Abstract
Cadmium (Cd) is probably the most damaging metal to plant species; with a long biological half-life, it can be taken up by plants, disrupting the cell homeostasis and triggering several metabolic pathways. Selenium (Se) improves plant defence systems against stressful conditions, but the biochemical antioxidant responses to Cd stress in tomato plants is poorly understood. To further address the relationship of Cd-stress responses with Se mineral uptake, Cd and Se concentration, proline content, MDA and H2O2 production, and the activity of SOD, APX, CAT and GR enzymes were analyzed in Micro-Tom (MT) plants submitted to 0.5 mM Cd. The results revealed different responses according to Se combination and Cd application. For instance, roots and leaves of MT plants treated with Se exhibited an increase in dry mass and nutritional status, exhibited lower proline content and higher APX and GR activities when compared with plants with no Se application. Plants submitted to 0.5 mM Cd, irrespective of Se exposure, exhibited lower proline, MDA and H2O2 content and higher SOD, CAT and GR activities. Selenium may improve tolerance against Cd, which allowed MT plants exhibited less oxidative damage to the cell, even under elevated Cd accumulation in their tissues. The results suggest that Se application is an efficient management technique to alleviate the deleterious effects of Cd-stress, enhancing the nutritional value and activity of ROS-scavenging enzymes in tomato plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a heavy metal that is toxic to living organisms. Due to bioaccumulation and its increasing use in industry, cadmium presents an increasing hazard (Alves et al. 2016, Edelstein and Ben-Hur 2018). Plants can easily uptake this heavy metal, which triggers drastic alteration in tissues and metabolic pathways (Ma et al. 2017, Zhu et al. 2018). Even at low concentrations, Cd can modify root morphology, limiting the root-to-shoot transport of nutrients (Lux et al. 2011) and decrease nutrient uptake because Cd competes with and can replace the uptake of calcium, copper, iron, manganese and zinc (Rabêlo et al. 2018). Moreover, Cd provokes uncontrolled oxidation that disrupt cell balance and cause electrolyte leakage, which activates biochemical responses by favouring the production of reactive oxygen species (ROS), disrupting the plant defence systems (Alves et al. 2017).
Plants are able to detoxify excessive oxidation caused by Cd by complex enzymatic and non-enzymatic mechanisms that protect plant cells against oxidative damage and restore the cell redox balance (Gratão et al. 2015). The first defence response at the cellular level involves antioxidant enzymes such as superoxide dismutase (SOD, EC 1.15.1.1), which dismute superoxide (O2−) to hydrogen peroxide (H2O2) (Noctor and Foyer 2016). Next, H2O2 can be eliminated by ascorbate peroxidase (APX, EC 1.11.1.11), catalase (CAT, EC 1.11.1.6), glutathione peroxidase (GPX, EC 1.11.1.9) (Noctor et al. 2018) and other peroxidases. Moreover, non-enzymatic mechanisms, such as proline, carotenoids, ascorbate and glutathione, are also involved in scavenging excess ROS in plant tissue (Zouari et al. 2016).
Plant-stress mechanisms may involve an interplay between interconnected networks of cellular responses and elements under Cd stress conditions that are not fully understood (Chmielowska-Bak et al. 2014). Exploring these mechanisms and developing strategies to reduce Cd accumulation and the damage it causes to plants is a fundamental issue. A recently studied strategy to decrease the oxidative damage and minimize the toxicity generated by Cd to plant tissues is the use of beneficial elements or substances to restore the photosynthetic capacity, improve the antioxidant response and enhance crop production, such as selenium (Se) (Feng et al. 2013, Alyemeni et al. 2018).
Application of Se at low concentrations promotes plant growth and alleviates the negative effects of Cd-induced stress by regulating ROS scavenger metabolism (Castillo-Godina et al. 2016; Alyemeni et al. 2018), abundant stress-responsive proteins (Sun et al. 2016) and delaying fruit senescence (Pezzarossa et al. 2014). Moreover, Se application can decrease Cd uptake in tomatoes (Abd et al. 2016) and translocation of Cd from root-to-shoot in rice plants (Wan et al. 2016). However, Se was not effective in preventing Cd uptake in rice with increased soil Cd concentrations (Huang et al. 2017). Therefore, further studies are needed to better understand the effects of Se on the biochemical mechanisms of Cd transport from root-to-shoot.
The miniature tomato cultivar Micro-Tom (MT) has been considered as a plant model based on growth at high plant density and production of viable fruits within a short life cycle (Meissner et al., 1997). Several studies have indicated the antioxidant potential of Se (Abd Allah et al. 2016, Castilo-Godina 2016); however, there is a lack of information regarding the biochemical antioxidant responses to Cd stress in tomato plants. To better understand the role of Se on the modulation of Cd-stress responses in tomato plants, a pot experiment was performed using Micro-Tom under different concentrations of Cd and Se.
Material and methods
Plant material and growth conditions
Seeds of the tomato (S. lycopersicum L.) cultivar Micro-Tom (MT) were sterilized using a sodium hypochlorite solution (5%), rinsed three times and sown in boxes filled with a 1:1 (by volume) mixture of commercial pot mix (Plantmax HT Eucatex, Brazil) and vermiculite supplemented with 1 g L−1 10:10:10 nitrogen–phosphorus–potassium and 4 g L−1 lime (MgCO3 + CaCO3). The boxes were maintained in a greenhouse. When two true leaves were completely formed, one seedling was transplanted per 0.350 L Leonard pot (Vincent 1975), a hydroponic cultivation system, where modified Hoagland’s nutrient solution (250 ml) rise up through cotton strings by capillarity to hum the sterilized sand and polystyrene (4:3). Thirty 6-day-old plants were grown in the same solution at four Se concentrations (0, 1, 5 and 10 µm of Na2SeO3) combined with or without Cd (0, 0.5 mM CdCl2), which was changed every 5 days.
After a period of 75 days post germination, corresponding to 39 days under Cd and Se exposure, samples of roots and leaves were rinsed with distilled water and kept in paper bags and dried in a drying oven (60 °C) until constant weight was achieved for dry mass determination and nutritional analysis. Samples of roots and leaves were harvested, rinsed and immersed in liquid N2 and stored at −80 °C for analysis of lipid peroxidation, H2O2 and proline concentration, enzyme extraction and protein determination.
Chlorophyll and carotenoids determination
The chlorophyll and carotenoids content was assayed spectrophotometrically. Fresh leaves (0.50 mg) were added in tube with 2 ml acetone (100%). After shaking for 72 h at 60 g at 4 °C the sample was read in 470 nm for carotenoids and 645 and 662 nm for chlorophyll a and b, respectively (Lichtentlaler 1987).
Nutritional analysis, Cd and Se content
At a controlled pressure of 2 MPa, dry root and leaf samples (200.0 mg DW) were milled and microwave digested in 2 mL 70% HNO3, 2 mL H2O2 and 2 mL Milli-Q water (18.2 MX cm a 25 °C) (Chilimba et al. 2011). The digested samples were diluted to 10 mL with Milli-Q water and stored. The presence and concentration of Cd, Se, P, K, Ca, Mg, S, B, Cu, Fe, Mn and Zn were determined by radial view inductively coupled plasma optical emission spectrometry (ICP-OES JobinYvon, JY50P Longjumeau, France) equipped with a nebulization chamber. A certified reference material from the National Institute of Standards and Technology (NIST SRM 1567a Wheat flour, Gaithersburg, USA) was used for quality control in the analysis of acid digests by ICP OES. The following emission lines were used: Cd II 228.80; Se II 196.09; P I 213.618 nm; K I 769.897 nm; Ca I 422.673 nm; Mg I 280.270 nm; S I 181.972 nm; B I 249.773 nm; Cu I 324.754 nm; Fe II 259.940 nm; Mn II 259.373 nm; Zn II 231.865 nm and Ni II 231.604 nm.
Lipid peroxidation
Lipid peroxidation was assessed by analyzing the content of thiobarbituric acid (TBA) reactive substances (TBARS). Fresh root and leaf mass (0.4 g) was ground with 20% (w/v) polyvinylpyrrolidone (PVPP) and 0.1% trichloroacetic acid (TCA). After centrifugation at 11,000 × g for 10 min, the supernatant was added to a solution of 20% TCA and 5% TBA and incubated in a water bath at 95 °C for 30 min. Samples were then placed in an ice bath for 10 min to stop the reaction and then centrifuged at 11,000 × g for 10 min. The concentration of malondialdehyde (MDA) equivalents was measured spectrophotometrically between 535 and 600 nm; data were calculated using an extinction coefficient of 1.55 × 10−5 mol−1 cm−1 (Gratão et al. 2012).
H2O2 content
Fresh plant tissue (0.4 g) was homogenized in trichloroacetic acid (0.1%) and centrifuged at 10,000 × g for 10 min. The supernatant was added to 100 mM potassium phosphate buffer (pH 7.50) and 1 M potassium iodide solution. This solution was incubated on ice for 1 h, the absorbance was measured at 390 nm, and the H2O2 content was determined using a known H2O2 concentration curve as a standard following the method of Gratão et al. (2012).
Proline content
Fresh leaves and root samples (0.5 g) were homogenized in 3% sulphosalicylic acid and filtered. The mixture filtrate was added with 1 mL each of acid ninhydrin and glacial acetic acid and was placed in boiling water for 1 h. Toluene (4 mL) was added to the mixture, and the absorbance was measured spectrophotometrically at 520 nm and converted to mmol g−1 fresh weight against standard proline. The proline content was determined as described by Bates et al. (1973).
Enzyme extraction and protein determination
Enzyme extraction was performed by Boaretto et al. (2014). Fresh leaves and roots (1.0 g) were homogenized in a chilled mortar with a pestle using an extraction buffer containing 100 mM potassium phosphate buffer (pH 7.5), 1 mM ethylenediamine tetraacetic acid (EDTA), 3 mM DL-dithiothreitol and 5% (w/v) insoluble polyvinylpolypyrrolidone in 3:1 and 2:1 volume/fresh weight ratio to leaves and roots, respectively. The homogenate was centrifuged at 10,000 × g for 30 min, and the supernatant was stored at −80 °C for further determination of SOD, CAT, GR and APX activities. The protein concentration was assayed following the method of Bradford (1976) using bovine serum albumin as a standard.
Superoxide dismutase assay
Superoxide dismutase (SOD, EC 1.15.1.1) activity was analyzed by activity staining using non-denaturing PAGE (Azevedo et al. 1998). Leaf and root extracts were inserted to non-denaturing-PAGE separation. After that, the gels were rinsed in distilled-deionized water and kept in the dark for 30 min in 50 mM potassium phosphate buffer (pH 7.8) containing 0.05 mM riboflavin, 0.1 mM nitro blue tetrazolium, 1 mM EDTA, and 0.3% N,N,N′,N′-tetramethylethylenediamine. The gels were rinsed with deionized water and were exposed to artificial light under the water until the achromatic bands of SOD activity were visible on a purple-stained gel. SOD isoenzymes were differentiated by their sensitivity or inhibition to 5 mM hydrogen peroxide (H2O2) or 2 mM potassium cyanide (Azevedo et al. 1998).
Catalase assay
The activity of Catalase (CAT, EC 1.11.1.6) was determined by checking the degradation of H2O2 at 240 nm over 1 min. Catalase activity (CAT) was measured spectrophotometrically at 25 °C in a reaction mixture containing 1 mL of 100 mM potassium phosphate buffer (pH 7.5) and 25 μL H2O2 (30% solution) (Nogueirol et al. 2015). CAT activity was expressed as mmol min−1 mg−1 protein.
Ascorbate peroxidase assay
Ascorbate peroxidase (APX, EC 1.11.1.11) was determined by monitoring the rate of ascorbate oxidation at 290 nm at 30 °C and expressed as nmol ascorbate min−1 mg−1 protein. APX activity was assayed spectrophotometrically following Gratão et al. (2012) in a reaction consisting of plant extract, 80 mM potassium phosphate buffer (pH 7.0) including 5 mM ascorbate, 1 mM EDTA, and 1 mM H2O2.
Glutathione reductase assay
The glutathione reductase (GR, EC 1.6.4.2) assay mixture consisted of 100 mM potassium phosphate buffer (pH 7.5) containing 1 mM 5,5′-dithiobis (2-nitrobenzoic acid), 1 mM glutathione disulfide (GSSG) and 0.1 mM NADPH. The rate of decrease of GSSG was monitored by the intensification in absorbance at 412 nm over 1 min. GR activity was measured spectrophotometrically at 30 °C and expressed as nmol min−1 mg−1 protein as described in Gratão et al. (2012).
Statistical analysis
The experimental design was randomized using six plants per treatment from three replicate pots in a factorial scheme of 4 × 2, following four Se concentrations and two Cd concentrations (presence or absence). The result of three independent replicates of each extract for plant growth, Cd concentration and nutritional analyses, TBARS and proline contents, CAT, APX and GR activities was expressed as the mean and standard error of the mean (±SEM). All data were tested for normality and homogeneity of variances using the Shapiro–Wilk and Bartlett tests, respectively. The data was submitted to one-way analysis of variance bifactorial (ANOVA) and when significant differences among averages were found, Tukey’s HSD test at P < 0.05 level of significance was applied. The statistical analyses were performed using Agro Estat® software (Barbosa and Maldonado 2009).
Results
Plant growth
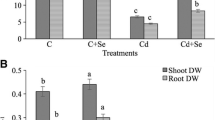
Over 75 days post germination, plants with 1 and 5 µm Se concentrations exhibited higher dry mass without Cd exposure. Root dry mass exhibited a significant increase of 44% (1 µm Se) and 45% (5 µm Se), whereas leaf dry mass was enhanced by 30% (1 µm Se) and 31% (5 µm Se), when compared to control plants (without Se). With respect to Cd exposure, no significant results were observed for plant growth, irrespective of Se concentration (Fig. 1).
Roots and leaves dry mass (g dry wt) in MT grown over 39-day period in the presence of 0 or 0.5 mM CdCl2 and 1, 5 or 10 µm Se. Data above x-axis represent leaves and below x-axis, roots. Different uppercase letters on the top of the columns indicates the difference between −Cd or +Cd exposure and lowercase indicates the difference between Se concentrations with significantly different at P < 0.05 by Tukey test
Chlorophyll and carotenoids content
The application of different Se concentrations not caused a significant alteration in chlorophyll and carotenoids contents, when compared with control plants. In the presence of Cd, chlorophyll and carotenoids content was lower with the application of 1 and 5 µm of Se, when compared with plant exposed to the same Se concentration without Cd exposure (Fig. 2).
Total chlorophyll content (µg cm−²) measured in leaves of in MT grown over 39-day period in the presence of 0 or 0.5 mM CdCl2 and 1, 5 or 10 µm Se. Data above x-axis represent leaves and below x-axis, roots. Different uppercase letters on the top of the columns indicates the difference between −Cd or +Cd exposure and lowercase indicates the difference between Se concentrations with significantly different at P < 0.05 by Tukey test
Cd and Se concentrations
Se concentration in MT roots and leaves was significantly higher with increasing Se concentrations. In the presence of CdCl2, Se concentration was lower in roots when compared to plants without Cd application, with 51% for 1 µm Se, 73% for 5 µm Se and 40% for 10 µm Se, whereas in leaves, Se concentration was 23, 56 and 77% lower, respectively (Table 1). When the Cd concentration was concerned, plants growing under 1 µm Se exhibited 121% higher in leaves (Table 1). Plants growing under 5 µm Se exhibited significant alterations, where Cd concentration in roots was 44% lower, whereas leaves exhibited 38% higher in Cd concentration, when compared with Cd exposure alone.
Nutritional analysis
Macronutrients and micronutrients were taken up and distributed differently depending on the Se concentration, Cd exposure treatment and the tissue analyzed (Table 2). Roots of plants treated with Se, irrespective of Se concentration, only Ca concentrations was lower. Phosphorous, K, Ca, Mg, Cu, Fe, Mn, Zn and Ni concentrations was significantly higher in leaves treated with Se, irrespective of Cd exposure (Tables 2 and 3). Cadmium reduced the concentrations of P, Ca, Mn and Ni in roots, while it increased the Fe concentration. However, Cd significantly reduced the P concentration and increased the Fe and Mn concentrations in the leaves of tomato plants (Tables 2 and 3). Plants following Cd and Se application exhibited higher in the P and Mn concentrations in roots, whereas the K, Ca, Mg, S, Fe and Ni concentrations decreased in roots. However, this pattern of nutrient content in roots was modified by Cd exposure (Tables 2 and 3).
Lipid peroxidation and H2O2 content
Lipid peroxidation, expressed as MDA content, exhibited interesting results, where Se applied alone did not influence MDA content in either roots or leaves. In the presence of Cd, plants with 0 µm Se application exhibited a significantly higher in MDA content in leaves when compared with the highest Se concentration. Leaves with 1 µm Se was 57% lower in MDA content in the presence of Cd when compared with 0 µm Se. Roots did not exhibit significant changes in MDA content among treatments (Fig. 3a).
Content of (a) lipid peroxidation measured as malondialdehyde (MDA) (nmol g−¹ fresh weight) and (b) hydrogen peroxide (H2O2) (µmol g−¹ fresh weight) in roots and leaves of MT, plants grown over 39-day period in the presence of 0 mM or 0.5 mM CdCl2 and 1, 5 or 10 µm Na2SeO3. Data above x-axis represent leaves and below x-axis, roots. Different uppercase letters on the top of the columns indicates the difference between −Cd or +Cd exposure and lowercase indicates the difference between Se concentrations with significantly different at P < 0.05 by Tukey test
The H2O2 content was higher in leaves with 1 µm Se, whereas leaves with 5 and 10 µm Se was significantly lower in H2O2 content. Leaves with 0 µm Se submitted to Cd exhibited 16% higher in H2O2 content when compared to control plants (0 mM Cd). When Se and Cd was applied, a significant reduction occurred in H2O2 content of leaves, mainly with 1 µm Se (Fig. 3b).
Proline content
Proline content was lower in leaves and roots for all Se concentrations (Fig. 4). Following treatment with CdCl2, leaves exhibited a significant decrease in proline content without Se application. On the other hand, leaves with 10 µm Se exhibited 17% higher in proline content.
Proline content (µmol g−¹ fresh weight) in roots and leaves of MT plants grown over 39-day period in the presence of 0 mM or 0.5 mM CdCl2 and 1, 5 or 10 µm Na2SeO3. Data above x-axis represent leaves and below x-axis, roots. Different uppercase letters on the top of the columns indicates the difference between −Cd or +Cd exposure and lowercase indicates the difference between Se concentrations with significantly different at P < 0.05 by Tukey test
Antioxidant enzyme activities
Three SOD isoenzymes were detected in leaves (Fig. 5a) and two in roots (Fig. 5b), which were characterized as Mn-SOD (SOD I), Fe-SOD (SOD II) and Cu/Zn-SOD (SOD III) (Supplementary Data 1). Different changes were observed between the tissues analyzed depending on the Se and Cd exposure treatment. For instance, the activity of SOD I exhibited the same pattern in leaves, irrespective of Se and Cd treatments, while leaves exhibited very low SOD III activity in the presence of Cd (Fig. 5a, line 5–8).
Superoxide dismutase (SOD) activity stained following non-denaturing polyacrylamide gel electrophoresis of leaves (a) and roots (b) isolated from non-grafted and grafted MT plants grown over 39-day period in the presence of 0 mM or 0.5 mM CdCl2. The lanes listed are: (S) bovine SOD standard; (1) MT (0 mM CdCl2 + 0 µm Na2SeO3), (2) MT (0 mM CdCl2 + 1 µm Na2SeO3), (3) MT (0 mM CdCl2 + 5 µm Na2SeO3), (4) MT (0 mM CdCl2 + 10 µm Na2SeO3), (5) MT (0.5 mM CdCl2 + 0 µm Na2SeO3), (6) MT (0.5 mM CdCl2 + 1 µm Na2SeO3), (7) MT (0.5 mM CdCl2 + 5 µm Na2SeO3), (8) MT (0.5 mM CdCl2 + 10 µm Na2SeO3).The SOD isoforms are (I) Mn-SOD, (II) Fe-SOD and (III) Cu/Zn-SOD
SOD III was affected by Cd exposure (Fig. 5a, lines 1–4) and was more pronounced in leaves with 0 mM Cd and 5 µm Se (line 3). In roots, SOD I was more pronounced in the presence of Cd (Fig. 5, line 5–8), although a slight increase was observed with Se application (line 6–8). Following Cd application, the activity of SOD II was lower in the roots (line 5–8), and higher with the presence of Se (line 6–8).
CAT (Fig. 6a), APX (Fig. 6b) and GR activities (Fig. 6c) are crucial for the detoxification of any excess H2O2 produced by SOD and/or by other metabolic processes. Leaves and roots treated with Se, irrespective of Se concentration, exhibited significantly higher CAT activity following Cd application, being more pronounced for 10 µm Se concentration. When APX activity was concerned, leaves with 5 µm Se exhibited 34% higher APX activity when compared with leaves without Se. Following Cd application, roots exhibited higher APX activity for all Se treatments, when compared with plants without Cd exposure. However, under Cd exposure, the application of 1, 5 or 10 µm Se induced lower APX activity, when compared with plants under Cd without Se application.
Antioxidant total enzyme activity. a CAT specific activity (mmol min−1 mg−1 protein), b APX specific activity (nmol min−1 mg−1 protein) and c GR specific activity (nmol min−1 mg−1 protein) in MT plants grown over 39-day period in the presence of 0 or 0.5 mM CdCl2 and 1, 5 or 10 µm Na2SeO3. Data above x-axis represent leaves and below x-axis, roots. Different uppercase letters on the top of the columns indicates the difference between −Cd or +Cd exposure and lowercase indicates the difference between Se concentrations with significantly different at P < 0.05 by Tukey test
The application of Se triggered differences in GR activity. Leaves with 5 µm Se exhibited significantly higher GR activity by 67% compared with other Se concentrations without Cd exposure. Nevertheless, GR activity increased by 62% in leaves and 70% in roots following Cd application. Moreover, leaves and roots with 10 µm Se under Cd exposure exhibited strict increases of 65 and 84%, respectively, when compared with treatments with 0 µm Se under Cd exposure (Fig. 6c).
Discussion
Environmental stresses may drastically affected plant development, growth and yield due to changes in metabolism. Selenium application has been used in agriculture as an efficient strategy to improve plant response against stress and to avoid or minimize crop loss or damages (Kaur et al. 2016, Alyemeni et al. 2018). On the other hand, the beneficial effect of Se is dose-dependent, and higher concentrations in soil may damage plant development. Therefore, it is essential to optimize the concentration to be applied to each crop.
Several studies have indicated that Se can promote an increase in plant biomass, as observed in the present work (Reis et al. 2018). The application of 1 µm and 5 µm Se efficiently promoted a gain in plant dry mass. For instance, roots exhibited higher dry mass of 44 and 45%, respectively, while leaf dry mass increased by 30 and 31%, respectively (Fig. 1). These data demonstrate that adequate Se concentration results in improves growth. The application of 1 µm and 5 µm Se concentrations increased plant growth, whereas chlorophylls and carotenoids contents were not altered (Fig. 2). The application of 10 µm Se had no effect on plant growth, which could indicate that higher Se concentrations limit plant growth (Silva et al. 2018). The application of Cd resulted in a decrease in root dry mass when compared with control plants (Fig. 1), since that well known that Cd damages plant growth (Ma et al. 2017, Zhu et al. 2018). Reduced growth by Cd stress could be related with reduced synthesis of photosynthetic pigments (Alyemeni et al., 2018). However, we not observed alterations in chlorophylls and carotenoids contents following Cd application (Fig. 2). Although a number of studies have indicated that Se treatment can reverse the reduction in growth caused by Cd in other crops, our treatments did not exhibit the same effect in MT plants, which could be related with lower chlorophylls and carotenoids contents, mainly with the application of 1 and 5 µm Se (Fig. 2).
The effect of Se on nutrient uptake is still controversial, but the beneficial effect of Se on nutrient uptake has been previously observed in many crops, such as cowpea (Silva et al. 2018), rice (Reis et al. 2017) and wheat (Yao et al. 2013). Several factors, such as the composition of the cell wall of the roots and biomass production, affect the uptake and transport of nutrients (Santos et al. 2017). Reis et al. (2018) reported increases in P, K, Cu and Zn concentrations in the leaves of rice plants supplied with Se. Moreover, cowpea under field conditions Silva et al. (2018) showed lower P, K, and Cu concentration in leaves and higher in Zn concentration in the leaves as a result of Se application. Se can act as a competitor with S for transporters involved in their uptake at the root plasma membrane (Zayed and Terry 1992), which can decrease S content.
The positive impact of Se on the nutritional status of plants under Cd stress can significantly contribute to growth because of its involvement in enzyme activation, signalling, and chlorophyll synthesis (Alyemeni et al. 2018). The interaction between Se and nutrients is a complex process, and sometimes, these elements may naturally present synergistic and antagonistic relations at the same time.
Although Se is non-essential to plants, in the form of selenite it can be easily taken up via phosphate transporters and metabolized to organic forms in roots, such as selenomethionine, selenocysteine and several other unidentified Se species (Li et al. 2008). We found that the Se concentration in MT roots and leaves was significantly higher with increasing Se concentrations (Table 1). The Se concentration in plant tissue is dependent on the Se concentrations in soils, Se source and nutrient solution (Li et al. 2018). The Se concentration was higher in roots than in leaves. The Se accumulation observed in our data is due to the characteristics of selenite. A study conducted by Lin et al. (2012) showed that selenite is poorly translocated to the above parts, which triggers a higher accumulation of Se in roots.
Cadmium toxicity clearly has negative effects on plant growth and can reduce root dry weight, root diameter and number of lateral roots (Rabêlo et al. 2018). In this study, we observed a reduction of 38% in root growth of 0 µm Se + 0.5 Cd application treatments when compared with control plants (without Cd) (Fig. 1). Cd can cause nutritional imbalances in plants due to (i) competition of Cd2+ with nutrients using the same uptake sites (Clemens and Feng 2016) and (ii) modification in the translocation of nutrients (Nazar et al. 2012). Moreover, Cd exposure causes biochemical and structural alterations in plants that can result in changes in the nutritional status of the plant (Alves et al. 2017).
The reduced concentration of nutriients in the roots of plants submitted to Cd (Tables 2 and 3) can be associated with reduced growth of these plants, as previously observed in other species (Rabêlo et al. 2018). For instance, Cd exposure decreased P, Ca, Zn and Mn contents in roots. Plants under Cd exposure may exhibit reduced Mn uptake due to Cd and Mn competition for the same transporters (Wu et al. 2016; Borges et al. 2019). In addition, our results showed that Cd increased the content of Fe in roots and leaves. Plants exposed to Cd toxicity in the root generally exhibit an increment of Fe concentration in roots (Gratão et al. 2015; Borges et al. 2019). The higher Fe content results from the formation of Fe plaques on the root surface of plants exposed to Cd due to the increase in the Fe2+ availability (Sebastian and Prasad 2015). Exposure to Cd may increase the Fe availability in the rhizosphere and consequently the concentration of Fe in leaves, as observed in the present work. Apart from nutritional dynamics, Cd can also cause oxidative damage.
Plants have a range of potential mechanisms that may be involved in avoiding damage to plant metabolism caused by Cd. As shown in Table 1, plants accumulate more Cd in roots than in leaves, which may be a natural defence response of plants to Cd toxicity. Our data revealed that the application of 5 µm Se efficiently reduced Cd content by 44% in roots. Se is favourable to plant growth under Cd-stress conditions (Wu et al. 2017). Se was not effective in avoiding damage to plant growth caused by Cd (Fig. 1), but Se reduced MDA and H2O2 contents in plants exposed to Cd. It can be due to Se improved the activity of enzymes and compounds related to ROS scavenging (Alyemeni et al. 2018). The reduced growth of tomato plants exposed to Cd (Fig. 1) is a typical symptom of phytotoxicity in response to changes in nutrient uptake (Nazar et al. 2012). Cd and Se are bound to thiol groups of the amino acid cysteine, and it should be noted the competition for a shared binding site in proteins could explain the reduced Cd uptake and defence mechanisms against Cd toxicity (Schützendubel et al. 2001).
However, the application of 1 and 10 µm Se did not decrease the Cd concentration in roots. When the Cd content was measured in the above parts of the plants, we observed that plants under Cd exposure treated with 1 µm Se exhibited 121% higher Cd content in leaves (Table 1). Wan et al. (2016) revealed that Cd uptake kinetics result in selenite promoting Cd influx and increased Cd uptake in rice roots during short-term exposure, proving no competition in uptake between Se and Cd on the root surface. Furthermore, the results of Lin et al. (2012) indicate that Se markedly decreased Cd accumulation in the leaves of rice plants. It is still unknown how Se can decrease heavy metal translocation. On the other hand, Se ions are co-transported with Cd ions by the same protein carriers, causing an increase of Cd in the above parts of plants (Zembala et al. 2010). These findings could explain why 1 µm Se induced high Cd concentrations in leaves. Cadmium exposure interfered Se concentrations in plant tissues. For instance, the presence of Cd caused a severe reduction of Se content in all plant tissues (Table 1). Cd is well known to induce the expression of sulfur-assimilating enzymes and sulfate uptake by roots (Yamaguchi et al. 2016), leading to increased Se uptake, mainly in the form of selenate, mediated by S transporters. However, in our work, plants exposed to Cd showed lower plant growth, reduction in Se content and nutrient uptake. This fact may be due to the Se source. Selenite is easily transformed into organic forms in roots; these forms are more difficult to transport above to parts of the plant, and they compete with other ions in the cell and do not cooperate to reduce Cd in leaves.
Cadmium triggers an overproduction of ROS, causing oxidative stress in fundamental cellular structures such as lipids and proteins, which leads to an imbalance in plant metabolism (Gratão et al. 2015, Alves et al. 2017). A number of recent studies indicate that low concentrations of Se can modulate oxidative stress caused by Cd exposure (Abd et al. 2016, Alyemeni et al. 2018). For instance, plants with Cd exhibited an intense increase in lipid peroxidation when compared to plants with higher concentrations of Se under Cd stress (Fig. 3a). Furthermore, leaves with 1 µm Se reduced MDA content by 57% in the presence of Cd. The attenuation of Cd-induced toxicity by Se may be attributed either to Se-triggered reactivation of membrane enzymes and subsequent restoration of metabolite transport or the competition of Se with Cd for some key specific binding sites, such as thiol groups of cysteine (Feng et al. 2013). Moreover, Se can induce ROS scavenging through dismutation of superoxide anions to form H2O2 without the involvement of superoxide dismutase (Cartes et al. 2010) or selenocompounds can quench superoxide anions and hydroxyl radicals directly (Xue et al. 1993).
H2O2 content was high in leaves treated with 1 µm Se application; however, at higher concentrations, the H2O2 content was lower. The presence of Cd can produce higher H2O2 production of 16% in leaves with 0 µm Se application when compared with control plants. The application of Se efficiently decreased H2O2 production triggered by Cd exposure, mainly at 1 µm Se application (Fig. 3b). These data reinforce the conclusion that Se application leads to a reduction in ROS production, which contributes to the maintenance of the structural and functional integrity of membranes even under Cd stress (Alyemeni et al. 2018).
Plants increase the synthesis and reduce the degradation of proline, a protective metabolite which contribute to the stabilization of protein molecules and membranes, as a direct response against stress (Zouari et al. 2016). Under Cd exposure, proline content was slight lower in leaves not treated with Se, which could indicate that Cd stress was enough to enhance proline synthesis (Fig. 4). On the other hand, leaves with 10 µm Se exhibited higher proline content of 17%. It is well known that proline has a fundamental role in osmoregulation and ROS scavenging (Kaur and Asthir 2015). Se can increase proline content by inducing up-regulation proline-synthesizing enzymes concomitant with a reduction of catabolic enzymes (Abd et al. 2016, Alyemeni et al. 2018).
Antioxidant enzymes allow plants to avoid oxidative stress and to survive Cd stress conditions (Gratão et al. 2015). Strong evidence indicates that Se can improve the activity of antioxidant enzymes under normal and stress conditions (Abd et al. 2016, Alyemeni et al. 2018, Handa et al. 2018). The antioxidant enzymes SOD, APX, CAT and GR were selected based on their responses in a number of reports and the role of Se during Cd stress in plants. These enzymes exhibited varied responses among Se applications, Cd exposures and tissue types. SOD, which converts O2 into H2O2, has been increased in several plant species when exposed to Cd and Se (Alyemeni et al. 2018). Different isoenzymes contribute to cell protection against different toxic substances, such as Cd.
In this study, three SOD isoenzymes (SOD I, II and III) were identified following non-denaturing PAGE analysis (Fig. 5), with a similar isoenzyme classification as previously reported by Gratão et al. (2015) and Alves et al. (2017) for MT plants. Mn-SOD (SOD I), Fe-SOD (SOD II) and Cu/Zn-SOD (SOD III) isoenzymes were detected in leaves (Fig. 5a), and Mn-SOD (SOD I) and Fe-SOD (SOD II) were identified in roots (Fig. 5b). The activity of SOD I in leaves was not affected by any treatment. SOD I was more prevalent in roots in the presence of Cd (Fig. 5, line 5–8), but a slight increase occurred with the application of Se (line 6–8). It is well known that Cd exposure can induce upregulation of Mn-SOD at both transcript and activity levels (Rodríguez-Serrano et al. 2006), which supports a slight increase in Mn-SOD activity in roots under Cd stress conditions. Moreover, Se can contribute to an increase in its activity and a reduction in lipid peroxidation. SOD II was lower in leaves and roots due to Cd exposure (Fig. 5a, b, line 5–8), but the application of Se slightly higher its activity in roots (Fig. 5b, line 6–8). Cd can affect the expression and regulation of this isoenzyme (Romero-Puertas et al. 2007). Moreover, we can observe that the Fe content in roots and leaves under Cd exposure was lower when compared to plants without Cd exposure (Table 3). SOD III in leaves was completely inhibited by Cd presence; therefore, this isoform was visible only in 0 mM CdCl2 treatments (Fig. 5a, lines 1–4). Plants treated with 5 µm Se exhibited higher Zn concentration (Table 3), which may improve Cu/Zn-SOD activity (SOD III) (line 3). Moreover, Se has synergistic effects on the transcription of the Cu/Zn-SOD isoform (Seppänen et al. 2003), which can provide extra strength to tomato plant defences to counteract Cd stress.
CAT (Fig. 6a), APX (Fig. 6b) and GR (Fig. 6c) activities are crucial for the detoxification of any excess H2O2 produced by SOD and/or by other metabolic processes. The current literature contains mixed results concerning changes in CAT activity with Se application. For instance, Alyemeni et al. (2018) observed that Se application increased CAT activity in tomatoes. However, a study conducted by Saidi et al. (2014) showed that only Se did not alter CAT activity in sunflowers. Our data are in accordance with this finding, where the application of Se did not alter CAT activity in either the root or the leaves (Fig. 6a). When Cd was applied, an increase was observed in all treatments and was more pronounced at 10 µm Se. Similar results were observed by Lin et al. (2012) for rice, Khan et al. (2015) for Brassica juncea and Alyemeni et al. (2018) in tomato plants.
The glutathione-ascorbate cycle is a fundamental metabolic pathway that converts H2O2 to H2O and O2. This cycle involves antioxidant metabolites, such as ascorbate, glutathione and NADPH, and key enzymes, such as APX and GR. In the present study, leaves with 1 µm and 5 µm Se exhibited a significant higher in APX activity. Under Cd exposure, we observed higher APX activity in the roots in 1, 5 and 10 µm Se treatments, which may protect plant metabolism by nullifying the effects of ROS generated by Cd stress. Furthermore, Khan et al. (2015) demonstrated that selenium induced the upregulation of APX and GR in B. juncea, thus protecting photosynthetic electron transport from cadmium-induced oxidative damage via the maintenance of the NADP/NADPH ratio.
GR activity was more pronounced in leaves with 5 µm Se compared with other Se treatments without Cd exposure. Nevertheless, when Cd was applied, GR exhibited 62% higher in leaves and 70% in roots when compared with control plants. Plants treated with 10 µm Se under Cd stress exhibited a considerable higher GR activity of 65 and 84%, respectively, in leaves and roots when compared with 0 µm Se application under Cd exposure (Fig. 6c). Wu et al. (2017) indicated that Se may play a role in enhancing the efficiency of GSH-AsA under Cd exposure, and higher activity of GR by Se application may induce higher levels of GSH and AsA due to Se boosting GSH synthesis, which has a regulatory role in methylglyoxal detoxification and upregulation of GR (Wu et al. 2017). The different patterns of SOD, CAT, APX, and GR activities indicate that Se plays a fundamental role in optimizing the performance of the antioxidant system for alleviating Cd stress.
Conclusion
Oxidative stress triggered by Cd causes a serious cell imbalance between ROS production and antioxidant enzymes, which leads to intense physiological disorders in plants. The results clearly indicate distinct trends in plants treated with Se under Cd exposure. Thus, our findings demonstrate that Se application is an efficient management technique that can be used to alleviate deleterious effects of Cd-stress conditions and enhance Cd tolerance by improving the nutritional status, exhibited higher proline content and activity of ROS-scavenging enzymes in MT plants. However, the exactly way that Se promotes these benefits in plants remains unknown. Thus, is necessary further studies exploring signalling molecules, hormones and other pathways to comprehend how Se acts in plants metabolism.
References
Abd AEF, Hashem A, Alqarawi AA (2016) Mitigation of cadmium induced stress in tomato. (Solanum lycopersicum L.) by selenium. Pak J Bot 48:953–961
Alves LR, Monteiro CC, Carvalho RF, Cury PR, Tezotto T, Azevedo RA, Gratão PL (2017) Cadmium stress related to root-to-shoot communication depends on ethylene and auxin in tomato plants. Environ Exp Bot 134:102–115
Alves LR, Reis AR, Gratão PL (2016) Heavy metals in agricultural soils: from plants to our daily life(a review). Cientifica 44:346–361
Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P (2018) Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 255:459–469
Azevedo RA, Alas RM, Smith RJ, Lea PJ (1998) Responses of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and catalase-deficient mutant of barley. Physiol Plant 104:280–292
Barbosa JC, Maldonado W Jr (2009) Software AgroEstat:Sistema de análises estatísticas de ensaios agronômicos. Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias, Jaboticabal, SP, Brasil
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Boaretto LF, Carvalho G, Borgo L, Creste S, Landell MGA, Mazzafera P, Azevedo RA (2014) Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiol Biochem 74:165–75
Borges KLR, Hippler FWR, Carvalho MEA, Nalin RS, Matias FI, Azevedo RA (2019) Nutritional status and root morphology of tomato under Cd-induced stress: comparing contrasting genotypes for metal-tolerance. Sci Hortic 246:518–527
Bradford MMA (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cartes P, Jara AA, Pinilla L, Rosas A, Mora ML (2010) Selenium improves the antioxidant ability against aluminium‐induced oxidative stress in ryegrass roots. Ann Appl Biol 156(2):297–307
Castillo-Godina RG, Foroughbakhch-Pournavab R, Benavies-Mendonza A (2016) Effect of selenium on elemental concentration and antioxidant enzymatic activity of tomato plants. J Agr Sci Technol 18:233–244
Chmielowska-Bak J, Gzyl J, Rucinska-Sobkowiak R, Arasimowicz-Jelonek M, Deckert J (2014) The new insights into cadmium sensing. Front Plant Sci 5:1–13
Chilimba ADC, Young SD, Black CR, Rogerson KB, Ander EL, Watts MJ, Lammel J, Broadley MR (2011) Maize grain and soil surveys reveal suboptimal dietary selenium intake is widespread in Malawi. Sci Rep 1:1–9
Clemens S, Feng MaJ (2016) Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol 67:1–12
Edelstein M, Ben-Hur M (2018) Heavy metals and metalloids: sources, risks and strategies to reduce their accumulation in horticultural crops. Sci Hortic 234:431–444
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Gratão PL, Monteiro CC, Carvalho RF, Tezotto T, Piotto FA, Peres LEP, Azevedo RA (2012) Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiol Biochem 56:79–96
Gratão PL, Monteiro CC, Tezotto T, Carvalho RF, Alves LR, Peters LJ, Azevedo RA (2015) Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. BioMetals 28:803–816
Handa N, Kohli SK, Sharma A, Thukral AK, Bhardwaj R, Alyemeni MN, Wijaya L, Ahmad P (2018) Protective role of selenium against chromium stress involving metabolites and essential elements in Brassica juncea L. seedlings. 3Biotech 8:66
Huang G, Ding C, Guo F, Li X, Zhang T, Wang X (2017) Underlying mechanisms and effects of hydrated lime and selenium application on cadmium uptake by rice (Oryza sativa L.) seedlings. Environ Sci Pollut Res 24:18926–18935
Kaur G, Asthir B (2015) Proline: a key player in plant abiotic stress tolerance. Bio Plant 59:609–619
Kaur S, Kaur N, Siddique KH, Nayyar H (2016) Beneficial elements for agricultural crops and their functional relevance in defence against stresses. Arch Agron Soil Sci 62:905–920
Khan MIR, Nazir F, Asgher M, Per TS, Khan NA (2015) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Li HF, McGrath SP, Zhao FJ (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102. https://doi.org/10.1111/j.1469-8137.2007.02343.x
Li X, Li B, Yang Y (2018) Effects of foliar selenite on the nutrient components of turnip (Brassica rapa var. rapa Linn.). Front Chem 6:42
Lichtentlaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Lin L, Zhou W, Dai H, Cao F, Zhang G, Wu F (2012) Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J Hazard Mater 235:343–351
Lux A, Martinka M, Vaculík M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37
Ma Q, Cao X, Tan X, Si L, Wu L (2017) Effects of cadmium stress on pakchoi (Brassica chinensis L.) growth and uptake of inorganic and organic nitrogenous compounds. Environ Exp Bot 137:49–57
Meissner R, Jacobson Y, Melamed S, Levyatuv S, Shalev G, Ashri A, Elkind Y, Levy A (1997) A new model system for tomato genetics. Plant J 12:1465–1472
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 3:1476–1489
Noctor G, Foyer CH (2016) Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol 171:1581–1592
Noctor G, Reichheld JP, Foyer CH (2018) ROS-related redox regulation and signaling in plants. Semin Cell Dev Biol 80:3–12
Nogueirol RC, Monteiro FA, Gratão PL, Borgo L, Azevedo RA (2015) Tropical soils with high aluminum concentrations cause oxidative stress in two tomato genotypes. Environ Monit Assess 187:73
Pezzarossa B, Rosellini I, Borghesi E, Tonutti P, Malorgio F (2014) Effects of Se-enrichment on yield, fruit composition and ripening of tomato (Solanum lycopersicum) plants grown in hydroponics. Sci Hortic 165:106–110
Rabêlo FHS, Lux A, Rossi ML, Martinelli AP, Cuypers A, Lavres JJ (2018) Adequate S supply reduces the damage of high Cd exposure in roots and increases N, S and Mn uptake by Massaigrass grown in hydroponics. Environ Exp Bot 148:35–46
Reis AR, Barcelos JPQ, Osório CRWS, Santos E, Lisboa LAM, Santini JMK, Santos MJD, Furlani Jr E, Campos M, Figueiredo PAM, Lavres J, Gratão PL (2017) A glimpse into the physiological, biochemical and nutritional status of soybean plants under Ni-stress conditions. Environ Exp Bot 144:78–87
Reis HPG, Barcelos JPQ, Furlani Junior E, Santos EF, Silva VM, Moraes MF, Putti FF, Reis AR (2018) Agronomic biofortification of upland rice with selenium and nitrogen and its relation to grain quality. J Cereal Sci 79:508–515
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, delRío LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisumsativum L.) roots: imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Romero-Puertas MC, Corpas FJ, Rodríguez-Serrano M, Gomez M, delRío LA, Sandalio LM (2007) Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. J Plant Physiol 164:1346–1357
Saidi I, Nawel N, Djebali W (2014) Role of selenium in preventing manganese toxicity in sunflower (Helianthus annuus) seedling. S Afr J Bot 94:88–94
Santos EF, Macedo FG, Zanchim BJ, Lima GPP, Lavres J (2017) Prognosis of physiological disorders in physic nut to N, P, and K deficiency during initial growth. Plant Physiol Biochem 115:249–258
Schützendubel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 127:887–898
Sebastian A, Prasad MNV (2015) Trace element management in rice. Agron 5:374–404
Seppänen M, Turakainen M, Hartikainen H (2003) Selenium effects on oxidative stress in potato. Plant Sci 165:311–319
Silva VM, Boleta EHM, Lanza MGDB, Lavres J, Martins JT, Santos EF, Broadley MR (2018) Physiological, biochemical, and ultrastructural characterization of selenium toxicity in cowpea plants. Environ Exp Bot 150:172–182
Sun H, Dai H, Wang X, Wang G (2016) Physiological and proteomic analysis of selenium-mediated tolerance to Cd stress in cucumber (Cucumis sativus L.). Ecotoxicol Environ Saf 133:114–126
Vincent JM (1975) Manual practico de rizobiologia, 1st ed. Hemisferio Sur, Buenos Aires
Xue T, Hou S, Tan J, Liu GL (1993) The antioxidative function of selenium in higher plants: II. Non-enzymatic mechanisms. Chin Sci Bull 38:356–358
Wan Y, Yu Y, Wang Q, Qiao Y, Li H (2016) Cadmium uptake dynamics and translocation in rice seedling: influence of different forms of selenium. Ecotoxicol Environ Saf 133:127–134
Wu Z, Liu S, Zhao J, Wang F, Du Y, Zou S, Huang Y (2017) Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ Exp Bot 133:1–11
Wu D, Yamaji N, Yamane M, Kashino-Fujii M, Sato K, Ma JF (2016) The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiol 172:1899–1910
Yamaguchi C, Takimoto Y, Ohkama-Ohtsu N, Hokura A, Shinano T, Nakamura T, Suyama A, Maruyama-Nakashita A (2016) Effects of cadmium treatment on the uptake and translocation of sulfate in Arabidopsis thaliana. Plant Cell Physiol 57:2353–2366
Yao X, Jianzhou C, Xueli H, Binbin L, Jingmin L, Zhaowei Y (2013) Effects of selenium on agronomical characters of winter wheat exposed to enhanced ultraviolet-B. Ecotoxicol Environ Saf 92:320–326
Zayed AM, Terry N (1992) Selenium volatilization in broccoli as influenced by sulfate supply. J Plant Physiol 140:646–652
Zembala M, Filek M, Walas S, Mrowiec H, Kornas A, Miszalski Z, Hartikainen H (2010) Effect of selenium on macro- and microelement distribution and physiological parameters of rape and wheat seedlings exposed to cadmium stress. Plant Soil 329:457–468
Zhu G, Xiao H, Guo Q, Zhang Z, Zhao J, Yang D (2018) Effects of cadmium stress on growth and amino acid metabolism in two compositae plants. Ecotoxicol Environ Saf 158:300–308
Zouari M, Ahmed CB, Elloumi N, Bellassoued K, Delmail D, Labrousse P, Abdallah FB, Rouina BB (2016) Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defence system of Oleaeuropaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol Environ Saf 128:195–205
Acknowledgements
The authors thank to Prof. Dr José Carlos Barbosa (Universidade Estadual Paulista—UNESP—Faculdade de Ciências Agrárias e Veterinárias. Departamento de Ciências Exatas, Brazil) for the collaboration in statistical analyses.
Funding
This work was funded by Fundação de Amparo à Pesquisa do Estadode São Paulo (FAPESP - Grant n°2017/04787-6); PLG also thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the research fellowship (Grant nº 314380/2018-3) – Brazil; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (L.R.A.) for the scholarship granted (Finance Code 001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Alves, L.R., Prado, E.R., de Oliveira, R. et al. Mechanisms of cadmium-stress avoidance by selenium in tomato plants. Ecotoxicology 29, 594–606 (2020). https://doi.org/10.1007/s10646-020-02208-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02208-1