Abstract
The effect of Cd on H2O2 production, peroxidase (POD) activity and root hair formation were analyzed in barley root. Cd causes a strong H2O2 burst in the root region 0–6 mm behind the root tip. POD activity was activated in root tip and raised toward the root base in Cd treated roots. In situ analyses showed that both elevated H2O2 production and POD activity are localized in the early metaxylem vascular bundles. Cd induces root hair formation in the region 2 to 4 mm behind the root tip that was not detected in control roots. These results suggest that Cd-induced root growth inhibition is at least partially the consequence of Cd-stimulated premature root development involving xylogenesis and root hair formation, which is correlated with shortening of root elongation zone and therefore with root growth reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing amount of metals in soils is a growing agronomic problem limiting crop productivity over the world. Cd is released to the environment in nature due to rock mineralization; however, this amount is negligible compare to Cd originating from anthropogenic sources (Gadd and White 1993). Cd is a non-essential metal that already at low concentration inhibits plant development. It is easily taken up and accumulated by plants therefore its concentration in plant tissues can excess Cd concentration in soil in several crop species including barley (Cutler and Rians 1974). Therefore, Cd exposure constitutes not only a decrease of crop productivity but also a hazard to human health as consequence of Cd transfer to the animals and humans through the food chain.
Cd induces perturbation in cellular metabolism mainly by inhibition of water and nutrient uptake, destruction of chloroplast function and inhibition of several enzyme activities manifested as visible general symptoms such as root and leaf growth reduction, and leaf chlorosis (Sanita di Toppi and Gabbrielli 1999; Benavides et al. 2005). Although the plant responses to Cd are only partially understood, the increasing number of publications suggests that some Cd induced toxicity symptoms are attributed to the oxidative damage arising from the imbalance in the generation or removal of reactive oxygen species (ROS). Both Cd induced generation of ROS (Olmos et al. 2003; Cho and Seo 2005) or Cd caused inhibition of enzymatic (Sandalio et al. 2001; Chen et al. 2003) or non-enzymatic antioxidants (Ranieri et al. 2005; Rellán-Ávarez et al. 2006) have been reported. The crucial role of ROS regulation in the cell at the presence of elevated concentration of toxic metals is supported also by observation that some heavy metal hyperaccumulator plants have increased antioxidant apparatus compared to non-accumulators (Iannelli et al. 2002; Ederli et al. 2004; Singh et al. 2006).
Although Cd cannot participate in Fenton reaction like redox metals (Fe, Cu), there is a strong evidence that Cd generates ROS indirectly. In cultured tobacco cells NADPH oxidase-like enzyme mediated ROS production was described as early step in the Cd induced response (Olmos et al. 2003). Peroxidase mediated production of H2O2 was also reported under several stress conditions including Cd treatment (Šimonovičová et al. 2004). One of the possible ways is the activation of “normal” physiological processes, however with temporal or/and spatial shifting compared to non-stressed condition. It was suggested previously that the extreme lignification is a general marker of several stresses in plants such as drought, wounding and allelopathy (Holm et al. 2003; Böhm et al. 2006; Fan et al. 2006). In most of these stress responses H2O2 plays crucial role not only as a substrate for peroxidases, but probably also as a signal molecule (Neil et al. 2002). Recently Cd-induced lignification was described, which is accompanied by elevated production of H2O2 in the newly formed protoxylem elements, in the root region that normally constitutes the elongation zone of pine root tip (Schützendübel et al. 2001). In the present study, we investigated the effect of Cd on H2O2 generation and peroxidase activity in the early metaxylem elements and analyzed the change in its isozyme profile in barley root tip.
Material and methods
Plant material and growth conditions
Seeds of barley (Hordeum vulgare L.) cv. Jubilant were imbibed either in distilled water (control) or in 1.0 mM CdCl2 for 4 h followed by germination between two sheets of filter papers (density 110 g/m2, Papírna Perštejn, Czech Rep.) moistened with respective imbibing solutions in Petri dishes (5 ml per sheet of filter paper of 18 cm in diameter) at 24°C in darkness. The uniformly germinating seeds (40 seeds per Petri dish) were transferred to fresh filter papers moistened with appropriate treatment solutions at an interval of 24 h. After 72 h individual barley root segments (2 mm in length) were obtained by gradual cutting of each root in the distances of 2, 4, 6 and 8 mm in the direction from the tip to the base. For histochemical staining 0.5 mm thick root sections were used from the appropriate distance from the root tip. The experiments were carried out in three independent series.
Hydrogen peroxide assay
Hydrogen peroxide (H2O2) production was monitored fluorimetrically using the Amplex Red Hydrogen Peroxide Assay Kit (Molecular Probes) according to manufacturer’s recommendations with minor modifications. Segments from barley root tips (five 2 mm long segments per reaction) were cut into 100 μl of the reaction buffer and after adding of 100 μl of the working solution (prepared according to manufacturer’s instructions) they were dark incubated for 30 min at 30°C. Fluorescence signal was recorded with the microplate reader (Synergy HT BIO-TEK, USA) using excitation at 530 nm and fluorescence detection at 590 nm.
Determination of POD activity
In the case of syringaldazine (SYR) as POD substrate segments from barley root tips (five 2 mm long segments per reaction) were incubated in 110 μl of 100 mM Na-acetate buffer (pH 5.2) containing 1 M NaCl for 5 min. Then 100 μl of this solution was added to the reaction mixture (100 μl) containing 0.5 mM H2O2 and 0.5 mM syringaldazine (from 5 mM stock solution in DMSO) in 0.1 M Na-acetate buffer, pH 5.2. Absorbance was measured with the microplate reader at 530 nm after 1 min at 30°C.
Using 4-methoxy-1-naphthol (4-MN) as a substrate five root segments were incubated in 60 μl of 100 mM Na-acetate buffer (pH 5.2) containing 1 M NaCl for 5 min. Then 50 μl of this solution was added to the reaction mixture containing 100 μl of 100 mM Na-acetate buffer (pH 5.2), 50 μl of 1 mM 4-MN (from 5 mM stock in 20% ethanol), and 50 μl of 0.04% H2O2. The reaction was initiated by adding of H2O2. The increase in absorbance at 595 nm was recorded with a microplate reader over a time period of one minute at 30°C.
Gel electrophoresis and POD staining
Proteins were extracted and concentrated by immersing the freshly excised root segments (from 40 seedlings) into 100 mM Na-acetate buffer (pH 5.2) containing 1 M NaCl and gently shaking for 15 min followed by filtration (cheesecloth), centrifugation at 10,000 × g for 10 min and ultrafiltration (Amicon Ultra, USA). The proteins were quantified with bovine serum albumin as the calibration standard by the method of Bradford (1976).
The basic proteins were separated under non-denaturing conditions on 7% slab polyacrylamide gels using the cathodic system (Reisfeld et al. 1962), while the acidic and neutral proteins on 7% slab polyacrylamide gels using the discontinuous buffer system (Laemmli 1970). Proteins (1 μg per lane) were loaded using Mini-Protean electrophoretic system (BIO-RAD, USA). The POD activity in the gels was detected after incubation of gel in 100 mM Na-acetate buffer (pH 5.2), 5 mM 4-MN (in ethanol) or 1 mM syringaldazine (from 10 mM stock solution in DMSO) and 10 mM H2O2 for 15 min at 30°C (Ferrer et al. 1990).
Histochemical stain
H2O2 production was monitored with the KI-starch reagent by immersing 0.5 mm thick root sections into 0.1 M KI, 4% starch and 0.1 mg/ml TMB (from 10× stock solution containing 1 mg TMB/1 ml methanol) for 2 h (Ros Barceló 1998).
Peroxidase activity was monitored by immersing of 0.5 mm thick root sections into same staining solution as was used for gel staining but H2O2 was avoided.
Results
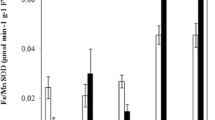
The analysis of H2O2 production in individual barley root segments (2 mm in length) revealed an increase of the amount of H2O2 from root tip towards the base in control roots (Fig. 1A). Cd greatly stimulates H2O2 production immediately behind the root tip. In the first 0–2 mm and second 2–4 mm segments and in the 4–6 mm segment behind the root tip three and two times more H2O2 was detected in comparison to control root segments (Fig. 1A). Opposite results were obtained by measuring POD activity, which raised towards the root base in Cd treated roots while control segments showed relatively low and stable level of POD activity measured with 4-MN as non-specific POD substrate (Fig. 1B). Using SYR as a POD substrate these significant differences disappeared between control and Cd treated root segments (Fig. 1C).
In our experiments staining of control root sections with starch/KI reagent yielded a weak stain in mucilage around the root cap (data not shown) and in the early metaxylem vascular bundles in the region from 4 mm behind root tip (Fig. 2A). In the Cd treated roots the marked H2O2 production in vascular bundles was detected already in the distance 2 mm from root tip. The profile of POD activity was similar to that of H2O2 production visualized by SYR as a substrate (Fig. 2B). This outermost part of the xylem was also stained with 4-MN and in similar manner to SYR staining (Fig. 2C).
One cathodic and three anodic Cd-induced POD isozymes were detected using 4-MN as substrate (Fig. 3). The cathodic Cd-induced POD isozyme was detected only in the first 2 mm segment and was not stained with SYR suggesting that this form is not involved in lignification processes. On the other hand one faintly SYR stained Cd-induced POD isozyme was detected, which was not stained with 4-MN. Among three anodic Cd-induced POD isozymes only one slow migrating anionic POD was stained also with SYR suggesting that this isozyme is probably involved in lignin synthesis.
Besides premature lignification of early metaxylem bundles of barley root Cd induces also formation of root hairs in the region 2 to 4 mm behind the root tip, which was not detected on control roots (Fig. 4).
Discussion
The reduction of root growth is a general and sensitive visual symptom indicating the presence of excessive metal concentration in root environment, which is toxic for given plant species. Due to the high binding capacity of cellulose filter paper to cations, a millimolar concentration is required to obtain free cations available for roots in micromolar concentration (Tamás et al. 2006a). In our previous work we showed that 1 mM concentration of Cd was required to obtain about 50% inhibition of root growth 72 h after the onset of imbibition on the filter paper (Tamás et al. 2006b). Therefore we used the same concentration and time of Cd treatment also in these experiments.
In our experiments strong correlation was found between Cd induced H2O2 production and POD activity using 4-MN or SYR as substrate in vascular bundles. In vitro using SYR as POD substrate this significant difference disappeared between control and Cd treatment roots in comparison with POD activity using 4-MN or to H2O2 production. However, it was observed that SYR as a lignin monomer analog specifically reacts only with POD localized in lignifying CW in situ. In vitro this specific feature of SYR is lost and this substrate is able to react with several other PODs isolated from non-lignifying tissues (Pang et al. 1989), which can explain the differences in results obtained in situ and in vitro.
Hydrogen peroxide accumulation is the major reason causing Cd phytotoxicity in Arabidopsis seedlings and in the efficient quenching of H2O2 by antioxidative system it is important component of Cd tolerant seedlings (Cho and Seo 2005). Similarly in roots of wheat, in spite of elevated presence of phytochelatins, the excess of Cd causes generation of considerable amounts of H2O2, which was not detected in leaves (Ranieri et al. 2005). The observed distribution of SYR oxidase activity identified within the xylem of barley roots correlates with the area believed to be actively lignifying (e.g. outermost part of the xylem) similarly as was reported in poplar xylem (Christensen et al. 2001).
The intense production of H2O2 and high POD activity are characteristic for young maturing vascular elements; while at the maturity stage they both rapidly decrease (Olson and Varner 1993). Our results demonstrate that Cd-induced changes in H2O2 production and POD activity in the region 2–8 mm behind the root tip are associated with premature maturation of root xylem elements. It was earlier observed that Cd induces electron dense depositions in differentiating xylem vessels, which are typical for later maturing tracheary elements at non-stressed conditions (Barceló et al. 1988a). Inhibition of growth of maize root by water deficit is associated with the shortening of the length of elongation zone as a consequence of increased lignin metabolism in the region 3–9 mm behind root tip (Fan et al. 2006). However, it was not detected at the region 0–3 mm behind the root tip, which explains partial maintenance of the root growth during drought stress. In contrast to drought stress Cd induces lignification immediately behind the root tip, which is probably associated with strong inhibition of root growth. In addition to lignification formation of cell wall ingrowths similar to transfer cells were observed in the hypodermis, suggesting a Cd-induced alteration of ion transport (Vázquez et al. 1992).
The Cd-induced abnormal premature lignification of epidermis and exodermis cell walls in absorption and elongation zone of root tip was reported in Phragmites australis without any significant structural modification (Ederli et al. 2004). This unusual lignification was interpreted as a part of a defense reaction that limits the entry of toxic metal. Schützendübel et al. (2001) reported that Cd induced premature lignification of xylem elements of pine roots in the distance from root tip, which normally constitutes the elongation zone. Authors suggested that Cd inhibits the systems involved in H2O2 removal therefore causes its accumulation that in turn may trigger the developmental program leading to xylogenesis. However, our results demonstrated that H2O2 accumulated strictly in the xylem elements during Cd treatment. Therefore Cd induced xylogenesis is not triggered by non-specific accumulation of H2O2, but probably Cd induces another signal, which triggers generation of H2O2 in xylem elements as first stage in their differentiation. The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH-oxidase-like enzyme, which may be further used by peroxidases for the synthesis of lignin (Ros Barceló 1998). In cultured tobacco cells Cd activates just the NADPH oxidase-like enzyme mediated ROS production (Olmos et al. 2003).
Several reports suggest that both acidic and basic peroxidases are involved in lignin synthesis depending on plant species (Ostegaard et al. 2000; Ros Barceló and Aznar-Asensio 2002). However, it has been also suggested that both cationic and anionic peroxidases are required for correct lignification of CW (Mäder et al. 1986).
Induction of lignin synthesis was described also under other stress conditions. Juglone (5-hydroxy-1,4-naphthoquinone) originated from black walnut affects growth of various plants probably by inhibition of root growth as consequence of enhanced lignification of root tissues (Böhm et al. 2006). Aluminium-induced root growth inhibition is correlated with elevated lipid peroxidation and lignin deposition, which are associated with an increase in H2O2 generation in wheat (Hossian et al. 2005). In Asparagus wound induced increased peroxidase activity and lignin production were localized in the vascular bundles during post-harvest storage (Holm et al. 2003).
Besides premature xylogenesis we described also Cd induced root hair formation. Similar results were reported in radish seedling where Cd induces premature death of epidermis and an increase in the number of root hairs (Vitória et al. 2003). In contrast to these results, the opposite effect was observed in the case of Cu treatment, which causes reduction in root hair formation already at concentration lower than that which caused a significant reduction in root fresh weight and appearance of typical Cu toxicity symptoms (Kopittke and Menzies 2006). Both xylem element lignification and root hair formation in the region that in control roots represents the elongation zone, suggest that Cd induces premature differentiation of barley root. Accelerated differentiation was also observed in the upper part of the plants where Cd induces senescence symptoms in leaf peroxisomes probably by activation of a metabolic transition of leaf peroxisomes into glyoxysomes (McCarthy et al. 2001). Similarly to peroxisome, chloroplasts from Cd treated plants show typical senescence features suggested that Cd probably induced premature leaf senescence (Barceló et al. 1988b; Sandalio et al. 2001).
In conclusion the above reported results suggest that Cd-induced root growth inhibition is at least partially the consequence of Cd-stimulated premature root development involving xylogenesis and root hair formation, which is correlated with shortening of root elongation zone and therefore with root growth reduction.
Abbreviations
- AOS:
-
Active oxygen species
- CW:
-
Cell wall
- 4-MN:
-
4-Methoxy-1-naphthol
- POD:
-
Peroxidase
References
Barceló J, Vázquez MD, Poschenrieder C (1988a) Cadmium-induced structural and ultarstructural changes in the vascular systems of bush bean stems. Bot Acta 101:254–261
Barceló J, Vázquez MD, Poschenrieder C (1988b) Structural and ultrastructural disorders in cadmium-treated bush bean plants (Phaseolus vulgaris L.). New Phytol 108:37–48
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
Bradford MN (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Böhm PAF, Zanardo FML, Ferrarese MLL, Ferrarese-Filho O (2006) Peroxidase activity and lignification in soybean growth-inhibition by juglone. Biol Plant 50:315–317
Chen YX, He YF, Luo YM, Yu YL, Lin Q, Wong MH (2003) Physiological mechanism of plant roots exposed to cadmium. Chemosphere 50:789–793
Cho U, Seo N (2005) Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci 168:113–120
Christensen JH, Overney S, Rohde A, Diaz WA, Bauw G, Simon P, van Motagu M, Boerjan W (2001) The syringaldazine-oxidizing peroxidase PXP 3–4 from poplar xylem: cDNA isolation, characterization and expression. Plant Mol Biol 47:581–593
Cutler JM, Rians DW (1974) Characterisation of cadmium uptake by plant tissue. Plant Physiol 54:67–71
Ederli L, Reale L, Ferrari F, Pasqualini S (2004) Responses induced by high concentration of cadmium in Phragmites australis roots. Physiol Plant 121:66–74
Fan L, Linker R, Gepstein S, Tanimoto E, Yamamoto R, Neumann PM (2006) Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolisms and progressive stelar accumulation of wall phenolics. Plant Physiol 140:603–612
Ferrer MA, Calderón AA, Muñoz R, Ros Barceló A (1990) 4-methoxy-α-naphthol as a specific substrate for kinetic, zymographic and cytochemical studies on plant peroxidase activities. Phytochem Anal 1:63–69
Gadd GM, White C (1993) Microbial treatment of metal pollution – a working biotechnology. Trends Biotechnol 11:353–359
Holm KB, Andreasen PH, Eckloff RM G, Kristensen BK, Rasmussen SK (2003) Three differentially expressed basic peroxidases from wound-lignifying Asparagus officinalis. J Exp Bot 54:2275–2284
Hossian MA, Hossian AKM, Kihara T, Koyama H, Hara T (2005) Aluminum-induced lipid peroxidation and lignin deposition are associated with an increase in H2O2 generation in wheat seedlings. Soil Sci Plant Nutr 51:223–230
Iannelli MA, Pietrini F, Fiore L, Petrilli L, Massacci A (2002) Antioxidant response to cadmium in Phragmites australis plants. Plant Physiol Biochem 40:977–982
Kopittke PM, Menzies NW (2006) Effect of Cu toxicity on growth of cowpea (Vigna inguiculata). Plant Soil 279:287–296
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680–685
Mäder M, Nessel A, Schloss P (1986) Cell compartmentation and specific roles of izoenzymes. In: Greppin H, Penel C, Gaspar T (eds) Molecular and physiological aspects of plant peroxidases. University Press, Geneva, Switzerland, pp 246–260
McCarthy I, Romero-Puertas MC, Palma JM, Sandalio LM, Corpas FJ, Gómez M, Del Río LA (2001) Cadmium induces senescence symptoms in leaf peroxisomes of pea plants. Plant Cell Environ 24:1065–1073
Neil S, Desikan R, Hancock J (2002) Hydrogen peroxide signaling. Curr Opin Plant Biol 5:388–359
Olmos E, Martínez-Solano JR, Piqueras A, Hellín E (2003) Early steps in the oxidative burst induced by cadmium in cultured tobacco cells (BY-2 line). J Exp Bot 54:291–301
Olson PD, Varner JE (1993) Hydrogen peroxide and lignification. Plant J 4:887–892
Ostegaard L, Teilum K, Mirza O, Mattsson O, Petersen M, Welinder KG, Mundy J, Gajhede M, Henriksen A (2000) Arabidopsis ATP A2 peroxidase. Expression and high-resolution structure of plant peroxidase with implication for lignification. Plant Mol Biol 44:231–243
Pang A, Catesson A, Francesch C, Rolando C, Goldberg R (1989) On substrate specificity of peroxidases involved in the lignification process. J Plant Physiol 135:325–329
Ranieri A, Castagna A, Scebba F, Careri M, Zagnoni I, Predieri G, Pagliari M, Sanita di Toppi L (2005) Oxidative stress and phytochelatin characterisation in bread wheat exposed to cadmium excess. Plant Physiol Biochem 43:45–54
Reisfeld RA, Levis UJ, Williams DE (1962) Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature 195:281–283
Rellán-Ávarez R, Ortega-Villasante C, Álvarez-Fernández A, del Campo FF, Hernández LE (2006) Stress responses of Zea mays to cadmium and mercury. Plant Soil 279:41–50
Ros Barceló A (1998) The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH-oxidase-like enzyme. Planta 207:207–216
Ros Barceló A, Aznar-Asensio GJ (2002) Basic peroxidases in cell walls of plants belonging to Asteraceae family. J Plant Physiol 159:339–345
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 127:887–898
Šimonovičová M, Huttová J, Mistrík I, Široká B, Tamás L (2004) Peroxidase mediated hydrogen peroxide production in barley roots grown under stress conditions. Plant Growth Regul 44:267–275
Singh N, Ma LQ, Srivastava M, Rathinasabapathi B (2006) Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L and Pteris ensiformis L. Plant Sci 170:274–282
Tamás L, Budíková S, Šimonovičová M, Huttová J, Široká B, Mistrík I (2006a) Rapid and simple method for Al-toxicity analysis in emerging barley roots during germination. Biol Plant 50:87–93
Tamás L, Bočová B, Huttová J, Mistrík I, Ollé M (2006b) Cadmium-induced inhibition of apoplastic ascorbate oxidase in barley roots. Plant Growth Regul 48:41–49
Vázquez MD, Poschenrieder C, Barceló J (1992) Ultrastructural effects and localization of low cadmium concentrations in bean roots. New Phytol 120:215–226
Vitória AP, Rodriguez APM, Cunha M, Lea PJ, Azevedo RA (2003) Structural changes in radish seedlings exposed to cadmium. Biol Plant 47:561–568
Acknowledgements
We wish to thank Margita Vašková for excellent technical assistance. This work was supported by the Grant agency VEGA, project No 2/4040/04.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ďurčeková, K., Huttová, J., Mistrík, I. et al. Cadmium induces premature xylogenesis in barley roots. Plant Soil 290, 61–68 (2007). https://doi.org/10.1007/s11104-006-9111-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9111-6