Abstract
Stroke is one of the main causes of mortality and disability in most countries of the world. The only way of managing patients with ischemic stroke is the use of intravenous tissue plasminogen activator and endovascular thrombectomy. However, very few patients receive these treatments as the therapeutic time window is narrow after an ischemic stroke. The paucity of stroke management approaches can only be addressed by identifying new possible therapeutic targets. Mitochondria have been a rare target in the clinical management of stroke. Previous studies have only investigated the bioenergetics and apoptotic roles of this organelle; however, the mitochondrion is now considered as a key organelle that participates in many cellular and molecular functions. This review discusses the mitochondrial mechanisms in cerebral ischemia such as its role in reactive oxygen species (ROS) generation, apoptosis, and electron transport chain dysfunction. Understanding the mechanisms of mitochondria in neural cell death during ischemic stroke might help to design new therapeutic targets for ischemic stroke as well as other neurological diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a medical emergency in which the diminution of blood flow to the brain ends in the death of brain cells. Stroke is of two types’ ischemic stroke and hemorrhagic stroke. Most of the stroke cases are ischemic in nature and contributes for about 87% of cases. It is considered second most common cause of mortality and the leading cause of adult disability all over worldwide. Stroke is also the third most common cause of impairment (4.5%) after ischemic heart disease (6.1%) (Thrift et al. 2014). According to the statistical data from the World Health Organization (WHO), it has been determined that 16.9 million people suffer from stroke each year. With the increasing lifespan of the population worldwide, the prevalence of stroke is expected to rise in the future. The prevalence of stroke in men is 1.5 times greater than in women (Ahangar et al. 2017). According to epidemiological surveys, the number of stroke survivors will keep on rising to 77 million by the year 2030 adding more to the socioeconomic burden.
Stroke is the third leading cause of death and more than 140,000 people die each year from stroke in the USA. Asia is one of the significant contributors of age-related neurological diseases that is driven by demographic changes and increased by the increasing prevalence of the vital modifiable risk factors. The people of Asia have been reported to have a higher risk of stroke and consequent fatality than their Caucasian counterpart. In some countries such as India, Pakistan, and Indonesia, high death rates are still recorded due to limited resources and poor public awareness in comparison to Japan, Korea, and China which have better post-stroke management strategies (Wasay et al. 2014).
In Europe, the age–related incidences of stroke vary from 94 to 288/100,000 per annum, with monthly mortality rates ranging from 12 to 36% (Mozaffarian et al. 2016). Approximately 1 million people of Europe suffering a stroke every year, due to aging population; the stroke cases are expected to increase dramatically to 1.5 million by 2030 (Bejot et al. 2016).
Pathogenesis of ischemic stroke
Among the neurological diseases related with aging, stroke is one of the common causes of disability and death worldwide (Thakur et al. 2016). The term “stroke” can be defined into three categories: brain ischemia, intracerebral hemorrhage, and subarachnoid hemorrhage. Global cerebral ischemia occurs when the blood flow to the brain is diminished affecting wide areas of the brain. This is often triggered by a cardiac arrest or an arrhythmia. The damage depends upon the span of time the circulation is blocked; the more time it takes for reperfusion, the greater the damage will occur (Karsy et al. 2017).
Brain ischemia can also classify as focal and multifocal stroke. Focal cerebral ischemia is characterized by the blockade of a cerebral vessel that supplies blood to a cerebral region of the brain, which in turn increases the risk of cell death. (Takatsuru et al. 2014). Reperfusion is essential for protecting the injured brain tissue, but it can also lead to reperfusion injury by exacerbating the damage despite restoring the circulation (Siket et al. 2016).
The reperfusion following stroke takes place in a variety of tissues including the brain, kidney, and heart. Further injury due to reperfusion is a result of the accumulation of excessive ROS and Ca2+ in the tissues. The mechanism involved in the reperfusion tissue damage occurs at both the cellular and molecular levels (Sims et al. 2017). The excessive generation of ROS overpowers the anti-oxidative defense system in the cells, which then becomes unable to scavenge free radicles leading to cellular demise. Reperfusion-promoted ROS damages the proteins, lipids, and DNA, which causes a direct damage to mitochondrial functions (Borutaite et al. 2010). The damage to the mitochondrial functions after reperfusion injury leads to reduced activity and more attenuation of ATP levels. Oxidative stress after reperfusion has also been associated with mitochondrial DNA damage. ROS may also interact and alter signal transduction that interferes with or initiates cell death by apoptosis or necrosis (Kiselyov et al. 2016). ROS and Ca2+accumulation increase the membrane permeabilization and cell death.
There is a paucity of treatments for stroke, partly owing to its complicated understanding of the diverse cellular and molecular cascades that occur after ischemic stroke. Experimental studies have already begun to determine the cellular and molecular mechanisms involved in stroke injury, in order to find pharmacologically active agents that target various pathways related to injury (Khoshnam et al. 2017). The diminution of blood supply that underlies a stroke results in degeneration and death of neurons because of an abrupt attenuation in oxygen and glucose. The understanding of cellular events that lead to ischemic neuronal injury begins long before. Synapses, which are often located at a relative distance from the neuronal body, are the sites where the neurodegenerative process may be progressed in ischemic stroke. In addition to its deteriorating effects on neurons, ischemic injury greatly affects glial cells including astrocytes, microglia, and oligodendrocytes (Song et al. 2017). Each type of glial cell plays an important role in modulating neuronal physiology and functions, and it is, therefore, essential to understand their responses to cerebral ischemia and their effect on brain cells (Taylor and Sansing 2013). Focal cerebral ischemia results in characteristic histopathological changes that manifest as a necrotic core of tissue at the center of the infarcted cortex in which all cells die rapidly. It is the cells in the penumbral region of the ischemic territory that can be prevented by therapeutic intervention after a stroke (Fan et al. 2017).
Experimental models of ischemic stroke
In order to study the molecular and cellular mechanisms involved in neuronal dysfunction in stroke, diverse animal as well as cell-culture models that simulate stroke like conditions have been developed (Buoncervello et al. 2017). The practice of animal models from past two decades have gained better understanding of complicated mechanisms involved in the ischemic stroke. The rodents such as rats are the most often used species, with a growing focus on larger species and even on non-human primates, for the investigations of finding a possible druggable targets for stroke. The rat is still the most commonly used because of its numerous advantages (Archer et al. 2017). The physiology and cerebral vasculature of rats is like that of human. The suitable size and more reproducibility make rats more promising models for the stroke research. Mice are also considered as suitable models for research because of their genetic similarity with the human genome (Azad and Haddad 2013). It has been widely used in the transgenic studies for the experiments of stroke mechanisms.
The animal models include the transient global forebrain ischemia model, in which the entire blood supply to the brain is blocked (Rehni et al. 2017). Another is the focal cerebral ischemia model, in which the middle cerebral artery is occluded, resulting in damage to the cerebral region of the brain.
The focal model can be transient or permanent, while transient is considered as more similar to clinical setting (Sommer 2017). The number of in vivo models from MCA (middle cerebral artery) to proximal occlusions in stroke varies widely in their applications for study purposes. The most frequent ones are middle cerebral artery occlusion (MCAO) models for their profound advantages and less surgical complicities (El Amki et al. 2015). The tMCAO or pMCAO (transient or permanent) are achieved by directly inserting a silicon-coated nylon suture into the internal carotid artery then advances to the circle of Willis and blocks the MCA origin. The time of occlusion determines the severity of ischemic damage and neuronal death. In the case of permanent pMCAO presentation, the suture is not removed from the artery and therefore no reperfusion injury occurs. The other craniotomy induced models to include dMCAO (distal middle cerebral artery occlusion) that directly exposes the MCA branches. The damage with dMCAO is less than that of pMCAO models. The other way of generating ischemic models is by using thrombin-induced clot formation autologous to that of the arteries (Ma et al. 2012). One more way to establish an ischemic model is the use of intracerebral injection of endothelin -1 (ET-1), which is a vasoconstrictor that reduces the blood flow. It has been reported that rats are the most reliable species for this model than mice. It has been credited to have made a substantial contribution for finding a novel neuroprotective mechanism for stroke (Rousselet et al. 2012).
Global ischemia models can develop by several ways. The highly predictable brain damage model is “four-vessel occlusion method” (4VO) which consists of a reversible cerebral carotid artery (CCA) occlusion. The other way of developing the global model is to occlude the two common carotid arteries of the brain (Neumann et al. 2013). Global ischemia is determined by the critical attenuation of cerebral blood flow to the whole brain.
The major focus of stroke research has been to identify the pathophysiology of ischemic neuronal injury with prime objective to improve functional outcomes. Neurons are more prone to ischemic injury than other types of cells because they always depend on a constant supply of energy. Neurons are excitable cells and express high levels of receptors for excitatory transmitters, which are concentrated in synapses (Schmidt and Minnerup 2016). The most critical components of tissue damage in stroke are the deprivation of oxygen and glucose. The most widely used in vitro models of stroke are oxygen-glucose deprivation model (OGD). The other ways to produce the in vitro models are glutamate-induced excitotoxicity and hydrogen peroxide insults. The studies on cultured neurons or brain slices have contributed immense to understand mechanisms of ischemic injury and neuroprotection (Skelding et al. 2014). The primary neuronal cultures provide a powerful access to study the molecules involved in the cascade of reactions within a brain cell. They are considered much better than secondary cultures for the purpose of investigating the molecular pathways (Roque and Baltazar 2017).
Excitotoxicity
Excitotoxicity is a specific form of neurotoxicity that arises when there is a prolonged release of glutamate neurotransmitter (Puyal et al. 2013). It acts on around 30–40% of synapses in the central nervous system (CNS) and is regulated in a systematic manner to maintain specific levels in the vesicles. Glutamate is one of the prime neurotransmitters of the brain crucial for synaptic transmission, critical for communication of neurons, synaptic growth, neuronal growth, brain development, and synaptic plasticity (Vandenberg and Ryan 2013). Glutamate acts through three families of receptors, a-amino-3-hydroxy-5-methyl-4 isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA), and kainate receptors for its actions (Bettler and Fakler 2017). Among these ionotrophic and metabotropic receptors, N-methyl-D-aspartate receptor (NMDAR) acts as a main point of target for the signaling processing of glutamate into diverse signaling pathways (Banerjee et al. 2016; Brassai et al. 2015). NMDAR is most often implicated in the neuronal death in ischemic stroke. Ischemia stimulation of NMDA receptors takes place by glutamate and glycine which acts as co-agonists. The co-agonistic binding of glycine is critical for this receptor to be stimulated by glutamate. Its stimulation causes the entry of Ca2+ into the cells as it is directly linked to the Ca2+ channels. Under physiological conditions, the cytosolic Ca2+ is stable and low, around 10,000 times lower than that of the extracellular space. But in pathological conditions, such as in ischemic reperfusion injury, the over-stimulation of the NMDA receptors causes more entry of Ca2+ into the cells thereby mediating the excitotoxicity. The accumulation of Ca2+ is highly toxic for the cells, promoting cerebral edema and activation of intracellular self-destruction cascade. Upon excitotoxicity-induced elevation of the Ca2+ level, mitochondria takes up the excessive Ca2+ that causes the swelling of the organelle and formation of the mtPTP. The accumulation of Ca2+ in the intracellular space potentiates the Ca2+ dependent processes that execute and activate the apoptotic and death pathways (Ma et al. 2017a).
Mitochondria and ischemic stroke
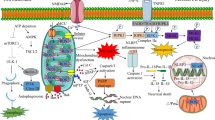
Mitochondria are at the center stage of cell survival and cell death, and the processes to pathophysiological conditions convert mitochondria from life-sustaining organelle to an activator of cell death ((Andrabi et al. 2019; Andrabi et al. 2015). (Vakifahmetoglu-Norberg et al. 2017). These organelles are the main players in the ischemic cell death not only by the impairment of ATP generation but also by playing a role in the cell death pathways such as apoptosis and necrosis (Jordan et al. 2011). Mitochondria are important for processes such as cellular bioenergetics, control intracellular Ca2+ homeostasis, and participate in important metabolic functions. Mitochondrion contains an outer phospholipid bilayer membrane, an intermembrane space, a complex inner phospholipid bilayer, and a mitochondrial matrix (Amigo et al. 2016). The outer membrane contains voltage-dependent anionic channels (VDACs), also known as mitochondrial porin proteins, which make it permeable to small molecules during normal physiological processes (Maldonado and Lemasters 2014). The intermembrane space is for vital roles such as the transportation of proteins across mitochondrial membranes, and oxidative phosphorylation (Andrabi et al. 2017). The inner membrane is freely permeable to oxygen, carbon dioxide, and water. It contains multiple folds called cristae which increase the surface area for vital chemical reactions. The inner mitochondrial matrix also contains the citric acid cycle reaction enzymes and substrates for several metabolic processes (Grimm and Eckert 2017).
Mitochondria produce energy and their numbers are high in brain cells, as they require a significant amount of ATP for the functioning (Fidaleo et al. 2017). Mitochondria use electron transport chain (ETC) to produce usable energy from electron donors like reduced nicotinamide adenine dinucleotide (NADH) through different oxidation/reduction reactions. These molecules transfer electrons and facilitate transmembrane proton transport resulting in an electrochemical gradient that drives adenosine triphosphate (ATP) synthesis in the mitochondria (Gollihue and Rabchevsky 2017). The ETC consists of five enzyme complexes that are comprised of integral inner membrane proteins, NADH-CoQ reductase, CoQ-cytochrome c reductase, Succinate-CoQ reductase, cytochrome c oxidase, and ATP synthase (complexes I-V) (Piotrowska and Bartnik 2014). The ubiquinone (CoQ) and cytochrome c are two freely diffusible molecules that reconcile the transfer of electrons between complexes, and oxygen is the final acceptor of electrons in this reaction that produces the electrochemical proton gradient across inner mitochondrial membrane (IMM) (Ponnalagu and Singh 2017). This ETC is an important source of ROS generation, especially after reperfusion, and wealth of evidence has demonstrated that mitochondrial ETC is the major source of ROS in reperfusion injury, which deteriorates the ischemic cell death (Tang et al. 2016). The substantial deficit in ATP production and increase of ROS after ischemia set the mitochondrial death pathways active via apoptosis (Bakthavachalam and Shanmugam 2017). The production of ATP in brain cells is abruptly reduced following the outset of ischemia, resulting in the impairment of membrane-bound channels that are very critical for normal functioning.
The experimentally documented results have shown that the levels of oxidative damage to proteins, lipids, and DNA in these stroke models are high. The mechanism by which lipid peroxidation disrupts neuronal ion homeostasis and induces apoptosis involves the production of a toxic aldehyde called 4-hydroxynonenal, which binds the membrane molecules impairing their normal functions (Andrienko et al. 2017). A major site of ROS production is the mitochondria in which formation of superoxide anion radical, a precursor to other potentially destructive ROS molecules include hydrogen peroxide, hydroxyl radical, and peroxynitrite. Mitochondrial dysfunction occurs as the consequences of oxidative stress, energy failure, and disruption of cellular calcium homeostasis. Reperfusion promoted mitochondrial ROS, and Ca2+ triggers the death pathways through the caspase-dependent or caspase-independent processes (Rodriguez-Lara et al. 2016). Mitochondria being the center for the release of various factors that induce the caspase-dependent (Cytochrome c) and caspase-independent (AIF) death (Thornton and Hagberg 2015). In ischemia, these events are quite dramatic and promote both apoptotic and necrotic death of brain cells.
Oxidative stress and ischemic stroke
Oxidative stress is one of the major detrimental factor of the pathophysiology of cerebral ischemia, and ROS generation normally occurs during many pivotal biological processes such as energy production, cell signaling, and gene transcription (Drose et al. 2016; Ertracht et al. 2014). Ischemia-elicited malfunctioning of the respiratory chain in mitochondria produces oxidative stress in the cell that may be overwhelming for mitochondrial defense system (Rekuviene et al. 2017). The less oxygen in mitochondria limits the oxidative phosphorylation, reducing the ATP production. This leads to the release of free radicals from the ETC and generation of ROS in the mitochondria. This ROS production is more after the reperfusion injury that damages the macromolecules of the cell and triggers the cellular processes ranging from alteration of cell signaling pathways to brain cell death.
Mitochondria are the great contributor of cellular ROS through various metabolic reactions that convert molecular oxygen into superoxide (O−2) and hydrogen peroxide (H2O2). These include oxidative phosphorylation and other enzymatic reactions, e.g., xanthine oxidase (XO), nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase, cyclooxygenases (COX), and oxidation of unsaturated fatty acids (Granger and Kvietys 2015). The ETC is the major source of O−2 generation, while mitochondria reduce the O2 to H2O by cytochrome c oxidase during oxidative phosphorylation. There are various sites in the mitochondria that partially reduce the oxygen to generate ROS, especially the ubiquinone-cytochrome c site has been considered as an extensive source of ROS in ischemia (Korge et al. 2017). Mitochondrial succinate formation during ischemia is also a considerable source of ROS in the ischemia injury (Wijermars et al. 2016). The accumulated succinate is rapidly re-oxidized driving major ROS formation, particularly after reperfusion due to reversal electron transport at complex I. This overload of free radicals includes hydroxyl radicals (OH), O2−, H2O2, nitric oxide (NO), and peroxynitrite (OONO−) (Thompson et al. 2012). The free radicle OH is another molecule that is generated from H2O2 in the presence of ferrous iron that has been reduced by O2−. H2O2 is accumulated by the dismutation of O2− or direct reduction of oxygen and is highly lipid soluble. These highly reactive free radicles not only damage the macromolecules but also activate the cellular pathways that cause the ischemic damage to the brain. Redox signaling can be referred to as the particular oxidative and reductive mediators of the cellular pathways by various free radicals such as O2−. RNS further elevates the ROS signaling in the cells. The NO produced by particular nitric oxide synthases (NOSs), such as neuronal (nNOS), inducible (iNOS), and endothelial (eNOS) bind with O2− to produce the nitrosative stress (Yang et al. 2017). The redox signaling is one of the important aspects after reperfusion and several molecules which are involved in the redox signaling. NADPH oxidase generates ROS in the brain that is critical for the host immune system. NADPH oxidase has been identified as a source of ROS formation in the brain cells after ischemic stroke (Rastogi et al. 2016). After reperfusion, NADPH oxidase produces ROS, which is sensitive to the hypoxic conditions that elicit its upregulation through the hypoxia inducible factor (HIF) (Ma et al. 2017b). There is growing evidence that a number of isoforms of NOX family is involved in a variety of neurological diseases. In addition to NADPH, xanthine oxidase (XO), another enzyme in the brain acts as a source of ROS by generating the O2−. Ischemia induces the activation of XO that causes the brain edema after ischemia/reperfusion. Some other enzymes that catalyze the production of ROS are COX, lipoxygenase, and cytochrome c P450 (Yagami et al. 2016).
Mitochondria permeability transition pore and ischemic stroke
Mitochondria permeability transition pore (mtPTP) is stated as a regulatory pore that controls the exchange of molecules between the mitochondria matrix and cytoplasm (Perez and Quintanilla 2017). The component of mtPTP that forms or regulates the pore is not completely elucidated. The most common view is that it is constituted of three proteins, one voltage-dependent anionic channel (VDAC) in the OMM and another is adenine nucleotide translocase (ANT) in the IMM. The third protein that is proposed to be a part of it is residing inside the mitochondrial matrix is called cyclophilin D. Cyclophilin D is vital for the regulation of mtPTP and plays a crucial role in the neuronal ischemic death (Alam et al. 2015). The other proteins that are linked to be associated with the pore are hexokinase and Bcl-2 family proteins. The long-debated hypothesis is that mtPTP transits through both mitochondrial inner and outer membranes and composed of both membrane proteins and matrix (Brenner and Moulin 2012). The emergence of inhibitors and binders to the mtPTP led to the discovery of the abovementioned basic components of the pore. The proteins ANT and VDAC form the multimeric pore by binding the contact sites of inner and outer mitochondrial membrane along with matrix protein cyclophilin D. Recently, experimental studies have demonstrated that the null mice of Vdac1-, Vdac3-, and Vdac1-Vdac3- exhibited a Ca2+ and oxidative stress from wild-type mice raising serious doubts about mtPTP model (Baines et al. 2007). Bcl-2 family proteins such as Bax and Bak are components of the pore in the outer mitochondrial membrane, and the current data strongly propose that dimers of ATP synthase form mtPTP, as well as cyclophilin D binds to the lateral stalk of ATP synthase (He et al. 2017). Cyclophilin D binds to the oligomycin sensitivity conferring protein (OSCP) subunit of ATP synthase which triggers the opening of mtPTP (Karch and Molkentin 2014). Due to the emergence of this new model of mtPTP, the association of ATP synthase cannot explain the regulation of mtPTP modulators such as bongkrekic acid and atractyloside (Karch and Molkentin 2014). Mitochondrial instability due to the prolonged opening of mtPTP is most often as a result of the ischemic injury-induced Ca2+ and ROS accumulation that underlies the necrotic death of the cells (Brenner and Moulin 2012). The identity of molecules that constitute and affect the mtPTP holds an important clinical value that is still a long lasting scientific debate as well as challenge (Hurst et al. 2017). Haworth and Hunter have observed that mtPTP is an evolutionarily conserved pore that is permeable to molecules of size 1.5 kDa during physiological conditions (Bernardi 2013). The opening of mtPTP is considered to be the primary and crucial event leading to mitochondrial death. The key mechanism of mtPTP is mediated through the accumulation of ROS and Ca2+ after the reperfusion (Izzo et al. 2016). The mtPTP, multimeric pore spanning through the mitochondrial membranes, is a non-particular pore that opens in the IMM when matrix Ca2+ concentrations are large, specifically when followed by oxidative stress. The mtPTP inhibitors such as cyclosporine A is neuroprotective in various neurological diseases such as ischemic stroke as shown by using conventional in vitro and in vivo models of stroke (Osman et al. 2011). Thus, therapeutic interventions aimed at mtPTP inhibition would be an important tool of neuroprotection in many neurodegenerative diseases.
Mitochondrial impairment and the formation of mtPTP release various proapoptotic factors from mitochondria to the cytosol such as proteins which include cytochrome c, Smac/DIABLO, and the serine protease HtrA2/Omi (Green and Llambi 2015). Ischemia/reperfusion promoted mitochondrial ROS, and Ca2+ causes the mtPTP formation through which cytochrome c is translocated to the cytosol and leads to cell death (Yuan et al. 2016).
Cytochrome c binds the cytosolic apoptosis promoting activation factor (Apaf1), and caspase-9 leads to the formation of apoptosome; it stimulates the activation of caspase-3, which then activates many substrate proteins such as poly (ADP-ribose) polymerase (PARP) (Shakeri et al. 2017). This PARP translocates into the nucleus via different mechanisms which cause damages to the nuclear DNA (Julien and Wells 2017). The formation of this mtPTP after injury propagates the release of another group of apoptotic proteins such as AIF, endonuclease G, and Bcl-2/adenovirus E1B (Shakeri et al. 2017). There is extensive evidence that has demonstrated that AIF proteins elicit the alternative process of death upon bioenergetics failure that impedes the caspase activation. The AIF binds to cyclophilin A in cytosol from where this conjugate is transferred to the nucleus and promotes DNA damage as well as cell death. Another protein, i.e., endonuclease G nuclear translocation is also associated with cell death but the link between AIF and endonuclease is not established. Lastly, another apoptotic protein BNIP3 mediating apoptosis that is independent of both caspases and AIF seems to be a novel cell death pathway in stroke (Cho and Toledo-Pereyra 2008). The clustering of procaspase-9 in this manner leads to caspase-9 activation, which is presumably an initiator of the cytochrome c-dependent caspase cascade, then activates caspase-3. On the other hand, excessive activation of PARP causes depletion of nicotinamide-adenine dinucleotide and ATP, which ultimately leads to cellular energy failure and necrotic cell death. Collectively, these studies unveil a strong assumption for concluding that cerebral ischemia activates the mitochondrial apoptotic pathway, characterized by changes in Bcl-2 family proteins, cytochrome c release, and caspase-like enzyme activation (Scheme 1). .
Conclusion
In spite of wealth of experimental evidences of research and remarkable number of promising data sets in the various laboratories on rodent models of stroke, no drug or neuroprotective agent has been conclusively effective in the stroke patients. The current therapeutics is limited to thrombolytics, which is only applicable to very few case of stroke patients (Chang and Prabhakaran 2017). The current therapies are limited to post stroke care management and symptomatic treatment. The failure of translating the preclinical therapies to clinical level might have various reasons that led to their failure. The major drawback is the narrow therapeutic window of the drugs that make them eligible for limited patients, and other factors are dose and route of administration and their possible adverse effects on the patients. The paucity of clinical trials to evaluate the long-term and dose-dependent studies is another drawback of bringing the medication from bench to bedside.
The development and discovery of new drugs is a major challenge for the ischemic stroke research. The mitochondrial pathways of cell death can be target for the inhibition of brain cell death in ischemic stroke. The mitochondrial dysfunctions such as energetic failure, mitochondrial ROS, Ca2+, and increase in the permeabilization seem to be promising targets to attenuate the loss of cellular damage. Mitochondrial dysfunction is an early step for cascade of events such as apoptotic and necrotic death of brain cells in ischemia stroke. Since cerebral ischemia is a multifactorial disease, effective treatment though at multiple dimensions might prove to be more effective. The therapies that have more translational efficacy might prove beneficial for designing the future candidates. The mitochondrial-dependent therapeutic agents that could provide neuroprotection against stroke might be help to improve disease conditions.
References
Ahangar AA, Saadat P, Heidari B, Taheri ST, Alijanpour S (2017) Sex difference in types and distribution of risk factors in ischemic and hemorrhagic stroke. Int J Stroke:1747493017724626. https://doi.org/10.1177/1747493017724626
Alam MR, Baetz D, Ovize M (2015) Cyclophilin D and myocardial ischemia-reperfusion injury: a fresh perspective. J Mol Cell Cardiol 78:80–89. https://doi.org/10.1016/j.yjmcc.2014.09.026
Amigo I et al (2016) Mitochondrial form, function and signalling in aging. Biochem J 473:3421–3449. https://doi.org/10.1042/bcj20160451
Andrabi SS, Parvez S, Tabassum H (2015) Melatonin and ischemic stroke: mechanistic roles and action. Adv Pharmacol Sci 2015:384750. https://doi.org/10.1155/2015/384750
Andrabi SS, Parvez S, Tabassum H (2017) Neurosteroids and ischemic stroke: progesterone a promising agent in reducing the brain injury in ischemic stroke. J Environ Pathol Toxicol Oncol 36:191–205. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2017017156
Andrabi SS, Ali M, Tabassum H, Parveen S, Parvez S (2019) Pramipexole prevents ischemic cell death via mitochondrial pathways in ischemic stroke. Dis Model Mech. https://doi.org/10.1242/dmm.033860
Andrienko TN, Pasdois P, Pereira GC, Ovens MJ, Halestrap AP (2017) The role of succinate and ROS in reperfusion injury - a critical appraisal. J Mol Cell Cardiol 110:1–14. https://doi.org/10.1016/j.yjmcc.2017.06.016
Archer DP, Walker AM, McCann SK, Moser JJ, Appireddy RM (2017) Anesthetic neuroprotection in experimental stroke in rodents: a systematic review and meta-analysis. Anesthesiology 126:653–665. https://doi.org/10.1097/aln.0000000000001534
Azad P, Haddad GG (2013) Genetic animal models of preconditioning. Transl Stroke Res 4:51–55. https://doi.org/10.1007/s12975-012-0218-1
Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD (2007) Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9:550–555. https://doi.org/10.1038/ncb1575
Bejot Y, Bailly H, Durier J, Giroud M (2016) Epidemiology of stroke in Europe and trends for the 21st century Presse medicale (Paris, France : 1983) 45:e391–e398. https://doi.org/10.1016/j.lpm.2016.10.003
Bakthavachalam P, Shanmugam PS (2017) Mitochondrial dysfunction - silent killer in cerebral ischemia. J Neurol Sci 375:417–423. https://doi.org/10.1016/j.jns.2017.02.043
Banerjee A, Larsen RS, Philpot BD, Paulsen O (2016) Roles of rresynaptic NMDA receptors in neurotransmission and plasticity. Trends Neurosci 39:26–39. https://doi.org/10.1016/j.tins.2015.11.001
Bernardi P (2013) The mitochondrial permeability transition pore: a mystery solved? Front Physiol 4:95. https://doi.org/10.3389/fphys.2013.00095
Bettler B, Fakler B (2017) Ionotropic AMPA-type glutamate and metabotropic GABAB receptors: determining cellular physiology by proteomes. Curr Opin Neurobiol 45:16–23. https://doi.org/10.1016/j.conb.2017.02.011
Brassai A, Suvanjeiev RG, Ban EG, Lakatos M (2015) Role of synaptic and nonsynaptic glutamate receptors in ischaemia induced neurotoxicity. Brain Res Bull 112:1–6. https://doi.org/10.1016/j.brainresbull.2014.12.007
Brenner C, Moulin M (2012) Physiological roles of the permeability transition pore. Circ Res 111:1237–1247. https://doi.org/10.1161/circresaha.112.265942
Buoncervello M, Marconi M, Care A, Piscopo P, Malorni W, Matarrese P (2017) Preclinical models in the study of sex differences. Clin Sci (London, England : 1979) 131:449–469. https://doi.org/10.1042/cs20160847
Borutaite V (2010) Mitochondria as decision-makers in cell death Environmental and molecular mutagenesis 51:406–416. https://doi.org/10.1002/em.20564
Chang P, Prabhakaran S (2017) Recent advances in the management of acute ischemic stroke. F1000Research 6. https://doi.org/10.12688/f1000research.9191.1
Cho BB, Toledo-Pereyra LH (2008) Caspase-independent programmed cell death following ischemic stroke. J Investig Surg 21:141–147. https://doi.org/10.1080/08941930802029945
Drose S, Stepanova A, Galkin A (2016) Ischemic A/D transition of mitochondrial complex I and its role in ROS generation. Biochim Biophys Acta 1857:946–957. https://doi.org/10.1016/j.bbabio.2015.12.013
El Amki M, Clavier T, Perzo N, Bernard R, Guichet PO, Castel H (2015) Hypothalamic, thalamic and hippocampal lesions in the mouse MCAO model: potential involvement of deep cerebral arteries? J Neurosci Methods 254:80–85. https://doi.org/10.1016/j.jneumeth.2015.07.008
Ertracht O, Malka A, Atar S, Binah O (2014) The mitochondria as a target for cardioprotection in acute myocardial ischemia. Pharmacol Ther 142:33–40. https://doi.org/10.1016/j.pharmthera.2013.11.003
Fan J, Dawson TM, Dawson VL (2017) Cell Death mechanisms of neurodegeneration. Adv Neurobiol 15:403–425. https://doi.org/10.1007/978-3-319-57193-5_16
Fidaleo M, Cavallucci V, Pani G (2017) Nutrients, neurogenesis and brain ageing: from disease mechanisms to therapeutic opportunities. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2017.05.016
Gollihue JL, Rabchevsky AG (2017) Prospects for therapeutic mitochondrial transplantation. Mitochondrion. https://doi.org/10.1016/j.mito.2017.05.007
Granger DN, Kvietys PR (2015) Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol 6:524–551. https://doi.org/10.1016/j.redox.2015.08.020
Green DR, Llambi F (2015) Cell death signaling. Cold Spring Harb Perspect Biol 7. https://doi.org/10.1101/cshperspect.a0060807
Grimm A, Eckert A (2017) Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. https://doi.org/10.1111/jnc.14037
He J, Ford HC, Carroll J, Ding S, Fearnley IM, Walker JE (2017) Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc Natl Acad Sci U S A 114:3409–3414. https://doi.org/10.1073/pnas.1702357114
Hurst S, Hoek J, Sheu SS (2017) Mitochondrial Ca2+ and regulation of the permeability transition pore. J Bioenerg Biomembr 49:27–47. https://doi.org/10.1007/s10863-016-9672-x
Izzo V, Bravo-San Pedro JM, Sica V, Kroemer G, Galluzzi L (2016) Mitochondrial Permeability transition: new findings and persisting uncertainties. Trends Cell Biol 26:655–667. https://doi.org/10.1016/j.tcb.2016.04.006
Jordan J, de Groot PW, Galindo MF (2011) Mitochondria: the headquarters in ischemia-induced neuronal death. Cent Nerv Syst Agents Med Chem 11:98–106
Julien O, Wells JA (2017) Caspases and their substrates. Cell Death Differ. https://doi.org/10.1038/cdd.2017.44
Karch J, Molkentin JD (2014) Identifying the components of the elusive mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111:10396–10397. https://doi.org/10.1073/pnas.1410104111
Karsy M, Brock A, Guan J, Taussky P, Kalani MY, Park MS (2017) Neuroprotective strategies and the underlying molecular basis of cerebrovascular stroke Neurosurgical focus 42:E3. https://doi.org/10.3171/2017.1.Focus16522
Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF (2017) Pathogenic mechanisms following ischemic stroke. Neurol Sci. https://doi.org/10.1007/s10072-017-2938-1
Kiselyov K, Muallem S (2016) ROS and intracellular ion channels Cell calcium 60:108–114. https://doi.org/10.1016/j.ceca.2016.03.004
Korge P, John SA, Calmettes G, Weiss JN (2017) Reactive oxygen species production induced by pore opening in cardiac mitochondria: the role of complex II. J Biol Chem. https://doi.org/10.1074/jbc.M116.768325
Ma Y, Zechariah A, Qu Y, Hermann DM (2012) Effects of vascular endothelial growth factor in ischemic stroke. J Neurosci Res 90:1873–1882. https://doi.org/10.1002/jnr.23088
Ma D, Feng L, Deng F, Feng JC (2017a) Overview of Experimental and Clinical Findings regarding the Neuroprotective Effects of Cerebral Ischemic Postconditioning. Biomed Res Int 2017:6891645. https://doi.org/10.1155/2017/6891645
Ma MW, Wang J, Zhang Q, Wang R, Dhandapani KM, Vadlamudi RK, Brann DW (2017b) NADPH oxidase in brain injury and neurodegenerative disorders. Mol Neurodegener 12:7. https://doi.org/10.1186/s13024-017-0150-7
Maldonado EN, Lemasters JJ (2014) ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 19(Pt a):78–84. https://doi.org/10.1016/j.mito.2014.09.002
Mozaffarian D et al (2016) Executive summary: heart disease and stroke statistics--2016 update: a report from the American Heart Association. Circulation 133:447–454. https://doi.org/10.1161/cir.0000000000000366
Neumann JT et al (2013) Association of MR-proadrenomedullin with cardiovascular risk factors and subclinical cardiovascular disease Atherosclerosis 228:451–459. https://doi.org/10.1016/j.atherosclerosis.2013.03.006
Osman MM, Lulic D, Glover L, Stahl CE, Lau T, van Loveren H, Borlongan CV (2011) Cyclosporine-A as a neuroprotective agent against stroke: its translation from laboratory research to clinical application. Neuropeptides 45:359–368. https://doi.org/10.1016/j.npep.2011.04.002
Perez MJ, Quintanilla RA (2017) Development or disease: duality of the mitochondrial permeability transition pore. Dev Biol 426:1–7. https://doi.org/10.1016/j.ydbio.2017.04.018
Piotrowska A, Bartnik E (2014) The role of reactive oxygen species and mitochondria in aging. Postepy Biochem 60:240–247
Ponnalagu D, Singh H (2017) Anion channels of mitochondria. Handb Exp Pharmacol 240:71–101. https://doi.org/10.1007/164_2016_39
Puyal J, Ginet V, Clarke PG (2013) Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. Prog Neurobiol 105:24–48. https://doi.org/10.1016/j.pneurobio.2013.03.002
Rastogi R, Geng X, Li F, Ding Y (2016) NOX activation by subunit interaction and underlying mechanisms in disease. Front Cell Neurosci 10:301. https://doi.org/10.3389/fncel.2016.00301
Rehni AK, Liu A, Perez-Pinzon MA, Dave KR (2017) Diabetic aggravation of stroke and animal models. Exp Neurol 292:63–79. https://doi.org/10.1016/j.expneurol.2017.03.004
Rekuviene E, Ivanoviene L, Borutaite V, Morkuniene R (2017) Rotenone decreases ischemia-induced injury by inhibiting mitochondrial permeability transition in mature brains. Neurosci Lett 653:45–50. https://doi.org/10.1016/j.neulet.2017.05.028
Rodriguez-Lara SQ, Cardona-Munoz EG, Ramirez-Lizardo EJ, Totsuka-Sutto SE, Castillo-Romero A, Garcia-Cobian TA, Garcia-Benavides L (2016) Alternative interventions to prevent oxidative damage following ischemia/reperfusion. Oxidative Med Cell Longev 2016:7190943. https://doi.org/10.1155/2016/7190943
Roque C, Baltazar G (2017) Impact of astrocytes on the injury induced by in vitro ischemia. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-017-0483-3
Rousselet E, Kriz J, Seidah NG (2012) Mouse model of intraluminal MCAO: cerebral infarct evaluation by cresyl violet staining. J Visualized Exp. https://doi.org/10.3791/4038
Schmidt A, Minnerup J (2016) Promoting recovery from ischemic stroke. Expert Rev Neurother 16:173–186. https://doi.org/10.1586/14737175.2016.1134324
Shakeri R, Kheirollahi A, Davoodi J (2017) Apaf-1: regulation and function in cell death. Biochimie 135:111–125. https://doi.org/10.1016/j.biochi.2017.02.001
Siket MS (2016) Treatment of Acute Ischemic Stroke Emergency medicine clinics of North America 34:861–882. https://doi.org/10.1016/j.emc.2016.06.009
Sims NR, Yew WP (2017) Reactive astrogliosis in stroke: Contributions of astrocytes to recovery of neurological function Neurochemistry international 107:88–103. https://doi.org/10.1016/j.neuint.2016.12.016
Skelding KA, Arellano JM, Powis DA, Rostas JA (2014) Excitotoxic stimulation of brain microslices as an in vitro model of stroke. J Visualized Exp:e51291. https://doi.org/10.3791/51291
Sommer CJ (2017) Ischemic stroke: experimental models and reality. Acta Neuropathol 133:245–261. https://doi.org/10.1007/s00401-017-1667-0
Song FE, Huang JL, Lin SH, Wang S, Ma GF, Tong XP (2017) Roles of NG2-glia in ischemic stroke. CNS Neurosci Ther 23:547–553. https://doi.org/10.1111/cns.12690
Takatsuru Y, Nabekura J, Koibuchi N (2014) Contribution of neuronal and glial circuit in intact hemisphere for functional remodeling after focal ischemia Neuroscience research 78:38–44. https://doi.org/10.1016/j.neures.2013.07.004
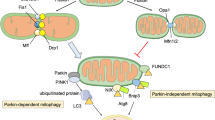
Tang YC, Tian HX, Yi T, Chen HB (2016) The critical roles of mitophagy in cerebral ischemia. Protein Cell 7:699–713. https://doi.org/10.1007/s13238-016-0307-0
Taylor RA, Sansing LH (2013) Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol 2013:746068. https://doi.org/10.1155/2013/746068
Thakur KT et al (2016) Neurological Disorders. In: Patel V, Chisholm D, Dua T, Laxminarayan R, Medina-Mora ME (eds) Mental, neurological, and substance use disorders: disease control priorities, vol 4, Third edn. 2016 International Bank for Reconstruction and Development / The World Bank, Washington DC. https://doi.org/10.1596/978-1-4648-0426-7_ch5
Thompson JW, Narayanan SV, Perez-Pinzon MA (2012) Redox signaling pathways involved in neuronal ischemic preconditioning. Curr Neuropharmacol 10:354–369. https://doi.org/10.2174/157015912804143577
Thornton C, Hagberg H (2015) Role of mitochondria in apoptotic and necroptotic cell death in the developing brain Clinica chimica acta. IntJ Clinical Chem 451:35–38. https://doi.org/10.1016/j.cca.2015.01.026
Thrift AG, Cadilhac DA, Thayabaranathan T, Howard G, Howard VJ, Rothwell PM, Donnan GA (2014) Global stroke statistics. Int J Stroke 9:6–18. https://doi.org/10.1111/ijs.12245
Vakifahmetoglu-Norberg H, Ouchida AT, Norberg E (2017) The role of mitochondria in metabolism and cell death. Biochem Biophys Res Commun 482:426–431. https://doi.org/10.1016/j.bbrc.2016.11.088
Vandenberg RJ, Ryan RM (2013) Mechanisms of glutamate transport. Physiol Rev 93:1621–1657. https://doi.org/10.1152/physrev.00007.2013
Wasay M, Khatri IA, Kaul S (2014) Stroke in South Asian countries. Nat Rev Neurol 10:135–143. https://doi.org/10.1038/nrneurol.2014.13
Wijermars LG, Schaapherder AF, Kostidis S, Wust RC, Lindeman JH (2016) Succinate accumulation and ischemia-reperfusion injury: of mice but not men, a study in renal ischemia-reperfusion. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 16:2741–2746. https://doi.org/10.1111/ajt.13793
Yagami T, Koma H, Yamamoto Y (2016) Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol Neurobiol 53:4754–4771. https://doi.org/10.1007/s12035-015-9355-3
Yang CH, Yen TL, Hsu CY, Thomas PA, Sheu JR, Jayakumar T (2017) Multi-targeting andrographolide, a novel NF-kappaB inhibitor, as a potential therapeutic agent for stroke. Int J Mol Sci 18. https://doi.org/10.3390/ijms18081638
Yuan J, Najafov A, Py BF (2016) Roles of caspases in necrotic cell death. Cell 167:1693–1704. https://doi.org/10.1016/j.cell.2016.11.047
Funding
This study is funded by the DST-PURSE program through grant (No. SR/PURSE Phase 2/39 (C)) and SERB-EMR grant (2016/001070/HS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Klaudia Brix
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andrabi, S.S., Parvez, S. & Tabassum, H. Ischemic stroke and mitochondria: mechanisms and targets. Protoplasma 257, 335–343 (2020). https://doi.org/10.1007/s00709-019-01439-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01439-2