Abstract
The present study reports the Agrobacterium rhizogenes-mediated hairy root induction in of an ethno-medicinally significant herb–Boerhaavia diffusa L., for elucidating the underlying competence regarding its biosynthetic (i.e. boeravinone B and eupalitin) and bioactivity (antibacterial, antioxidant and anti-inflammatory) potentials. Host plant-specific receptiveness towards A. rhizogenes strains and disparity in compatibility threshold of leaf and nodal explants were evident. Only leaf explants responded, attaining hairy root induction with the ATCC 15834 followed by A4 and SA79 strains in reducing order of transformation efficiency. The growth behaviours differed amongst independent rhizoclones, and two clones of A4 (RBH) and ATCC 15834 (RBT8) origin demonstrated higher growth potentials. Polymerase chain reaction amplification of rol genes confirmed their transformed nature. Optimization of the appropriate solvent and reverse phase high-performance liquid chromatography parameters relating to the targeted metabolite production in the selected RBH and RBT8 clones revealed higher accumulation of eupalitin with the RBH clone having the best result of 1.44 times greater yield over the control root. Compared to the selected rhizoclones, the control roots however showed higher boeravinone B content. Devising a modified “stirred-tank” reactor through equipping with marine impellers and ring spargers facilitated high-density RBH root biomass yield with 6.1-fold and 1.15-fold yield increment of the boeravinone B and eupalitin respectively compared to shake-flask cultures. Considering the control roots, the RBH clone revealed analogous antioxidant/antibacterial activities with improved anti-inflammatory potential. The hairy root mediated higher production of boeravinone B and eupalitin could be achieved for the first time in bioreactor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Boerhaavia diffusa L. (Nyctaginaceae) is one of the most renowned traditional herbs in India that is found in age-old applications in the Ayurveda and Unani systems of medicines (Riaz et al. 2014). Its various parts and especially the roots (Rajpoot and Mishra 2011) have predominantly been used in the Indian traditional systems of medicines for the treatment of diverse human ailments, such as jaundice, spleen enlargement, oedema, anaemia, leprosy, skin diseases, rheumatism and ocular disorders (Murti et al. 2010; Chaudhary and Dantu 2011). Advanced pharmacological studies have relentlessly displayed its notable therapeutic applications by virtue of the identified hepatoprotective, anticonvulsant, antioxidant, adaptogenic, antistress and antihyperglycemic potentials (Mishra et al. 2014). The latest discoveries of the potential anticancer, immunomodulatory and radioprotective activity of B. diffusa have highlighted its beneficial efficacies in cancer therapy (Mishra et al. 2014). Demonstration of antispasmodics/spasmolytic activity of the methanolic root extracts of this plant has further shown the potent use of this time-honoured medicinal plant for the treatment of involuntary muscle spasm caused due to various muscle disorders, including the debilitating symptoms of torticollis, paraplegia and palsy/glossal palsy (Borrelli et al. 2006; Mishra et al. 2014). A recent report has ignited new expectations about the cardio-protective potential of the ethanolic extract B. diffusa by highlighting its efficacious impact against mitochondrial dysfunction, which has formerly been proven to play a crucial role in triggering the development of cardiac hypertrophy and heart failure (Prathapan et al. 2014). The revelation of such multifaceted pharmacokinetics of this plant through the advent of latest tools of combinatorial chemistry is not only the additional therapeutic applications of this ancient medicinal plant in adjunct to its traditional uses, but also boosted its demand in the pharmaceutical industries for future drug development in the years to come.
B. diffusa has been reported to be a rich reservoir of diverse secondary metabolites, which includes flavonoids, isoflavonoids (rotenoids), alkaloids, triterpenoids and phenolic glycosides (Mishra et al. 2014). Amongst this vast repository of metabolites, boeravinone B and eupalitin have attracted recent research attention as marker molecules owing to their promising anticancer properties. Boeravinone B, a major rotenoid of B. diffusa, has been reported to posses anticancer (Ahmed-Belkacem et al. 2007) and in vitro COX-1/2 inhibitory activities in addition to in vivo anti-inflammatory potential (Bairwa et al. 2013a, b). On the other hand, eupalitin has also exhibited significant cytotoxicity and anti-proliferative property against human colorectal tumour cells as it induced apoptosis in cells through activation of caspases 3/7 (Ghalib et al. 2013).
The demand of the B. diffusa is mostly fulfilled by mass scale collection of this plant from natural habitats leading to invariable depletion of this plant species as well as adulteration of its raw materials to comply with its escalating demand (Gomes et al. 2013). Propagation by seeds and cuttings is normally practiced, but low germination percentage/poor seed viability and perennial nature of the above-ground tissues necessitated biotechnological interventions for round-the-year supply of plant materials independent of seasonal fluctuations and environmental/pathological constraints. Literature survey revealed some reported attempts on direct and callus-mediated propagation of B. diffusa (Bhansali et al. 1978; Shrivastava and Padhya 1995; Nagarajan et al. 2005; Sudarshana et al. 2008; Sahu and Khalkho 2012).
Moreover, since roots are the chief source of the therapeutically active constituents of B. diffusa (Sahu et al. 2014), their exclusive authentic collection implies utmost significance to comply with the characteristic pharmaceutical traits of the raw material. However, the uninhibited adulteration of its roots with that of Trianthema portulacastrum imparts serious practical challenge, which cannot only inadvertently jeopardize the overall bioactivity quotient of its metabolites but also can inflict detrimental effects on the curative benefits of its roots (Gomes et al. 2013). To this end, Agrobacterium rhizogenes-mediated hairy root cultures present an excellent platform for sustainable production of authentic root biomass, unharmed of the problem of cell-culture linked genetic/biochemical instability or normally endured seasonal/pathological constraints (Georgiev 2014; Ludwig-Muller et al. 2014). As a result of concerted global research efforts for more than three decades, hairy root has now been recognized as the most proficient alternative as sources of valuable phytomolecules of industrial demand, as it also adheres to the commercial obligation of biosynthetic stability, cost efficiency, long-term production uniformity and amenability for scale-up cultivation in bioreactors (Ono and Tian 2011). In recent years, a universal consensus has finally been achieved concerning the industrial applicability of hairy root culture as a strategic production alternative of raw materials and their associated metabolites through the advent of a Swiss company called ROOTec Bioactives (Talano et al. 2012). It is however disheartening to note that the hairy root culture system is relatively underutilized in B. diffusa and phytochemically the same remained uncharted so far. Only a handful of reports are presently available concerning the establishment of both adventitious root culture as well as A. rhizogenes-mediated hairy root cultures in this plant (Shrivastava and Padhya 1995; Jenifer et al. 2012; Sahu et al. 2013), which assigns ample scope for further studies.
In the background of this information, the present study was undertaken to evaluate the impending competence of the A. rhizogenes-induced hairy root cultures of B. diffusa relating to their biosynthetic as well as bioactivity potentials. Generation of different strains of A. rhizogenes-mediated individual rhizoclones followed by judicious screening and selection of the elite clone(s) with best yield potential in terms of biomass as well as boeravinone B and eupalitin accumulation constitutes the primary research objective of the present study. Consequent tailoring of the rate-limiting factors pertaining to both the extraction /quantitation of the targeted molecules as well as bioreactor scale-up procedures was also under taken to pave the way for industrial exploitation of this system involving this universally reputed medicinal plant—B. diffusa.
Materials and methods
Plant material and in vitro establishment
Axenic shoot cultures of B. diffusa L. were generated to serve as explant source for hairy root (HR) induction by collecting young nodal segments from the field-grown plant of CSIR-CIMAP. Sterilization of the explants was done according to our previously reported protocol (Pandey et al. 2015), and the cultures were established on semi-solid Murashige and Skoog’s (Murashige and Skoog 1962) basal medium, supplemented with 3 % (w/v) sucrose + 0.8 % (w/v) agar (Merck, India) and (1–2 mg/l) 6-benzylaminopurine or kinetin (Sigma-Aldrich). Individual microshoots, generated through auxiliary bud proliferation, were excised (2.5–3.5 cm) after 4–5 weeks of culture initiation and transferred to half-strength MS basal medium supplemented with indole-3-butyric acid (IBA) (0.5 to 2.0 mg/l) for rooting. The rooted plantlets were maintained through regular subculturing at 4–6 weeks intervals to serve as stock cultures for all hairy root induction experiments. All the cultures were maintained at 25 ± 2 °C under 16/8 h light/dark photoperiod.
Chemicals
Benzyl amino purine (BAP), IBA and kinetin (Kn) were purchased from Sigma-Aldrich (St. Louis, MO, USA). High-performance liquid chromatography (HPLC) grade acetonitrile, methanol and water were purchased from Merck (Mumbai, India). Boeravinone B and eupalitin standards were purchased from Natural Remedies Pvt. Ltd., Bangalore, India.
Bacterial strains used
Three strains of A. rhizogenes, viz, A4 (a kind gift from Prof. D. Tepfer, INRA, Versailles Cedex, France), ATCC 15834 and SA79 (kind gift from Dr. Alok Sinha, NIPGR, New Delhi, India) were used in the present study, which were grown in liquid YMB (Hooykaas et al. 1977) medium (OD600nm = 1.0) following our earlier reported protocol (Pandey et al. 2014).
Induction and establishment of hairy roots
The induction of hairy roots was done using the third and fourth leaves and apical portion of stem segments (1.0 cm in length) of 8-week-old, in vitro-established B. diffusa plants following standard protocol. In addition to the needle-pricking method, immersion of the cut-ends of both leaf and nodal explants in bacterial suspension for 10 min followed by co-cultivation for 2–4 days on basal MS medium was also carried out for optimizing the maximum root induction rate. After 2–4 days of co-cultivation with the individual bacterial strain (depending upon the manifestation of bacterial growth on the media surface), the explants were transferred onto the basal MS medium containing 1.0 g/l of cefotaxime (Alkem, India) under dark conditions. Analogous explants, wounded in similar method but without any bacterial inoculation, were cultured under uniform conditions as controls.
The emerging HR clones were initially transferred to the half and full strengths of semi-solid MS medium containing 3 % (w/v) sucrose to facilitate their proliferation and were subsequently established through transferring them separately to liquid half and full strengths of MS medium with the same concentration of antibiotic by incubating on a rotary shaker in the dark at 25 ± 2 °C under constant agitation (80 rpm). The antibiotic concentration was progressively lowered and finally completely omitted after 8 months of establishment through checking the total elimination of A. rhizogenes contamination.
Growth kinetic studies and characterization
The growth properties of 20 independently generated hairy root clones, irrespective of their strain-specific origin, were evaluated on the basis of total root elongation (cm), lateral branching/centimetre of primary roots and fresh weight (FW) increment after 10 days of their cultivation in liquid MS medium containing 3 % sucrose. On the basis of promising growth potentials, four rhizoclones were selected and were subjected to growth performance analysis at 12 days interval from 12 to 60 days of culture. The transformed nature of these independently generated putative hairy root clones was confirmed through polymerase chain reaction (PCR) according to the earlier published protocol (Pandey et al. 2014) using rol gene-specific (B and C) primers.
Scaling up of hairy root culture in bioreactor
The best performing HR clone of B. diffusa (i.e. RBH) was selected for scale-up studies in a 10 l bioreactor (BioFlow 3000, M/s New Brunswick Scientific, USA). Since the selected RBH clone was found amenable to grow as submerged culture in liquid medium, it was cultivated in the liquid-phase culture environments of the aforesaid bioreactor through certain modifications following our earlier patented procedure (Banerjee et al. 2003). In short, the presently optimized amendments mainly included incorporation of (i) an autoclavable nylon mesh support, tightened to the central shaft of the glass culture vessel for providing anchorage to the roots; (ii) two standard ring spargers at both sides of the central nylon mesh and (iii) with or without two marine impellers. Basically, for the cultivation of the selected RBH rhizoclone of B. diffusa, the bioreactor has been modified to give the configurations of either a bubble-column reactor (without any impeller) with two standard ring spargers at both sides of the central nylon mesh or that of a modified “stirred-tank” reactor through employing dual spargers on either side of the central support mesh and two marine impellers, placed at 3–4 cm above these two spargers on each side of the central support.
The impeller speed was kept constant at 75 rpm after an initial wait of 5 days to allow proper anchorage of the inoculated roots on the mesh surface. Sterile gas from an air pump was added using a flow meter after passing through an air filter (Whatman USA; 0.22 μm) to obtain regulated mixing and aeration (2.5 l/min) during the entire run period. The pH and temperature of the cultures (maintained at 5.8 and 25 °C, respectively) were monitored by connecting a pH electrode and temperature sensor to the bioreactor. The initial inoculum (10.23 ± 1.3 g FW) of RBH hairy root clone was inoculated in the bioreactor containing 4 l of half-strength MS medium. Biomass accumulation was monitored by fresh and dry weight determinations after 48 days of cultivation.
Quantification of metabolites by HPLC
The biosynthetic potentials of two independent HR clones of B. diffusa (i.e. RBH of A4 origin and RBT8 of ATCC 15834 origin) and control roots from in vivo-grown plants (>2 years old) were analyzed through quantification of two targeted metabolites (boeravinone B and eupalitin) using reverse phase HPLC (RP HPLC) (Waters Corporation, USA). The RP HPLC conditions were optimized for accurate quantification of these two metabolites using C18 column (250 × 4.6 mm, Waters) following certain modifications of an earlier reported protocol (Pandey et al. 2015). Concisely, the mobile phase consisted of solvent A as acetonitrile and solvent B as water (75:25) in isocratic elution mode with flow rate of 0.8 ml/min. All the extracts and standard stock solutions were prepared and purified according to our previously reported method (Pandey et al. 2015). The injection volume and detector wavelength were 15 μl and 274 nm, respectively. The identifications of the targeted compounds in the samples were done by comparing their UV absorbance and retention time with that of the authentic standards. Quantitative analyses of boeravinone B and eupalitin were based on their respective calibration curves.
Biological activity
Estimation of antioxidant enzymes
Fresh control roots from in vivo-grown plants (>2 years old) and RBH clone of B. diffusa (200 mg) were powdered in liquid nitrogen and homogenized in 1.2 ml of 0.2 M potassium phosphate buffer (pH 7.8 with 0.1 mM EDTA). The homogenate was centrifuged at 15,000 × g for 20 min at 4 °C, and the supernatant was collected and preserved at 4 °C for further use.
Ascorbate peroxidase (APX) activity was estimated according to the method of Nakano and Asada (1981) with some modifications. A gradual decrease in optical density at 290 nm was observed and noted for 3 min. In brief, 250 μl of the reaction cocktail was containing 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.5 mM H2O2 and 5 μl of the root homogenate. Results were expressed in terms of millimolar of ascorbate/min/g FW, which was calculated with the help of extinction coefficient of 2.8 mM/cm for reduced ascorbate.
The amount of catalase enzyme was quantified by the method of Aebi and Lester (1984) with slight modifications which is based on the principle of breakdown of H2O2. Decrease in absorbance at 240 nm for 3 min was observed and recorded with UV/vis spectrophotometer (Spectramax Plus 384 with Softmax pro v 5.3 software–Molecular Device Corp, USA). For the catalase estimation, 300 μl of assay mixture containing 5 μl of root extract, 50 mM potassium phosphate buffer (pH 7.0) and 10 mM H2O2 solution was dispensed in microplate and the absorbance was recorded. The results were expressed in terms of mM of H2O2/min/g FW, which was calculated with the help of molar extinction coefficient of H2O2 (40 mM/cm at 240 nm).
The estimation of superoxide dismutase (SOD) is based on the ability of enzyme to inhibit the autoxidation of pyrogallol in the presence of EDTA in the pH range from 7.9 to 10.6 (Marklund and Marklund 1974). The reaction mixture (300 μl) containing 280 μl of 50 mM phosphate buffer (pH7.9) with 1 mm of EDTA was taken and mixed with 10 μl of pyrogallol (2 mM), and the increase in absorbance was recorded for 5 min at 420 nm. The 50 % inhibition corresponds to 1U of superoxide dismutase and it is expressed as unit per milligram of fresh weight of extract.
Non-enzymatic antioxidant activity
The total phenolic content in the extracts of B. diffusa RBH hairy root clone and control roots from in vivo-grown plants (>2 years old) was determined using the method of Singleton and Rossi (1965) and expressed in terms of gallic acid equivalence (Luqman et al. 2012).
The total flavonoid content was determined using a spectrophotometric method as described by Meda et al. (2005). The extracts were added at different concentrations (10, 25, 50, 100 μg/ml) to 10 % AlCl3 and 1 M potassium acetate followed by the recording of the optical density at 415 nm. The total flavonoid content was determined using a standard curve with quercetin as the standard, and the results were expressed in terms of quercetin equivalent (Ahmad et al. 2014).
The free radical scavenging activity of RBH hairy root clone and control roots from in vivo-grown plants (>2 years old) was determined with 2,2-diphenyl-1-picrylhydrazyl (DPPH) according to the method of Chung et al. (2002). Different concentrations (10, 25, 50, 100 μg/ml) of extracts were added to 100 mM Tris-HCl buffer (pH 7.4) and 500 μM of DPPH. The reaction cocktail was incubated for 30 min in the dark at room temperature, and the absorbance was measured at 517 nm against a buffer blank. The scavenging potential was calculated with respect to control and expressed in percentage.
Antibacterial activity
The antibacterial activity of CHCl3, EtOAc and MeOH extracts of RBH hairy root clone and control roots from in vivo-grown plants (>2 years old) was determined against Gram positive (Staphylococcus aureus, Streptococcus mutans) and Gram negative (Escherichia coli and Pseudomonas aeruginosa) bacteria, according to the method described by Luqman et al. (2008).
Anti-inflammatory activity
The effect of MeOH extract of RBH hairy root clone and control root from in vivo-grown plants (>2 years old) was applied on the production of pro-inflammatory markers i.e. pro-inflammatory cytokines (TNF-α and IL-6) and nitric oxide. The quantification of pro-inflammatory cytokines through ELISA from cell culture supernatant was carried out as per the method described by Singh et al. (2014), and the levels of nitric oxide production in the supernatant were carried out according to a previously reported method (Sharma et al. 2014).
Statistical analysis
The values were expressed as mean ± SD of three independent tests in duplicates (n = 3). The mean, SD and Pearson’s coefficients were calculated with the help of MS Office Excel version 2007. The Welch's corrected t test was used for the antioxidant activities to compare the enzymatic activity of RBH clone to normal root as calculated in GraphPad Instat version 3.06. The anti-inflammatory data were also expressed as mean ± SEM and analyzed statistically using GraphPad Prism 4. ANOVA followed by Tukey’s multiple comparison test was used to assess the statistical significance of unstimulated vs LPS-stimulated cells as well as vehicle-treated stimulated cells vs extract treatment-stimulated cells. Differences with a p value of <0.05 were considered significant.
Results
The establishment of protocol for rapid in vitro multiplication of B. diffusa L. through auxiliary bud proliferation seemed an essential prerequisite to execute genetic transformation studies. A maximum of 4.5 ± 0.5 shoots was produced from a single nodal segment of field-grown plant after 4 weeks of transfer to MS medium supplemented with 1.0 mg/l BAP (data not shown). The maximum number of root formation was observed with half-strength MS medium supplemented with 1.0 mg/l IBA, which resulted into the establishment of complete plantlets to be used as explant source for the genetic transformation studies.
Induction and establishment of hairy roots
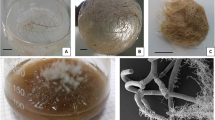
The influence of three different strains of A. rhizogenes, namely A4, ATCC 15834 and SA79, in inducing hairy roots on the stem segments and leaf explants of B. diffusa was investigated. Only the leaf explants of B. diffusa showed susceptibility towards A. rhizogenes-mediated hairy root induction where roots emerged after 4 weeks of inoculation (Fig. 1a, b), while the stem segments failed to reveal any result even after the addition of acetosyringone. The transformation frequency in B. diffusa differed amongst the tested strains, and the maximum root induction was noted with the ATCC 15834 strain (46.82 ± 1.2 %), which was moderately higher than that with the A4 strain (42.31 ± 2.14 %), while the SA79 strain revealed the minimum (21.03 ± 3.1 %) root induction frequency (Fig. 1e).
Induction and establishment of hairy roots in B. diffusa with different strains of A. rhizogenes. a, b Hairy root emergence at the cut ends with the (a) ATCC 15834 and (b) A4 strain respectively. c Best elongation and lateral branching in a selected HR clone (RBH) of A4 origin. d Successful establishment of RBH rhizoclone in half-strength liquid MS medium. e Specific transformation frequency with individual A. rhizogenes strain relating to leaf explants (magnification (a–d) = 1 cm)
In addition to the observed bacterial strain preferentiality and explants’ specificity, the applied method of wounding as well as the duration of co-cultivation has also been found to impact the overall hairy root induction process in B. diffusa. As opposed to the needle-pricking method, the immersion of cut-ends of leaf explants in bacterial suspension for 10 min followed by 2 days of co-cultivation was found most ideal for achieving the maximum root induction frequency in B. diffusa, and threefold higher root induction frequency could be obtained regardless of the bacterial strains used (Fig. 1a, b).
Growth kinetics analysis and confirmation of Rol genes through PCR
The morphology and the growth behaviour of the induced hairy root clones originating through different bacterial strains were found to differ significantly, and the maximum growth potential (i.e. root elongation + lateral branching) was observed amongst four hairy root clones out of 20 individually generated rhizoclones, irrespective of their strain specific origin (Fig. 2), which were subjected to growth performance analysis. Two hairy root clones of A4 (RBH) and ATCC 15834 (RBT8) origin demonstrated higher growth potential as compared to two other clones of ATCC 15834 (RBT3) and SA79 (RBSA1) origin and accumulated maximum biomass (i.e. GI 722.5 ± 18.45 and 483.5 ± 17.83 FW respectively) on the 48th day of culture in half-strength liquid MS medium (Fig. 2). Then again, the last two clones (i.e., RBT3 and RBSA1) attained their maximum growth (i.e. GI 160.5 ± 16.3 and 94.5 ± 6.93 FW respectively) on the 36th day of culture in the same medium (Fig. 2).
The maximum lateral branching, root elongation and rapid multiplication in liquid medium could be obtained with the RBH clone of A4 origin (Fig. 1c, d) followed by the RBT8 clone of the ATCC 15834 bacterial origin, which were selected for further studies concerning their potential to synthesize the two targeted metabolites (i.e. boeravinone B and eupalitin) at the optimum growth phase (48 days of culture) with respect to that in the control roots of field-grown plants (>2 years old). PCR analysis revealed the presence and expression of the rol B and rol C genes (Fig. 3a, b) in all the four selected rhizoclones of B. diffusa, indicating the integration of the Ri T-DNA as against their absence in the control roots.
Quantification of secondary metabolites
The RP HPLC-based quantitative analysis of the crude methanol, chloroform and ethyl acetate extracts of untransformed control roots of field-grown plants and the two hairy root clones (RBH and RBT8) with respect to the standard eupalitin and boeravinone B revealed the best resolution at 274 nm with the retention time of 4.019 and 4.574 min respectively, which remained consistent to that of the standard boeravinone B and eupalitin (Fig. 4a, b), having peak purity of >98 %. Ethyl acetate demonstrated better extractability in case of boeravinone B, whereas methanol showed best results relating to eupalitin (Fig. 5). An improved accumulation of boeravinone B could be noted in the untransformed control root extracts in comparison to that in both the hairy root clones at their 48th day of cultivation (Fig. 5). The highest yield of boeravinone B (0.027 ± 0.001 mg/g DW) could be obtained from the ethyl acetate extract of the untransformed control roots of field-grown plants, which was ∼5 times higher than that in either of the two rhizoclones (extracted with the same solvent) at their maximum levels of synthesis (Fig. 5). On the contrary, both the rhizoclones demonstrated enhanced production of eupalitin over that in the untransformed control root, and amongst the two, the RBH rhizoclone demonstrated the higher accumulation of the same, which was 1.44 times greater than that in the control root (Fig. 5).
Scaling up of selected rhizoclone in bioreactor
In view of the fact that the selected RBH hairy root clone of B. diffusa was amenable to grow as submerged culture in liquid medium (Fig. 1d), it was cultivated in a modified 10 l bioreactor (BioFlow 3000, M/s New Brunswick Scientific, USA), fitted with a central nylon mesh to provide anchorage (Fig. 6a, b); and parameters, such as presence or absence of impeller, position of sparger, airflow rate and impeller speed, were optimized through independent batch cultures following our earlier patented procedure (Banerjee et al. 2003).
Up-scaling of the RBH rhizoclone of B. diffusa in bioreactor. a Bioreactor setup of BioFlow 3000 (with 10-l capacity–M/s New Brunswick Scientific, USA). b Biomass enhancement after 48 days of cultivation in half-strength MS medium. . (The arrow indicates the position of the nylon mesh, incorporated for the anchorage and distribution of HR mass). c Root mass accumulation at harvesting
The growth of the hairy roots was too little (3.8-fold in FW) in the bubble-column reactor since the hairy roots formed intertwined clusters at isolated places in this configuration during the present study. On the contrary, the modified stirred-tank reactor of the present study equipped with central nylon-mesh support, marine impellers and standard ring spargers facilitated high-density culture of the RBH hairy root (Fig. 6b, c) with the best biomass yield (GI = 730.49 ± 10.89), which was moderately superior to that noted in the 250-ml shake flasks (GI = 721.67 ± 17.21). This result not only indicates the effectiveness of the presently optimized process but also calls for further optimization to boost up the biomass accumulation.
The RP HPLC-based quantitative analysis of boeravinone B and eupalitin in the crude ethyl acetate extracts of untransformed control root and the RBH hairy root clone in both shake-flask and 10 l bioreactor indicated almost 6.1-fold enhancement in the boeravinone B content in the bioreactor-grown culture compared to the shake flask culture, which even marginally (1.11-fold) superseded the yield in the control roots (Table 1). Likewise, compared to shake flask, the yield increment of eupalitin in the RBH clone was also enhanced by 1.15-fold through bioreactor up-scaling, which was 2.38-fold higher over that in the control roots (Table 1).
Biological activity assay
The biological activity of the selected RBH rhizoclone of B. diffusa was evaluated on the basis of enzymatic/non-enzymatic antioxidant levels, antimicrobial efficacy and anti-inflammatory potential compared with that of the untransformed control roots of field-grown plant.
Enzymatic antioxidant activity
Evaluation of enzymatic antioxidant levels (i.e. SOD and CAT) in the selected RBH rhizoclone of B. diffusa revealed 1.79- and 1.41-fold higher SOD and CAT activities respectively with reference to the same in the control roots (Table 2). Contrarily, the APX level was found significantly higher (8.95-fold) in the control roots over that in the RBH rhizoclone (Table 2).
Non-enzymatic antioxidant activity
Amongst the different tested extracts, the EtOAc extract of control root revealed the highest amount of phenolic compounds (16.63 ± 0.120 GAE), whereas its maximum level in the RBH hairy root clone (7.93 ± 0.33 GAE) could be obtained in the CHCl3 extract at 100 μg/ml concentration (Fig. 7a). The extracts of B. diffusa RBH HR clone and control root were also analyzed relating to total flavonoid content which showed similar trend of higher flavonoid content at 100 μg/ml concentration in the EtOAc extract (20.7 ± 0.87 QE) of control root (Fig. 7b). On the other hand, the RBH hairy root clone showed its highest total flavonoid content (8.15 ± 0.24 QE) in MeOH extract (Fig. 7b).
Estimation of the antioxidant potential of the RBH rhizoclone (HR) of B. diffusa in a dose-dependent manner in comparison to that in the control roots (CR), extracted with three different solvents. a Total phenolics. b Total flavonoids. c Free radical scavenging (DPPH) activity. (The values are mean ± SD of three independent experiments in replicates at each concentration)
All the tested extracts of B. diffusa control root and RBH hairy root clone demonstrated significant radical scavenging (DPPH) properties (Fig. 7c). The MeOH extract of control root demonstrated highest scavenging potential of 97.91 ± 1.06 % at 100 μg/ml concentration. Amongst the RBH hairy root extracts, the CHCl3 extract exhibited the best DPPH radical scavenging potential with percent inhibition of 91.50 ± 0.60 at the same concentration (Fig. 7c).
Antibacterial activity
The magnitude of inhibition differed amongst the different extracts (MeOH, CHCl3 and EtOAc) used with EtOAc proving superiority to others (Fig. 8). The zone of inhibition (ZOI) pertaining to the responding extracts ranged from 2 to 7 mm at concentration of 100 μg/disc (Fig. 8). All the tested extracts of control and RBH hairy root clone showed inhibitory activity against Staphylococcus aureus. Noticeably, in the case of S. aureus, the EtOAc extracts of RBH clone showed maximum growth inhibition (7 mm ZOI) followed by the same solvent extract of the control roots (6 mm ZOI). On the other hand, the EtOAc extract of control and RBH clone showed comparable inhibition against Streptococcus mutans and Pseudomonas aeruginosa (Fig. 8). In the case of Escherichia coli, both the RBH clone and control roots in all the tested solvents showed effective growth inhibition (2–7 mm ZOI) with the highest activity in the CHCl3 extract of control root (Fig. 8).
Antibacterial activity of the RBH rhizoclone (HR) of B. diffusa against pathogenic Gram-positive (Staphylococcus aureus, Streptococcus mutans) and Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacteria in comparison to that in the control roots (CR), extracted with three different solvents. The values are the means of three replicates ± SD
Quantification of pro-inflammatory mediators
In an attempt to evaluate the potential of the selected RBH rhizoclone pertaining to this specific function, the production of pro-inflammatory cytokines (TNF-α, IL-6) and nitric oxide (NO) was analyzed. The levels of TNF-α, IL-6 and NO were significantly (p < 0.05) increased in LPS-stimulated cells compared to the un-stimulated control cells. The MeOH extracts of RBH hairy root clone and control root exhibited significant (p < 0.05) inhibition of pro-inflammatory cytokines (Table 3) and NO (Fig. 9) production when compared with vehicle-treated LPS-stimulated cells. When the anti-inflammatory activity of root extracts was compared, the RBH clone displayed higher potential compared to that of the control root.
Discussion
Induction and establishment of hairy roots
Seeing that A. rhizogenes strain specificity together with plant genotype and explants factors has played a crucial role in determining the overall success of the underlying transformation events (Ooi et al. 2013), premeditated optimization of such decisive factors seemed realistic to reap the best advantage of applying this hairy root technology in B. diffusa. The presently observed exclusive susceptibility of leaf explants and total failure of stem segments towards A. rhizogenes-mediated hairy root induction contradict the earlier findings of Sahu et al. (2013, 2014), who have demonstrated notable susceptibility of intermodal segments of in vivo-grown B. diffusa plants towards the peak limit of hairy root induction. The disparity in the maturity levels of the utilized explants could be one of the causes for the observed difference in susceptibility of the nodal segments, as the maturation status of explants predominantly impacts the availability of functionally competent cells (as well as their internal hormonal levels), which essentially serve as ideal targets for hairy root induction (Potrykus 1990).
The currently documented preferentiality of B. diffusa leaf explants towards the three selected A. rhizogenes strains reiterates earlier observations where the transformation efficiencies of the ATCC 15834 and A4 strains have differed according to the plant system under study, and both their supremacies as well as failures have been documented earlier (Fu et al. 2005; Ooi et al. 2013; Setamam et al. 2014). In tandem, the prevailing lesser compatibility threshold of the SA79 strain towards B. diffusa substantiated a previous report involving Catharanthus roseus (Batra et al. 2004). However, higher virulence of the SA79 strain over other A. rhizogenes strains including the A4 could also be noted in case of Bacopa monnieri (Bansal et al. 2014) contradicting our present observation. Host plant-specific receptiveness towards designated A. rhizogenes strain, in combination with the compatibility threshold of certain explants, might primarily be accountable for the presently observed trend, which has already been exemplified in several earlier studies (Zehra et al. 1999; Akramian et al. 2008; Roychowdhury et al. 2013).
The presently noted better outcome of the cut-end immersion technique of leaf explants in bacterial suspension as opposed to the conventionally used needle-pricking method clearly indicated that apart from the recognized bacterial strain and explant specificities, the applied method of wounding with the co-cultivation duration can exert a controlling influence on the overall hairy root induction process in B. diffusa. The requirement of an accurate time duration and optimum cell surface area for maximum interaction with metabolically active cells and unsuppressed signal transduction leading to T-DNA transfer/integration necessitates optimization of wounding process and co-cultivation duration, which have already been highlighted earlier in several instances (Swain et al. 2012; Bansal et al. 2014).
Growth kinetics analysis
The observed range of morphological and growth behaviour diversities in the ensuing hairy root clones has offered the privilege of selecting four promising hairy root clones out of 20 individually generated rhizoclones, irrespective of their strain-specific origin. The prevalence of intra-clonal divergence has further facilitated the selection of two best performing rhizoclones of A4 (RBH) and ATCC 15834 (RBT8) origin, demonstrating higher growth potential as compared to the two other clones of ATCC 15834 (RBT3) and SA79 (RBSA1) origin. Such observation has yet again reiterated the unanimously acknowledged fact that differential interaction of the Ri T-DNA with the host plant genome, in parallel with the variance in integration sites and copy numbers, imparts enormous chance of generating variability within the resultant rhizoclones in terms of growth and productivities (Roychowdhury et al. 2013). Strategic screening and selection have ultimately proved to be genuinely realistic for making a choice amongst the cream of the crop to fulfil the entire purpose of generating functionally eminent, elite hairy root clone in B. diffusa.
Quantification of secondary metabolites
The RP HPLC-based quantification of eupalitin and boeravinone B contents in the two selected hairy root clones (RBH and RBT8) of B. diffusa has not only revealed their potential to synthesize both the molecules of therapeutic significance but also proved superior to control roots of field-grown plants in terms of eupalitin production, which signifies the future bearing of the present study in terms of anticancer drug development since eupalitin has already proved efficacious as an anticancer molecule for treating human colorectal tumour cells (Ghalib et al. 2013).
Scaling up of selected rhizoclone in bioreactor
Typically, growing the three dimensional structure of hairy roots in the containment of bioreactors under defined conditions reflects the need of optimizing a combination of factors to achieve competitive performance (Stiles and Liu 2013). Most of these factors are principally governed by certain technical issues that entail serious challenges and require special attention to comply with the changeable specifications which inadvertently differs from plant to plant owing to the variable morphology and textures of the resultant hairy roots (Eibl and Eibl 2008; Georgiev 2014).
On the basis of the observed amenability of the selected RBH hairy root clone to grow as submerged culture in liquid medium, the optimization of the bioreactor up-scaling parameters was carried out in a modified 10 l bioreactor at two different modes, i.e. either a bubble-column reactor (without any impeller) or a modified stirred-tank reactor with dual spargers and marine impellers, which has evidently demonstrated the better performance of the latter in terms of root biomass yield, while the former revealed poor root-yielding threshold. The formation of intertwined clusters of root mass at isolated places within the reactor vessel can account for the presently perceived inferior biomass production during up-scaling in the bubble-column reactor, which also corroborated the earlier finding where conventional bubble-column reactor design failed to deliver positive result involving Azadirachta indica hairy roots (Srivastava and Srivastava 2013). Such unsatisfactory performance might be due to inadequate fluid mixing and oxygen mass transfer limitations as observed under several other instances (Stiles and Liu 2013).
Conversely, the efficiency of the presently optimized modified stirred-tank reactor of the present study (equipped with central nylon mesh support, marine impellers and standard ring spargers) became strongly evident as it not only facilitated high-density biomass growth of the RBH hairy root clone but also elevated the production of both boeravinone B and eupalitin to surpass their levels of the same in the shake flask-grown cultures as well as in the control roots of the >2-year-old field-grown plants. Good fluid mixing, adequate oxygen transfer and low shear stress might be the contributing factors for such enhanced biomass and metabolite accumulations in the presently modified bioreactor, because optimization of these parameters has been designated as essential prerequisite in many instances for improving the yield potentials of hairy roots in an optimized bioreactor environment (Stiles and Liu 2013).
In the present scenario, the credit can be attributed to the combined use of the marine impellers and the spargers, which functioned optimally in agreement with our earlier findings (Banerjee et al. 2003). The marine impellers ensured suitable oxygen mass transfer at high root-grown areas through its axial flow generation pattern without causing detrimental shear damage because of its low shear stress level (Zhong et al. 1994). Moreover, the presently adopted improvisation technique of supplying air through the spargers and at the same time prevention of bubbles from coalescing owing to the presence of the marine impellers proved highly effective for surpassing the performance of the shake flask cultures as demonstrated earlier (Banerjee et al. 2003). Literature survey corroborates the present findings by revealing the successful use of either polypropylene mesh or perforated Teflon mesh as a support for anchorage of roots in different types of reactors including bubble-column or stirred-tank reactors of 3-l capacity for hairy root cultivation (Srivastava and Srivastava 2013; Patra and Srivastava 2014). Noticeably, the present study reports the optimization parameters in a 3.3 times bigger volume bioreactor imposing additional challenges of mass transfer limitations pertaining to O2 and nutrient supply at higher root-growing areas. Overcoming this bottleneck through the use of the marine impellers and spargers at specific position (even within the root growing zone) without causing any shear damage highlights the significance of these observations which can be implemented for commercial advantages.
Biological activity
The biological activity potential of the selected RBH rhizoclone of B. diffusa was analyzed to ascertain its worth in comparison to that of the control roots of field-grown plant. B. diffusa is also recognized by its vernacular name of “Punarnava”, the meaning of which in Sanskrit to English translation signifies its therapeutic potential as it symbolizes the plant “which rejuvenates the body” (Awasthi and Verma 2006). The “rejuvenation” power of any plant system has shown a direct bearing on its “antioxidant-making” efficiency (Matkowski 2008). Hence, the antioxidant activity of the presently established RBH rhizoclone of B. diffusa was determined to comprehend its healing merit in the background of the control roots, which is otherwise widely renowned for such functions (Mishra et al. 2014).
Enzymatic antioxidant activity
The overproduction of SOD and CAT and decreased level of APX in the RBH clone as compared to control root (p < 0.05) indicate better antioxidant utility of the presently raised rhizoclone, which might have happened due to Ri T-DNA-induced activation of ROS signalling cascade (Allen et al. 1995). B. diffusa leaves are also testified to have noticeable SOD and catalase activities as documented earlier (Premkumar et al. 2010).
Non-enzymatic antioxidant activity
A considerable augmentation of free radical scavenging activity was evident in the extracts of B. diffusa in a concentration-dependent mode. In accordance to the proven facts that the most important exogenous antioxidants are normally rich in polyphenols and flavonoids (Larson 1988; Berger 2005), the tested extracts were also found to contain high amounts of phenolic and flavonoid group of molecules which might support the antioxidant potential of B. diffusa extracts with correlation coefficient value from 0.04 to 0.88. Comparatively lower amount of total phenolics in the RBH clone of B. diffusa over that of the control roots can be attributed to the maturation levels of the two (as the hairy root was 16.2-fold lower age) as well as the influence of environmental factors. The DPPH scavenging activity of the RBH hairy root clone is comparable to that of control roots of field-grown plant, which also substantiates an earlier report showing noticeable DPPH scavenging activity of B. diffusa normal root (Khalid et al. 2011).
Antibacterial activity
There are only a handful reports available regarding the antibacterial activity of B. diffusa root extract (Mishra et al. 2014). Different solvents have been used to screen the antibacterial property of both root and aerial parts of B. diffusa with variable results involving both Gram-negative and Gram-positive bacteria (Murti et al. 2010; Ramachandra et al. 2012; Malhotra et al. 2013; Sahu et al. 2013). Enhanced antimicrobial activity of RBH hairy root clone than that of its control roots against S. aureus has corroborated a previous report demonstrating better performance of Maytenus senegalensis hairy roots than the control roots against the same bacterium (Jain et al. 2008). Apparently, no direct correlation could be established between the presently measured metabolite contents and the antibacterial potentials of either of the control or hairy root clone. However, since the reported effectiveness of B. diffusa’s use as an adjuvant to chemotherapy on pulmonary tuberculosis has generated renewed interest in novel activities of this plant (Kant et al. 2001), the better or comparable antibacterial efficacy of the RBH clone of B. diffusa than that of its control roots (15.21 times older) against S. aureus, Streptococcus mutans and P. aeruginosa for the first time unearthed new possibilities, expanding the scope of such hairy root’s application in generating new remedy involving other pathogenic bacteria.
Anti-inflammatory activity
Pro-inflammatory mediators, namely TNF-α, IL-6 and NO in LPS-induced macrophages, are known to have profound effects on the regulation of immune reactions, haematopoiesis and inflammation (Christiaens et al. 2008). Several inflammatory mediators are induced by oxidative stress. This study, to the best of our knowledge, provides the first clue that the RBH extracts of B. diffusa exhibit superior anti-inflammatory activity than the control roots by inhibiting the production of pro-inflammatory mediators in LPS-induced inflammation in macrophages. The overproduction of these cytokines has been implicated in the pathogenesis of many disease processes in mammals. The control of macrophage overproduction of these mediators should greatly facilitate the treatment of many inflammation-linked diseases (Shishodia and Aggarwal 2006). The selected B. diffusa rhizoclone can therefore be regarded as a suitable substitute of the field-grown roots which can perpetuate the potential to rejuvenate the cells by decelerating oxidative stress and inflammation. B. diffusa normal root extracts have earlier been noted to exhibit anti-inflammatory activity as well (Mehrotra et al. 2002; Manu and Kuttan 2009).
Conclusions
What is noteworthy of our present overall findings is that the biosynthetic potential and the bioactivity profile of the established hairy root clones of B. diffusa parallel that of control roots of field-grown plants with 15.21-fold time saving. Besides circumventing the limitations of production instabilities owing not only to the environmental/seasonal/pathological constrains but also to the obligation of prolonged growth requirement (>2 years), the currently developed rhizoclones of B. diffusa for the first time endorsed its practical utility relating to the synthesis of two therapeutically significant high-demand bioactive molecules bearing immense potentials in future drug development. To the best of our knowledge, this study also provides the first evidence of superior anti-inflammatory activity of the hairy root extracts compared to that of the control roots, indicating its potential to rejuvenate the cells by decelerating oxidative stress. The current strategically tailored protocols further ascertained improvements in the synthesis of the two targeted molecules through efficient up-scaling in bioreactor, which added much commercial merit to this study in view of the contemporary HR-based commercial venture in the name of Rootec® (Talano et al. 2012). In view of the credible assertion of the stability of hairy root cultures in sustaining long-term productivity of desired metabolites along with settlement of cost-related concerns as documented recently (Pandey et al. 2014), the present findings bear distinctive significance to sustain the boosted demand of this time-honoured medicinal plant for future drug development in the years to come.
References
Aebi H, Lester P (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad A, Singh DK, Fatima K, Tandon S, Luqman S (2014) New constituents from the roots of Oenothera biennis and their free radical scavenging and ferric reducing activity. Ind Crops Prod 58:125–132
Ahmed-Belkacem A, Macalou S, Borrelli F, Capasso R, Fattorusso E, Taglialatela-Scafati O, Pietro AD (2007) Nonprenylated rotenoids, a new class of potent breast cancer resistance protein inhibitors. J Med Chem 50:1933–1938
Akramian M, Tabatabaei S, Mirmasoumi M (2008) Virulence of different strains of Agrobacterium rhizogenes on genetic transformation of four Hyoscyamus species. Am-Eur J Agric Environ Sci 3:759–763
Allen RG, Keogh BP, Gerhard GS, Pignolo R, Horton J, Cristofalo VJ (1995) Expression and regulation of superoxide dismutase activity in human skin fibroblasts from donors of different ages. J Cell Physiol 165:576–587
Awasthi LP, Verma HN (2006) Boerhaavia diffusa–a wild herb with potent biological and antimicrobial properties. Asian Agri History 10:55–68
Bairwa K, Singh IN, Roy SK, Grover J, Srivastava A (2013a) Rotenoids from Boerhaavia diffusa as potential anti-inflammatory agents. J Nat Prod 76:1393–1398
Bairwa K, Singh IN, Roy SK, Grover J, Srivastava A (2013b) Correction to rotenoids from Boerhaavia diffusa as potential anti-inflammatory agents. J Nat Prod 76:2364
Banerjee S, Kukreja AK, Verma PC, Kahol AP, Kumar S (2003) U.S. patent no. 6,589,780. US Patent and Trademark Office, Washington, DC
Bansal M, Kumar A, Reddy MS (2014) Influence of Agrobacterium rhizogenes strains on hairy root induction and ‘bacoside A’ production from Bacopa monnieri (L.) Wettst. Acta Physiol Plant 36:2793–2801
Batra J, Dutta A, Singh D, Kumar S, Sen J (2004) Growth and terpenoid indole alkaloid production in Catharanthus roseus hairy root clones in relation to left- and right-termini-linked Ri T-DNA gene integration. Plant Cell Rep 23:148–154
Berger MM (2005) Can oxidative damage be treated nutritionally? Clin Nutr 24:172–183
Bhansali RR, Kumar A, Arya HC (1978) In vitro induction of adventitious shoots on stem explants of Boerhaavia diffusa L. Curr Sci 47:551–552
Borrelli F, Ascione V, Capasso R, Izzo AA, Fattorusso E, Taglialatela-Scafati O (2006) Spasmolytic effects of nonprenylated rotenoid constituents of Boerhaavia diffusa roots. J Nat Prod 69:903–906
Chaudhary G, Dantu PK (2011) Morphological, phytochemical and pharmacological studies on Boerhaavia diffusa L. J Med Plants Res 5:2125–2130
Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM (2008) Inflammatory processes in preterm and term parturition. J Reprod Immunol 79:50–57
Chung YC, Chang CT, Chao WW, Lin CF, Chou ST (2002) Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J Agric Food Chem 50:2454–2458
Eibl R, Eibl D (2008) Design of bioreactors suitable for plant cell and tissue cultures. Phytochem Rev 7:593–598
Fu CX, Zhao DX, Xue XF, Jin ZP, Ma FS (2005) Transformation of Saussurea involucrata by Agrobacterium rhizogenes: hairy root induction and syringin production. Process Biochem 40:3789–3794
Georgiev MI (2014) Design of bioreactors for plant cell and organ cultures. In: Paek KY, Murthy HN, Zhong JJ (eds) Production of biomass and bioactive compounds using bioreactor technology. Springer, New York, London
Ghalib MR, Mehdi SH, Hashim R, Sulaiman O, Foong FH, Ahamed BM, Majid A, Ahmed F (2013) Eupalitin from Asparagus falcatus (Linn.) has anticancer activity and induces activation of caspases 3/7 in human colorectal tumor cells. J Med Plants Res 7:1401–1405
Gomes AD, Vaidya VV, Bhagat RD, Gadgil JN (2013) Separation and quantification of pharmacologically active markers boeravinone B, eupalitin-3-o-β-d galactopyranoside and β-sitosterol from Boeravia diffusa Linn. and from marketed formulation Int. J Pharm Pharm Sci 5:953–956
Hooykaas PJJ, Klapwjik PM, Nuti MP, Schilperoort RA, Rorsch A (1977) Transfer of the A. tumefaciens Ti plasmid to avirulent Agrobacteria and Rhizobium ex planta. J Gen Microbiol 98:477–484
Jain N, Light ME, Van Staden J (2008) Antibacterial activity of hairy-root cultures of Maytenus senegalensis. S Afr J Bot 74:163–166
Jenifer U, Cecilia FK, Ravindhran R (2012) In vitro adventitious root and hairy root cultures in Boerhaavia diffusa L. Int J Curr Res 4:65–67
Kant S, Agnihotri MS, Dixit KS (2001) Clinical evaluation of Boerhaavia diffusa as an adjuvant in the treatment of pulmonary tuberculosis. Phytomedica 2:89–94
Khalid M, Siddiqui HH, Fareed S (2011) In vitro estimation of the antioxidant activity and phytochemical screening of Boerhaavia diffusa root extract. Asian J Traditional Med 6:259–266
Larson RA (1988) The antioxidants of higher plants. Phytochemistry 27:969–978
Ludwig-Muller J, Xu J, Agostini E, Georgiev M (2014) Advances in transformed root cultures for root biofactory and phytoremediation research. Root Eng Soil Biol 40:387–405
Luqman S, Dwivedi GR, Darokar MP, Kalra A1, Khanuja SPS (2008) Antimicrobial activity of Eucalyptus citriodora essential oil. Int J Essent Oil Ther 2:69–75
Luqman S, Srivastava S, Kumar R, Maurya AK, Chanda D (2012) Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evid Based Complement Alternat Med pp.12
Malhotra D, Khan A, Ishaq F (2013) Phytochemical screening and antibacterial effect of root extract of Boerhaavia diffusa L. (Family Nyctaginaceae). J Appl Nat Sci 5:221–225
Manu KA, Kuttan G (2009) Immunomodulatory activities of Punarnavine, an alkaloid from Boerhaavia diffusa. Immunopharmacol Immunotoxicol 31:377–387
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Matkowski A (2008) Plant in vitro culture for the production of antioxidants—a review. Biotech Adv 26:548–560
Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 9:571–577
Mehrotra S, Mishra KP, Maurya R, Srimal RC, Singh VK (2002) Immunomodulation by ethanolic extract of Boerhaavia diffusa roots. Int Immunopharmacol 2:987–996
Mishra S, Aeri V, Gaur PK, Jachak SM (2014) Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhavia diffusa Linn. Biomed Res Int 2014:1–19
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murti K, Panchal MA, Lambole V (2010) Pharmacological properties of Boerhaavia diffusa–a review. Int J Pharm Sci Rev Res 5:107–110
Nagarajan SM, Suresh T, Rajasekaran S, Kannan TMS, Kulothungan S (2005) In vitro micropropagation of Boerhaavia diffusa L. Geobios-Jodhpur 32:169–172
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–280
Ono NN, Tian L (2011) The multiplicity of hairy root cultures: prolific possibilities. Plant Sci 180:439–446
Ooi CT, Syahida A, Stanslas J, Maziah M (2013) Efficiency of different Agrobacterium rhizogenes strains on hairy roots induction in Solanum mammosum. World J Microbiol Biotechnol 29:421–430
Pandey P, Kaur R, Singh S, Chattopadhyay SK, Srivastava SK, Banerjee S (2014) Long-term stability in biomass and production of terpene indole alkaloids by hairy root culture of Rauvolfia serpentina and cost approximation to endorse commercial realism. Biotechnol Lett 36:1523–1528
Pandey H, Pandey P, Singh S, Gupta R, Banerjee S (2015) Production of anti-cancer triterpene (betulinic acid) from callus cultures of different Ocimum species and its elicitation. Protoplasma 252:647–655
Patra N, Srivastava AK (2014) Enhanced production of artemisinin by hairy root cultivation of Artemisia annua in a modified stirred tank reactor. Appl Biochem Biotechnol 174:2209–2222
Potrykus I (1990) Gene transfer to plants: assessment and perspectives. Physiol Plant 79:125–134
Prathapan A, Vineetha VP, Raghu KG (2014) Protective effect of Boerhavia diffusa L. against mitochondrial dysfunction in angiotensin II induced hypertrophy in H9c2 cardiomyoblast cells. PLoS One 9(4):e96220–e96220
Premkumar P, Priya J, Suriyavathana M (2010) Evaluation of antioxidant potential of Andrographis echioides and Boerhavia diffusa. Int J Curr Res 3:59–62
Rajpoot K, Mishra RN (2011) Boerhaavia diffusa roots (Punarnava mool)–review as rasayan (rejuvenator/antiaging). Int J Res in Pharm Biomed Sci 2:1451–1460
Ramachandra YL, Ashajyothi C, Rai P (2012) In vitro antibacterial potential of Boerhaavia diffusa. IJAPBC 1(3):420–424
Riaz H, Raza SA, Hussain S, Mahmood S, Malik F (2014) An overview of ethnopharmacological properties of Boerhaavia diffusa. Afr J Pharm Pharmacol 8:49–58
Roychowdhury D, Majumder A, Jha S (2013) Agrobacterium rhizogenes-mediated transformation in medicinal plants: prospects and challenges. In: Chandra S, Lata H, Varma A (eds) Biotechnology for medicinal plants: micropropagation and improvement. Springer, Berlin Heidelberg, pp 29–68
Sahu PR, Khalkho AS (2012) Callus induction and in vitro multiplication of Boerhaavia diffusa–milestone medicinal plant of Jharkhand. Biogeosciences 7:123–127
Sahu L, Jenaab S, Swainc SS, Sahoob S, Chand PK (2013) Agrobacterium rhizogenes-mediated transformation of a multi-medicinal herb, Boerhaavia diffusa L.: optimization of the process and anti-microbial activity against bacterial pathogens causing urinary tract infections. Front Life Sci 7:197–209
Sahu L, Ray DK, Chand PK (2014) Proton induced X-ray emission (PIXE) technique for determining multi-element composition of transformed hairy root cultures of Boerhaavia diffusa L. An important medicinal herb. J Radioanal Nucl Chem 300:345–354
Setamam N, Sidik NJ, Rahaman ZA, Zain C (2014) Induction of hairy roots by various strains of Agrobacterium rhizogenes in different types of Capsicum species explants. BMC Res Notes 7:414
Sharma S, Chattopadhyay SK, Singh M, Bawankule DU, Kumar S (2014) Novel chemical constituents with anti-inflammatory activity from the leaves of Sesbania aculeata. Phytochemistry 100:132–40
Shishodia S, Aggarwal BB (2006) Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the down regulation of Akt, I kappa B kinase activation and NF-kappa B-regulated gene expression. Oncogene 25:1463–1473
Shrivastava N, Padhya MA (1995) Punarnavine profile in the regenerated roots of Boerhavia diffusa L. from leaf segments. Curr Sci 68:653–656
Singh M, Hamid AA, Maurya AK, Prakash O, Khan F, Kumar A, Aiyelaagbe OO, Negi AS, Bawankule DU (2014) Synthesis of diosgenin analogues as potential anti-inflammatory agents. J Steroid Biochem Mol Biol 143:323–33
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Srivastava S, Srivastava AK (2013) Production of the biopesticide azadirachtin by hairy root cultivation of Azadirachta indica in liquid-phase bioreactors. Appl Biochem Biotechnol 171:1351–1361
Stiles AR, Liu CZ (2013) Hairy root culture: bioreactor design and process intensification. Adv Biochem Eng Biotechnol 134:91–114
Sudarshana MS, Niranjan MH, Girisha ST (2008) In vitro flowering, somatic embryogenesis and regeneration in Boerhaavia diffusa Linn.–a medicinal plant. Global J Biotechnol Biochem 3:83–86
Swain SS, Sahu L, Pal A, Barik DP, Pradhan C, Chand PK (2012) Hairy root cultures of butterfly pea (Clitoria ternatea L.): Agrobacterium × plant factors influencing transformation. World J Microbiol Biotechnol 28:729–739
Talano MA, Oller AL, Gonzalez PS S, Agostini E (2012) Hairy roots, their multiple applications and recent patents. Recent Pat Biotechnol 6:115–133
Zehra M, Banerjee S, Sharma S, Kumar S (1999) Influence of Agrobacterium rhizogenes strains on biomass and alkaloid productivity in hairy root lines of Hyoscyamus muticus and H. albus. Planta Med 65:60–63
Zhong JJ, Fujiyama K, Seki T, Yoshida T (1994) A quantitative analysis of shear effects on cell suspension and cell culture of Perilla frutescens in bioreactors. Biotechnol BioEng 44:649–654
Acknowledgments
The authors wish to express their sincere gratitude to the Director of CSIR-CIMAP, for providing the research facilities to carry out this work. Thanks are also due to the University Grants Commission (UGC), Department of Science & Technology (DST), Council of Scientific & Industrial Research (CSIR) and the Indian Council of Medical Research (ICMR)–New Delhi, India, for the financial support in the form of fellowships to RG, PP, SS, DKS and AS, respectively. Sincere appreciation is also due to the Academy of Scientific and Innovative Research, CSIR-CIMAP. The authors gratefully acknowledge the kind support from Dr. Prema Vasudeva. This work was supported by grants from the University Grants Commission (UGC–GAP 211).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Gupta, R., Pandey, P., Singh, S. et al. Advances in Boerhaavia diffusa hairy root technology: a valuable pursuit for identifying strain sensitivity and up-scaling factors to refine metabolite yield and bioactivity potentials. Protoplasma 253, 1145–1158 (2016). https://doi.org/10.1007/s00709-015-0875-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0875-5