato cv. Micro-Tom, under Cd stress, exhibited stomatal closure, a reduced root diameter and leaf epidermis disintegration, indicating the impact of Cd on gas exchange and nutrient uptake capacity.
The miniature tomato cultivar Micro-Tom (MT) exhibits a short life cycle and limited growth, making this as a model plant for agronomical studies (Meissner et al. 1997), especially regarding experimental approaches to understand the isolated and combined effects of abiotic stress on plant survival and productivity. Although several studies have indicated the beneficial effects of Se in plants to minimize Cd stress effects, there is no specific information available regarding how Se can act in protecting the ultrastructural aspects of chloroplast membranes, conserving photosynthetic capacity and plant growth. Thus, to better understand the role of Se in structural changes and photosynthetic attributes during Cd stress, we analyzed the structural aspects of chloroplasts in MT tomato plants, plant attributes related to root and leaf biomass and photosynthetic rates. We expected to find plants under Cd stress and without Se application to show damage to their chloroplast structure, leading to a reduction in photosynthesis, which would decrease the biomass production of the whole plant.
Material and methods
Plant material and growth conditions
Seeds of tomato (Solanum lycopersicum L.) cultivar Micro-Tom (MT) were sterilized with sodium hypochlorite solution (5%), rinsed three times and sown in boxes filled with a mixture of commercial pot mix (Plantmax HT Eucatex, Brazil) and vermiculite (1:1 by volume) supplemented with 1 g L −1 10:10:10 nitrogen-phosphorus-potassium and 4 g L −1 lime (MgCO3 + CaCO3). When two true leaves were completely expanded, each seedling was transplanted to one Leonard pot (0.35 L) (Vincent 1975), a hydroponic cultivation system with sterilized sand and polystyrene (4:3), and a Hoagland’s nutrient solution (250 ml). Thirty-day-old plants were grown in a greenhouse with the same nutritive solution supplemented with either Se form (0 or 1 μM of Na2SeO3—selenite or Na2SeO4—selenate) combined with Cd (0 or 0.5 mM CdCl2) (Alves et al. 2019). The nutritive solution was replaced every 5 days. All of these procedures were performed in 2018 using installations from the Plant Physiology Laboratory and a greenhouse of the Department of Applied Biology from the Faculty of Agrarian and Veterinary Sciences, Jaboticabal, São Paulo, Brazil.
Photosynthetic rates
Sixty days after germination, gas exchange parameters under saturating light were assessed at the beginning of the rainy season (October 2018). The measurements were taken using a portable system to measure CO2 and H2O exchange (LcPro-SD, ADC BioScientific Ltd., Hoddesdon, UK). The gas exchange measurements were made under a constant light intensity of 1200 μmol m−2 s−1 emitted by a blue–red LED light source. The measurements were made using the terminal leaflet of the fully expanded leaves from nodes 4 to 5. The leaf temperature was maintained at 29.00 ± 0.46 °C. Measurements were performed under ambient concentrations of CO2 (between 413 and 427 ppm). The maximum area-based rate of light-saturated CO2 assimilation (A), leaf transpiration (E) and stomatal conductance (gs) were measured after a stabilization period (3–5 min). Data collection was performed during the morning, between 08:00 and 10:00.
Sample preparation for light microscopy (LM) and transmission electron microscopy (TEM)
For histological characterization, samples of leaves were processed for light (LM) and transmission electron microscopy (TEM). Small pieces of leaf blade were collected immediately after the photosynthetic measurements were taken and fixed in a modified Karnovsky solution (2% glutaraldehyde, 2% paraformaldehyde and 5 mM CaCl2 in 0.05 M sodium cacodylate buffer, pH 7.2) (Karnovsky 1965) for 48 h. The samples were then rinsed in cacodylate buffer (0.1 M) and post fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer, pH 7.2, at room temperature for 1 h. The samples were dehydrated in a graded acetone series and embedded in Spurr epoxy resin (EMS, Electron Microscopy Sciences, Hatfield, PA, USA) for 48 h. Semithin sections (120–200 nm) were placed onto glass slides, stained with toluidine blue (2% in water) for 5 min, rinsed in distilled water and air dried.
The sections were permanently mounted in Entellan®, observed and documented using an upright light microscope (Axioskop 40 Carl Zeiss, Jena, Germany).
Ultrathin sections of leaves (60–90 nm) were obtained with an ultramicrotome (Porter Blum MT2- DuPont-Sorvall) equipped with a diamond knife, collected on copper grids (300 mesh) and stained with uranyl acetate (2.5%) followed by lead citrate (0.1%) (Reynolds 1993). Sections were observed at 80 kV under a transmission electron microscope (JEM1400 JEOL, Tokyo, Japan), and the images were digitalized.
Root samples were fixed as described above and dehydrated in an ethanol series (35 to 100%) at 30-min intervals. The infiltration was performed at 4 °C using ethanol:infiltration medium (glycol methacrylate, Historesin kit, Leica, Heidelberg, Germany) (3:1, 1:1 and 1:2, minimum of 2 h each step), followed by infiltration in 100% infiltration medium for 48 h. Polymerization was performed at room temperature for 48 h. Serial histological sections (5 mm thick) were obtained with a rotary microtome (Leica RM 2155, Nussloch, Germany), stained with 1% acid fuchsin and 0.05% (w/v) toluidine blue in water, mounted in Entellan® synthetic resin (Merck, Darmstadt, Germany), covered with coverslips and observed under an optical microscope (Axioskop 40 Carl Zeiss, Jena, Germany).
The effects of Se and Cd on the leaf blade thickness (μm) and root diameter were determined with three samples of three different roots and leaves. The tissues were measured using the ImageJ 1.46r program (Rasband 2012).
Biomass
After a period of 60 days post germination, samples of roots and leaves were collected to determine the total biomass produced. Samples were rinsed with distilled water and dried at 60 °C until a constant weight was reached, and the biomass produced was determined with an analytical scale.
Statistical analysis
The experimental design was completely randomized, with three replicates, two Se forms and the presence or absence of Cd. The results from the three independent replicates of root and leaf dry mass, maximum area-based rate of light-saturated CO2 assimilation (A), leaf transpiration (E) and stomatal conductance (gs), leaf mesophyll thickness and root diameter were expressed as the mean and standard error of the mean (±SEM). Multiple comparisons of means with Duncan’s test followed the application of an individual ANOVA for each characteristic at a 0.05 level of significance. The statistical analyses were performed using AgroEstat® software (Barbosa and Maldonado Júnior 2009).
Results and discussion
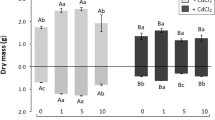
Although several studies have indicated the beneficial effects of Se in plants, there is no specific information available regarding how Se can protect the ultrastructural aspects and conserve photosynthetic capacity and plant growth. In our study, the application of either selenite or selenate forms under normal conditions at 1 μM improved root and shoot dry mass in comparison with those of the control plants (Table 1). However, Cd decreased plant growth. Although Se application has been shown to restore plant growth under Cd stress (Alyemeni et al. 2018), in our study, there were no differences in the root or shoot dry mass of plants treated with Cd and Se (Table 1). Similar results were reported for Brassica chinensis (Yu et al. 2019). Therefore, Se is not always effective at preventing losses in plant growth under Cd stress. This could be due to the impairment of photosynthetic attributes as a result of Cd stress that are not restored by Se application.
When photosynthetic attributes were analyzed, leaf transpiration (E), stomatal conductance (gs) and maximum area-based rate of light-saturated CO2 assimilation (A) increased with Se application in comparison with the values observed in the control plants (Table 1). Our data are in accordance with those found by Djanaguiraman et al. (2010), who reported that Se application increased photosynthetic attributes in sorghum because Se tends to minimize damage to the chloroplasts, enhancing the chlorophyll content. These positive responses may be related to the fact that Se can reduce ROS production, leading to the capacity to restore the structure of damaged chloroplasts and stimulate the production of other vital metabolites (Feng et al. 2013a).
Plants exposed to Cd exhibited a decrease in photosynthetic rate (Table 1). This response can be explained by an imbalance in cell homeostasis caused by Cd, which can destroy chloroplasts, leading to a decreased electron transport rate (Gratão et al. 2015). The use of either form of Se during Cd stress was not effective in preventing the damage caused by Cd to the photosynthetic attributes. Thus, Se application may not be effective in preventing the reduction in E, gs and A in MT plants grown under Cd stress as a result of damage caused by Cd in chloroplasts.
Histological analyses revealed that both Se forms led to an increase in the size of the intercellular spaces in the mesophyll (Fig. 1c, e), resulting in thicker leaves (Fig. 2) and increased dry mass (Table 1) when compared with those in the control plants (Fig. 1a). The increase in thickness observed in plants treated with Se might be related to the increased photosynthetic and transpiration rates also observed (Table 1). Otherwise, Cd stress caused a serious reduction in leaf mesophyll thickness (Figs. 1b and 2). When Se was applied to plants under Cd stress, we observed a decrease in leaf thickness when compared with those under only Cd application (Figs. 1d–f and 2). A study conducted by Feng et al. (2013b) demonstrated that Se application induced Cd uptake and reduced growth in rice plants exposed to high Cd concentrations (89–178 μM). In accordance with this information, in our study, neither Se form was effective in reversing the negative effects caused by exposure to 0.5 mM Cd on plant growth and development.
Tomato leaves from plants of cultivar Micro-Tom grown under CdCl2 stress with selenate and selenite application, observed in cross-section by light microscopy. a control; b–f supplemented with 0.5 mM CdCl2 (b); 1 μM selenate (c); 0.5 mM CdCl2 + 1 μM of selenate (d); 1 μM of selenite (e); 0.5 mM CdCl2 + 1 μM selenite (f). ep = epidermis; is intercellular space; pp. palisade parenchyma. Bars = 100 μm
In roots, Se application did not cause significant changes in root diameter when compared with that in the control plants (Figs. 3 and 4). On the other hand, Se increased the size of the intercellular spaces (Fig. 3c, arrows). The presence of Cd caused a reduction in root diameter, and Se application was ineffective in restoring the root diameter in plants under Cd stress (Figs. 3 and 4). Selenite is more effective than selenate in activating the antioxidant system and decreasing Cd uptake (Wan et al. 2016) and translocation to the aerial parts, which causes a higher accumulation of Se in roots (Lin et al. 2012). Although selenite and selenate exhibited distinct trends in their effects on plant metabolism, neither Se forms were able to restore root diameter in plants concomitantly exposed to Cd.
Cross sections of Micro-Tom roots grown under CdCl2 stress with selenate or selenite application, observed by light microscopy. a control; b–f supplemented with 0.5 mM CdCl2 (b); 1 μM selenate (c); 0.5 mM CdCl2 + 1 μM of selenate (d); 1 μM of selenite (e); 0.5 mM CdCl2 + 1 μM selenite (f). cc central cylinder; co cortical cell; arrows: intercellular spaces. Bars = 200 μm
The ultrastructural analysis (TEM) of palisade parenchyma cells shows differences among treatments (Fig. 5). Cells treated with Se exhibited an increased thickness of the middle lamella (Fig. 5h, i, n, o). This increased thickness of the middle lamella in plants treated with Se is interesting because priming with Se could be a strategy for improving plant tolerance before heavy metal exposure. The exposure of plants to Cd caused alterations to the ultrastructure of the chloroplast, increased the number of mitochondria, peroxisomes, starch grains and plastogloboli and caused disorganization of the thylakoids and middle lamella and the presence of autophagic bodies (Fig. 5d–f).
Ultrastructure of palisade parenchyma cells from plants grown under CdCl2 stress with selenate and selenite application, observed by transmission electron microscopy. a–c control; supplemented with 0.5 mM CdCl2 (d–f); 1 μM selenate (g–i); 0.5 mM CdCl2 + 1 μM selenate (j–l); 1 μM selenite (m–o); 0.5 mM CdCl2 + 1 μM selenite (p–r); ch chloroplast; mi mitochondria; ml middle lamella; pe peroxisome; pl plastoglobuli; sg starch grain; th thylakoids; small arrows autophagosomes. Bars: a, d, g, j, m, p = 5 μm; b, e, h, k, n, q = 1 μm; c, f, i, l, o, r = 0.5 μm
The increased number of mitochondria may be a consequence of the cellular need for higher amounts of energy, which is necessary for the Cd detoxification process (Zorov et al. 2014). The chloroplast ultrastructure changes may be caused by an increase in the production of ROS, which, at high concentrations in the cellular environment, can cause oxidative damage to cellular ultrastructure and function (Alyemeni et al. 2018).
Stressful conditions have direct effects on chloroplast ultrastructure and the biosynthesis of sugars and starch (Verma and Dubey 2001). In this study, the effect of Cd on the ultrastructure of chloroplasts involved thylakoid disorganization, with dilated thylakoid membranes, and an increased size and number of plastogloboli and starch grains (Fig. 5d–f). The changes in chloroplast ultrastructure could indicate metabolic dysfunction, leading to a reduction in the photosynthetic rate (Table 1). The decrease in photosynthetic attributes can, in turn, be related to reduced carbon fixation (Devi et al. 2007) and consequently a lower number of starch grains (Yue et al. 2018). However, our data reveal an increase in the number of starch grains (Fig. 5d–f) in the presence of Cd. Starch is a major metabolite and product of photosynthesis and is involved in several essential biological activities in the plant response against Cd stress, including osmoregulation and detoxification (He et al. 2011). Thus, the increase in starch grains may play a role in increasing responses against Cd toxicity in MT leaf cells.
Cd stress also increases the number and size of plastoglobuli and peripheral vesicles (Hakmaoui et al. 2007), as also observed in this study (Fig. 5d–f, j–l, p–r). The increased numbers and size of plastoglobuli in chloroplasts is a consequence of stress exposure (Olmos et al. 2006). For instance, plastoglobuli may function in the synthesis and recycling of lipophilic products formed by oxidative metabolism during stress (Olmos et al. 2006). These chloroplast changes are visible effects of Cd stress, as previously reported in tomato (Gratão et al. 2009), and wheat (Yue et al. 2018). Disintegration of chloroplasts caused by Cd stress compromises the photosynthetic rate; thus, maintenance of ultrastructural integrity is fundamental for plant growth and development (Yue et al. 2018). At low concentrations, selenium affects positive activities of enzymes related to sucrose and starch metabolism (Kaur et al. 2018). However, plants treated with both forms of Se under Cd stress exhibited similar changes to those observed in plants exposed to Cd only. Thus, the disintegration of chloroplasts and quantity of starch grains under Cd stress was not altered by Se application, and the potential benefits caused by Se possibly do not reverse the structural damage to palisade parenchyma cells caused by Cd.
Conclusions
Recently, several studies reported the use of Se as an effective strategy to prevent damage caused by abiotic stresses, including Cd exposure. Although biochemical responses have been widely reported, the structural changes underlying this interplay have not been elucidated until now. We found that plants treated with both Se forms exhibited improvements in photosynthetic attributes and growth, which are related to structural modifications to leaves. In contrast, Cd stress caused severe damage to chloroplasts and other cell structures, which resulted in decreased photosynthetic rates and affected growth. The application of Se to plants under stress did not reverse the cell damage caused by Cd. Although our results did not find that Se may induce ultrastructural and physiological changes (i.e., alleviation) during Cd stress, Se plays an important role under nonstress conditions due to the identification of specific Se-induced structural changes that could provide benefits to plant development. We conclude that the novel information provided by this study had not yet been described in plant anatomical studies and represent a fundamental step towards obtaining a better understanding of the structural changes caused by Se and the elucidation of its role in plant metabolism.
References
Abd Allah EF, Hashem A, Alqarawi AA (2016) Mitigation of cadmium induced stress in tomato (Solanum lycopersicum L.) by selenium. Pak J Bot 48:953–961
Ali B, Qian P, Jin R, Ali S, Khan M, Aziz R, Zhou W (2014) Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biol Plant 58:131–138. https://doi.org/10.1007/s10535-013-0358-5
Alves LR, Reis AR, Gratão PL (2016) Heavy metals in agricultural soils: from plants to our daily life (a review). Cientifica. 44:346–361. https://doi.org/10.15361/1984-5529.2016v44n3p346-361
Alves LR, Monteiro CC, Carvalho RF, Cury PR, Tezotto T, Azevedo RA, Gratão PL (2017) Cadmium stress related to root-to-shoot communication depends on ethylene and auxin in tomato plants. Environ Exp Bot 134:102–115. https://doi.org/10.1007/s10534-015-9867-3
Alves LR, Reis AR, Prado ER, Lavres J, Pompeu GB, Azevedo RA, Gratão PL (2019) New insights into cadmium stressful-conditions: role of ethylene on selenium-mediated antioxidant enzymes. Ecotoxicol Environ Saf 186:109747. https://doi.org/10.1016/j.ecoenv.2019.109747
Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P (2018) Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma. 255:459–469. https://doi.org/10.1007/s00709-017-1162-4
Ashraf MA, Akbar A, Parveen A, Rasheed R, Hussain I, Iqbal M (2018) Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol Biochem 123:268–280. https://doi.org/10.1016/j.plaphy.2017.12.023
Barbosa JC, Maldonado Júnior W (2009) Software AgroEstat: Sistema de análises estatísticas de ensaios agronômicos. Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias, Jaboticabal
Castillo-Godina RG, Foroughbakhch-Pournavab R, Benavies-Mendonza A (2016) Effect of selenium on elemental concentration and antioxidant enzymatic activity of tomato plants. J Agr Sci Tech 18:233–244
Devi R, Munjral N, Gupta AK, Kaur N (2007) Cadmium induced changes in carbo- hydrate status and enzymes of carbohydrate metabolism, glycolysis and pentose phosphatepathway in pea. Environ Exp Bot 61:167–174. https://doi.org/10.1016/j.envexpbot.2007.05.006
Djanaguiraman M, Prasad PVV, Seppänen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48:999–1007. https://doi.org/10.1016/j.plaphy.2010.09.009
Djebali W, Zarrouk M, Brouquisse R, El Kahoui S, Limam F, Ghorbel H, Chaibi W (2005) Ultrastructure and lipid alterations induced by cadmium in tomato (Lycopersiconsculentum) chloroplast membranes. Plant Biol 7:358–368. https://doi.org/10.1055/s-2005-837696
Edelstein M, Ben-Hur M (2018) Heavy metals and metalloids: sources, risks and strategies to reduce their accumulation in horticultural crops. Sci Hortic 234:431–444. https://doi.org/10.1016/j.scienta.2017.12.039
Feng R, Wei C, Tu S (2013a) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68. https://doi.org/10.1016/j.envexpbot.2012.09.002
Feng R, Wei C, Tu S, Ding Y, Song Z (2013b) A dual role of Se on Cd toxicity: evidences from the uptake of Cd and some essential elements and the growth responses in paddy rice. Biol Trace Elem Res 151:113–121. https://doi.org/10.1007/s12011-012-9532-4
Gratão PL, Monteiro CC, Rossi ML, Martinelli AP, Peres LE, Medici LO, Azevedo RA (2009) Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ Exp Bot 67:387–394. https://doi.org/10.1016/j.envexpbot.2009.06.017
Gratão PL, Monteiro CC, Tezotto T, Carvalho RF, Alves LR, Peters LJ, Azevedo RA (2015) Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. BioMetals. 28:803–816. https://doi.org/10.1007/s10534-015-9867-3
Haghighi M, Sheibanirad A, Pessarakli M (2016) Effects of selenium as a beneficial element on growth and photosynthetic attributes of greenhouse cucumber. J Plant Nutr 39:1493–1498. https://doi.org/10.1080/01904167.2015.1109116
Hakmaoui A, Ater M, Boka K, Baron M (2007) Copper and cadmium tolerance, uptake and effect on chloroplast ultrastructure. Studies on Salixpurpurea and Phragmites australis. Z Naturforsch C 62:417–426
Hawrylak-Nowak B, Hasanuzzaman M, Matraszek-Gawron R (2018) Mechanisms of selenium-induced enhancement of abiotic stress tolerance in plants. In: Hasanuzzaman M, Fujita M, Oku H, Nahar K, Hawrylak-Nowak B (eds) Plant Nutrients and Abiotic Stress Tolerance. Springer, Singapore, pp 269–295
He J, Qin J, Long L, Ma Y, Li H, Li K, Jiang X, Liu T, Polle A, Liang Z, Luo ZB (2011) Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus × canescens. Physiol Plant 143:50–63. https://doi.org/10.1111/j.1399-3054.2011.01487.x
Jarvis SC, Jones LHP, Hopper MJ (1976) Cadmium uptake from solution by plants and its transport from roots to shoots. Plant Soil 44:179–191. https://doi.org/10.1007/BF00016965
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative in high osmolality for use in electron microscopy. J Cell Biol 27:137–138A
Kaur M, Sharma S, Singh D (2018) Influence of selenium on carbohydrate accumulation in developing wheat grains. Commun Soil Sci Plant Anal 49:1650–1659. https://doi.org/10.1080/00103624.2018.1474903
Lin L, Zhou W, Dai H, Cao F, Zhang G, Wu F (2012) Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J Hazard Mater 235:343–351. https://doi.org/10.1016/j.jhazmat.2012.08.012
Meissner R, Jacobson Y, Melamed S, Levyatuv S, Shalev G, Ashri A, Elkind Y, Levy A (1997) A new model system for tomato genetics. Plant J 12:1465–1472. https://doi.org/10.1046/j.1365-313x.1997.12061465.x
Olmos E, Kiddle G, Pellny TK, Kumar S, Foyer CH (2006) Modulation of plant morphology, root architecture, and cell structure by low vitamin C in Arabidopsis thaliana. J Exp Bot 57:1645–1655. https://doi.org/10.1093/jxb/erl010
Rasband WS (1997-2012) ImageJ. U.S. National Institutes of Health, Bethesda, http://imagej.nih.gov/ij/
Reynolds ES (1993) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208–212
Shanying HE, Xiaoe Y, Zhenli HE, Baligar VC (2017) Morphological and physiological responses of plants to cadmium toxicity: a review. Pedosphere 27:421–438. https://doi.org/10.1016/S1002-0160(17)60339-4
Shekari L, Aroiee H, Mirshekari A, Nemati H (2019) Protective role of selenium on cucumber (Cucumis sativus L.) exposed to cadmium and lead stress during reproductive stage role of selenium on heavy metals stress. J. Plant Nutr 42:529–542. https://doi.org/10.1080/01904167.2018.1554075
Sun H, Dai H, Wang X, Wang G (2016) Physiological and proteomic analysis of selenium-mediated tolerance to Cd stress in cucumber (Cucumis sativus L.). Ecotoxicol Environ Saf 133:114–126. https://doi.org/10.1016/j.ecoenv.2016.07.003
Verma S, Dubey RS (2001) Effect of cadmium on soluble sugars and enzymes of their metabolism in rice. Biol Plant 44:117–123. https://doi.org/10.1023/A:1017938809311
Vincent JM (1975) Manual Practico de Rizobiologia, 1st edn. Hemisferio Sur, Buenos Aires
Wan Y, Yu Y, Wang Q, Qiao Y, Li H (2016) Physiological and proteomic analysis of selenium-mediated tolerance to cd stress in cucumber (Cucumis sativus L.). Ecotoxicol Environ Saf 133:127–134. https://doi.org/10.1016/j.ecoenv.2016.07.003
Yin H, Qi Z, Li M, Ahammed GJ, Chu X, Zhou J (2019) Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol Environ Saf 169:911–917. https://doi.org/10.1016/j.ecoenv.2018.11.080
Yu Y, Fu P, Huang Q, Zhang J, Li H (2019) Accumulation, subcellular distribution, and oxidative stress of cadmium in Brassica chinensis supplied with selenite and selenate at different growth stages. Chemosphere. 216:331–340. https://doi.org/10.1016/j.chemosphere.2018.10.138
Yue JY, Wei XJ, Wang HZ (2018) Cadmium tolerant and sensitive wheat lines: their differences in pollutant accumulation, cell damage, and autophagy. Biol Plant 62:379–387. https://doi.org/10.1007/s10535-018-0785-4
Zhu G, Xiao H, Guo Q, Zhang Z, Zhao J, Yang D (2018) Effects of cadmium stress on growth and amino acid metabolism in two Compositae plants. Ecotoxicol Environ Saf 158:300–308. https://doi.org/10.1016/j.ecoenv.2018.04.045
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94:909–950. https://doi.org/10.1152/physrev.00026.2013
Acknowledgments
We thank Centro de Microscopia e Imagem (FOP/UNICAMP) for the access to the transmission electron microscope.
Funding
This work was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP—Grant no. 2017/04787–6)—Brazil; PLG also thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the research fellowship (Grant no. 314380/2018–3)—Brazil; and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (L.R.A) for the scholarship granted (Finance Code 001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Bhumi Nath Tripathi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alves, L.R., Rossatto, D.R., Rossi, M.L. et al. Selenium improves photosynthesis and induces ultrastructural changes but does not alleviate cadmium-stress damages in tomato plants. Protoplasma 257, 597–605 (2020). https://doi.org/10.1007/s00709-019-01469-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01469-w