Abstract
Seed priming is a method by which seeds are subjected to different stress conditions to impart stress adaptation in seedlings germinating and growing under stressful situations. Drought stress is a major reason behind failure of crops. We studied the effects of hydropriming, dehydration priming (induced by PEG), and osmopriming (induced by NaCl and KH2PO4) on subsequent germination, growth and anti-oxidant defense mechanisms of 2-week-old rice seedlings under continuing dehydration stress. Unprimed seeds grown in PEG showed significantly lower germination and growth along with significantly higher reactive oxygen species (ROS) and lipid peroxidation levels. Among the priming methods, 5 % PEG priming was found to be the best in terms of germination and growth rate along with the lowest amount of ROS and lipid peroxidation (malondialdehyde [MDA]) values. MDA levels were reduced significantly by all of the priming methods. Hence, reduction of lipid peroxidation may be a key factor underlying the drought tolerance produced by the priming treatments. Glutathione peroxidase (GPX) activity seemed to bear an excellent correlation with oxidative stress resistance through seed priming. The PEG priming produced minimum peroxidative damage and superior germination and growth rate along with efficient GPX activity, overexpressed MnSOD and maintenance of HSP70 expression in normal as well as in drought condition. Therefore, in PEG-primed seeds the existence of robust protective mechanisms is definitely indicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most crucial functions of plants is to respond and adapt to stress for self-defense through expression of certain genes (Mittal et al. 2012; Timperio et al. 2008). Drought stress is one of the main causes for crop yield reduction in the majority of agricultural regions of the world. Drought stress induces several physiological, biochemical and molecular responses in crop plants, which may sometimes help them to adapt to limiting environmental conditions (Shehab et al. 2010). Like drought, several different abiotic stresses result in water stress (e.g., salt and cold stresses) (Carvalho 2008). Various abiotic stresses lead to the overproduction of reactive oxygen species (ROS) in plants which are highly reactive and toxic and cause damage to proteins, lipids, carbohydrates and nucleic acids. The ROS comprise both free radicals such as, superoxide radicals (O2 · −), hydroxyl radical (OH−), perhydroxy radical (HO2 −) and alkoxy radicals (RO−), and non-radical forms such as, hydrogen peroxide and (H2O2), and singlet oxygen (1O2) (Gill and Tuteja 2010). Injury caused by ROS, known as oxidative stress, is one of the major damaging factors in plants exposed to environmental stresses such as drought (Price et al. 1989) and desiccation (Senaratna et al. 1985a,b).
The predominantly rice-growing areas in Asia are often threatened by severe abiotic stresses, of which the most common is drought. Rice is a drought-susceptible crop for its small root system, and mild water stress causes rapid stomatal closure. As a result, reduction in photosynthesis, accumulation of organic acids and osmolytes, and changes in carbohydrate metabolism are the typical physiological and biochemical responses to drought stress (Ji et al. 2010). Drought stress during vegetative growth, flowering and terminal period of rice cultivation, can interrupt floret initiation resulting spikelet sterility or grain filling (Kamoshita et al. 2004). During reproductive growth rice is very sensitive to drought stress; even moderate stress can result in drastic grain yield reduction (Hsiao 1982; O’Toole 1982). According to Kramer and Boyer (1995), drought stress suppresses leaf expansion, tillering and midday photosynthesis and reduces photosynthetic rate and leaf area due to early senescence. Mostajeran and Rahimi-Eichi (2009) proposed that though soil drying is unfavorable to plant growth but may not be unfavorable to grain production if the plant is tolerant to drought stress and is capable to cope with the stress condition prior to grain filling process. Thus, drought is a major production constraint in cultivation of rice and particularly to rainfed areas the problem is severe. So increasing the crop stability under different extent of drought conditions in order to minimize the poor yield is of strategic importance for rice in guaranteeing food for the future.
The oxidative stress tolerance is genetically controlled for crop improvement using conventional breeding and selection, transgene production or adopting physiological approaches like seed-priming. Among various strategies pre-sowing priming treatment of the seeds is an easy, low-cost, low risk and effective approach to overcome the environmental stress problems (Wahid and Shabbir 2005; Ashraf and Foolad 2005). Priming is a controlled hydration process followed by redrying that allows metabolic activities to proceed before radical immergence (Khan 1992; Sivritepe et al. 2003, 2005). Various priming strategies, which include osmopriming, halopriming, and hormonal priming or hydropriming, are used to induce pre-germination changes. These changes usually have profound effects on germination rate and uniformity in emergence of seedlings specifically under stressful conditions (Wahid et al. 2007). Examples of good field performance in response to seed priming have been recorded in the literature. Seed priming with potassium salts enhanced emergence and yield of winter wheat planted deep into summer fallow (Giri and Schillinger 2003); hydropriming improved seed germination and seedling emergence of lentil in the field (Ghassemi-Golezani et al. 2008); exogenous application of H2O2 increased chilling tolerance by mung bean seedlings (Murphy et al. 2002). However the mechanisms inducing stress tolerance are still not known. Some components of the antioxidant defense system have been identified in a study describing the effect of seed-priming on Sorghum seeds during salt stress (Oliveira et al. 2011) and in rice seeds during flooding (Ella et al. 2011). Hydropriming was found to be the more effective in comparison to chemical and hormonal priming in promoting stress tolerance in seedlings of Brassica juncea subjected to stress after 15 days of growth under normal conditions (Srivastava et al. 2010). However, experimental data on the correlation between oxidative stress and seedling growth under drought stress subsequent to seed-priming are lacking. Therefore there is strong interest in identifying the molecular markers in response to seed priming to better understand the process.
Drought stress-induced free radicals of ROS cause stress dependent lipid peroxidation and membrane deterioration in plants (Ratnayaka et al. 2003; Upadhyaya and Panda 2004; Nair et al. 2008). Lipid peroxidation may be used as an indicator of increased oxidative damage (Jagtap and Bhargava 1995; Gill and Tuteja 2010). Garg and Manchanda (2009) reported that during Lipid peroxidation products are formed from polyunsaturated precursors which include small hydrocarbon fragments such as ketones, malondialdehyde (MDA) and related compounds. Yasar et al. (2010) found that MDA amount may be considered as a parameter for drought tolerance characteristic in green bean.
The antioxidative defense systems to keep ROS under control may work through glutathione (GSH) which is a water-soluble, low-molecular-weight antioxidant. Glutathione peroxidases (GPXs) are a large family of diverse isozymes that use GSH to reduce H2O2 and organic lipid hydroperoxides to enhance protection from oxidative stress in plants (Noctor et al. 2002). Overexpression of GPX has been found to enhance abiotic stress tolerance in transgenic plants (Gill and Tuteja 2010). GPXs are efficient scavengers of H2O2 and lipid hydroperoxides using GSH as a reducing agent. However it has also been suggested that in plants they preferably use thioredoxin as a reductant (Herbette et al. 2002; Jung et al. 2002). The major scavenging mechanisms also include superoxide dismutase (SOD) (Bowler et al. 1992), enzymes, metabolites from the ascorbate–glutathione cycle (Noctor and Foyer 1998), and catalase (CAT) (Willekens et al. 1997). SOD is the frontline enzyme in ROS attack since it rapidly scavenges superoxide, one of the first ROS to be produced, dismutating it to oxygen and H2O2 (Bowler et al. 1992). Plants generally contain Cu/Zn-SOD in the cytosol, Fe-SOD in chloroplast and Mn-SOD in mitochondria (Bowler et al. 1994). Pastore et al. (2007), while working with durum wheat, a species well adapted to drought stress, stated that mitochondria could play a central role in cellular adaptation to abiotic stresses. In fact, in the plant cell, mitochondria represent a major source of ROS production and consequent oxidative damage, as reported by Alscher et al. (1997) under two abiotic stresses — drought and salinity, and also indicated by proteomic studies (Sweetlove et al. 2002; Bartoli et al. 2004; Taylor et al. 2005). Wang et al. (2005) while working with transgenic rice stated expression of Mn-SOD can improve drought tolerance.
CATs are tetrameric heme containing enzymes which directly dismutate H2O2 into H2O and O2 and is indispensable for ROS detoxification during stressed conditions (Garg and Manchanda 2009). CAT located exclusively in the peroxisomes, might function as a bulk remover of excess ROS production under stress. It has lower affinity for H2O2 and so its activity may only be enhanced under severe drought stress (Carvalho 2008). Luna et al. (2004) and Sairam et al. (2002) found CAT activity significantly increased under severe drought condition in wheat.
Molecular chaperones provide further protection against heat and other stresses through the repair of denatured proteins. Increased expressions of Heat Shock Proteins (HSPs) like HSP70, HSP101 and small HSP classes were observed in drought acclimated rooted cuttings of loblolly pine (Wimmer et al. 1997). The 70-kDa HSP (HSP70) is the most abundant and best characterized of the heat shock protein family. The overexpression of HSP70 genes correlates positively with the acquisition of thermotolerance and also results in enhanced tolerance to salt, water and high-temperature stress in plants (Wang et al. 2004).
Crop plants with the acquired ability to scavenge and/or control the level of cellular ROS through seed priming may be useful in future to withstand harsh environmental conditions. We have identified the cellular components in the anti-oxidant defense system in rice, a major crop plant in many parts of the world, which may definitely add to the knowledge base on improvement of growth and yield of crop plants.
Materials and methods
Seed
Rice seeds (Oryza sativa ‘Kshitish’ [IET4094]) were obtained from the Chinsura Rice Research Centre, Hoogly, India. Khitish was among 46 Indian varieties released by All-India Coordinated Rice Improvement Project for worldwide testing of breeding lines over a wide range of climatic, cultural, soil, pest and disease conditions and Khitish was released in Pakistan, Paraguay and Malawi. This Indian variety produced stable high yields in the international tests. It has the ability to produce high yields even at higher ambient temperature at reproductive phase (Prasad et al. 2001). The variety is photoperiod-insensitive and is recommended both for winter and summer season. The seed is long, slender and the plant height ranges from 80 to 100 cm (dwarf), it is a good plant type with erect flag leaf and high tillering ability. The period of crop duration from sowing to harvest varies between 115 and 120 days in summer and between 150 and 155 days in winter and the average yield is 4,500 kg/ha. The seed is recommended both for rainfed and irrigated uplands and that is why this variety has been selected for this particular study.
The seeds were surface sterilized with 0.05 % HgCl2 solution for 15 min, thoroughly washed distilled water and then properly blotted. These seeds were used for the following steps.
Priming treatment and seed germination and growth
We used 5 % and 10 % solutions of potassium di hydrogen phosphate (KH2PO4), Polyethelene glycol-6000 (PEG-6000), and sodium chloride (NaCl) in water were used for priming solutions of the seeds. Autoclaved distilled water was used for hydropriming. About 20 g of soaked seeds were taken in 50 ml. of priming solutions as well as in 50 ml of water. Seeds were primed in dark at 24 °C for 24 h with constant gentle agitation. After 24 h, the imbibed soaked seeds were surface dried by blotting paper and then allowed to air dry in shade for 20 h at room temperature. The primed seeds can be kept for 45 days for subsequent germination. These primed as well as untreated unprimed seeds used as control were taken for the next step of germination process. The unprimed seeds were imbibed in autoclaved distilled water in room temperature for 3 h before giving to germination.
A total of 200 seeds (both unprimed and primed) were plated on moist blotting paper in a Petri dish and were given for germination at 28 °C for 3 days in dark. The seeds were germinated in autoclaved distilled water for normal growth and 5 % PEG-6000 solution in water (drought) to impose the desiccation stress to the seeds.
After 3 days of germination, the percentage of sprouted seeds was calculated to observe the effectiveness of the priming treatment on drought. The unsprouted seeds were removed from the Petri dishes. The sprouted seeds were allowed to grow at 30 °C for 2 weeks with 13 h of light and11 h of darkness in 24 h period. After every 4 days the blotting papers of the Petri dishes were replaced to avoid the accumulation of PEG in Petri dishes. After 2 weeks, the root and shoot growth of the seedlings were measured. Every experiment was replicated three times.
Treatments given to the seeds are summarized in the following scheme:
Growth medium | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
Unprimed (Control) | Priming | |||||||
Hydrpriming | KH2PO4 | NaCl | PEG-6000 | |||||
5 % | 10 % | 5 % | 10 % | 5 % | 10 % | |||
H2O | √ | √ | √ | √ | √ | √ | √ | √ |
5 % PEG-6000 | √ | √ | √ | √ | √ | √ | √ | √ |
Protein extraction
Protein was extracted from the leafy portion of the 2-week-old seedlings using TRI reagent (Ambion, California, USA) according to the manufacturer’s protocol. Total protein estimations were made according to Lowry et al. (1951).
ROS detection
Fluorimetry was used for the detection of ROS with fluorescent probes in unprimed and primed seedling cell lysate. 2′,7′-Dichlorodihydrofluorescein diacetate (DCFHDA) is a relatively non-fluorescent molecule which was added in cell lysate (8 μg protein) at a concentration of 12.5 μM and was incubated at 37 °C for 30 min. Activity of cellular esterases cleaves DCFH-DA into 2′,7′-dichlorodihydrofluorescein (DCFH2). ROS can oxidise fluorescent probe DCFH2 to 2′,7′-dichlorofluorescein (DCF). Accumulation of DCF fluorescence in the reaction mixture was measured in a Hitachi F-7000 fluorescence spectrophotometer by observing an increase in fluorescence at 530 nm when the sample was excited at 506 nm. Fluorescence at 530 nm was assumed to be proportional to the amount of ROS in the system.
Measurement of lipid peroxidation
Lipid peroxidation of the unprimed and primed seedlings grown in normal condition (water) and in drought (PEG) was measured using TBARS Assay Kit (Cat no. 10009055) of Cayman Chemical Company following the company’s protocol. Absorbance was measured in Epoch Microplate Reader. Lipid peroxidation has been expressed as μM MDA μg−1 of protein.
Measurement of glutathione
GSH was measured by using Glutathione assay kit (Cat. no. 703002) of Cayman Chemical Company according to company’s protocol. Absorbance was measured in Epoch Microplate Reader. As the sample loaded were low in protein content, (<1 mg ml−1), the deproteination step was not included in the assay. GSH of the samples were expressed as GSH μg−20 μg of protein.
Measurement of glutathione peroxidase
GPX activity has been measured by Glutathione Peroxidase assay kit (Cat. no. 703102) of Cayman Chemical Company according to company’s protocol. Absorbance was measured in Epoch Microplate Reader. GPX activity has been expressed as nmol min−1 μg−20 of protein.
Protein immunoblotting
12 % Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was run with 1× Tris glycine buffer (25 mM Tris, 250 mM glycine, 0.1 % SDS) at 20 mA current with voltage 130 V to resolve 20 μg cellular protein for Western blot analysis. After electrophoresis, the gel was transferred to PVDF membrane at 300 mA current in a semi-wet transfer system for 90 min. Membrane was blocked with 3 % bovine serum albumin (BSA) in TBST (25 mM Tris–Cl, pH 7.5, 0.15 M NaCl pH 7.40; 0.05 % Tween 20) for 2 h at room temperature. Then the blot was treated with primary antibody solution with specific dilution as described by manufacturer in TBST containing 3 % BSA for overnight at 4 °C accompanied by constant shaking. The three primary antibodies were mixed for detecting the expression of three proteins from a single blot ; mouse anti-HSP70 antibody (BD-Pharmingen) at 1:1,000 dilution for observing HSP70 expression (molecular weight, 70 kDa), mouse anti-Actin antibody (Abcam) at 1:500 dilution for internal actin control (molecular weight ~42 kDa) and mouse anti-MnSOD antibody (BD-Pharmingen) at 1:1,000 dilution for observing Mn-SOD expression (molecular weight ~18–20 kDa). For another blot two primary antibodies were mixed together for detection of two proteins from a single blot; rabbit anti-catalase antibody (Cal Biochem) at 1:500 dilution to observe CAT expression (molecular weight 60 kDa) and mouse anti-Actin antibody (Abcam). Then the membrane was treated with appropriate secondary antibody (anti mouse and mixture of anti rabbit and anti mouse, respectively, for two types of blots) conjugated with alkaline phosphatase (ALP), at dilution as described by the manufacturer (1:1,000) in TBST at room temperature with constant shaking. The membrane was then washed four times with TBST and developed using NBT/BCIP, the substrate for ALP.
Quantification of the Western blot
Western blots were scanned by a UMAX Astra Scanner and the band intensities were quantified by using Image J software.
Statistics
Student’s t-test was carried out using Origin pro8 software. Correlation coefficient was calculated using Microsoft Office Excel.
Results
Effect of priming on germination of rice seeds and growth of rice seedlings
The rate of germination of seeds grown in water and 5 % PEG-6000 is given in Table 1. Priming definitely enhanced the germination percentage both in normal and in drought condition except in the cases of priming with 10 % KH2PO4 and 10 % NaCl. In drought, hydropriming, 5 % KH2PO4 and 10 % PEG priming resulted in 80–90 % seed germination while seeds primed in 5 % of NaCl and 5 % PEG performed approximately similar germination percentage as the control seeds (60–70 % germination).
The seedling growth of the unprimed control and the differently primed plants grown in water and 5 % PEG-6000, were measured at the end of 2 weeks of their growth period. These data are depicted in Fig. 1a,b. The unprimed control grown in water demonstrated significantly (shoot *p < 0.001, root *p < 0.001) better growth than unprimed control seedlings grown in PEG (Fig. 1b). Priming with water, KH2PO4, NaCl and PEG resulted in a considerably higher growth rate than the unprimed control seedlings in normal as well as in drought condition (Fig. 1b). In case of drought the primed seedlings demonstrated compared to unprimed control 114 % (*p < 0.001), 257 % (*p < 0.001), 193 % (*p < 0.001), 282 % (*p < 0.001), 187 % (*p < 0.001), 388 % (*p < 0.001), and 346 % (*p < 0.001) higher rate of vegetative growth in cases of hydropriming, 5 % KH2PO4, 10 % KH2PO4, 5 % NaCl, 10 % NaCl, 5 % PEG, and 10 % PEG priming, respectively (Fig. 1a,b). The priming treatments also enhanced the root growth in drought exposed plants. Particularly very high growth rate was observed in 5 % KH2PO4, 5 % NaCl and 5 % PEG priming. In drought all the priming conditions showed significant root growth enhancement than the unprimed control seedlings (*p < 0.05, hydropriming; *p < 0.001, 5 % KH2PO4; *p < 0.001, 10 % KH2PO4; *p < 0.001, 5 % NaCl; *p < 0.001, 10 % NaCl; *p < 0.001, 5 % PEG; *p < 0.001, 10 % PEG).
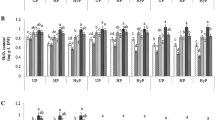
a Morphological development of 2-week-old unprimed and primed seedlings. (i) Picture of the 2-week-old unprimed (Control) and primed seedlings grown in water and 5 % PEG-6000. Uppermost panel depicts the effect of hydropriming. Second (from top) left-hand panel depicts the effect of 5 % KH2PO4 priming. Second (from top) right-hand panel depicts the effect of 10 % KH2PO4 priming. Third (from top) left-hand panel depicts the effect of 5 % NaCl priming. Third (from top) right-hand panel depicts the effect of 10 % NaCl priming. Last (from top) left-hand panel depicts the effect of 5 % PEG priming. Last (from top) right-hand panel depicts the effect of 10 % PEG priming. (ii) Picture of unprimed and primed seedlings which were subsequently grown under dehydration stress conditions. In a row-control unprimed (CON), hydroprimed (HY), 5 % PEG-6000 (PEG), 10 % PEG-6000 (PEG), 5 % KH2PO4, 10 % KH2PO4, 5 % NaCl, 10 % NaCl (all grown in 5 % PEG-6000). b Comparative analysis of vegetative growth and lipid peroxidation (MDA levels) in stressed seedlings. Vegetative growth (shoot and root lengths) of 2-week-old Control unprimed (CON) grown under water and PEG-600, hydroprimed (HY), 5 % KH2PO4, 10 % KH2PO4, 5 % NaCl, 10 % NaCl, 5 % PEG-6000 (PEG) and10% PEG-6000 (PEG) primed seedlings grown under PEG-6000. The error bars for vegetative growth represent the SD value of 50 randomly selected seedlings. The error bars of MDA values represent the SD value of three experiments. c Comparison of ROS levels (DCF Fluorescence) of 2-week-old unprimed and primed stressed seedlings. Two-week-old Control unprimed (CON) grown under water and PEG-6000, hydroprimed (HY), 5 % KH2PO4, 10 % KH2PO4, 5 % NaCl, 10 % NaCl, 5 % PEG-6000 (PEG) and 10 % PEG-6000 (PEG) primed seedlings grown under PEG-6000. The error bars represent the SD value of three experiments
The lipid peroxidation (Fig. 1b) was found to be highest in unprimed seedlings facing drought (about 3.02 μM MDA μg−1 protein), amongst all the experimental conditions. It showed significant (*p < 0.05) difference from unprimed control seedlings grown in water. Also in general, the lipid peroxidation in unprimed seedlings was higher than the primed ones. All the primed seedlings showed significantly lesser amount (*p < 0.05) of MDA production in normal condition (about 0.11–0.48 μM MDA μg−1 protein) as well as in drought (about 0.06–0.25 μM MDA μg−1 protein). However, priming with 5 % PEG showed the least lipid peroxidation both in the case of normal and drought grown seedlings. Figure 1b and Table 2 also highlight a negative correlation between lipid peroxidation and root and shoot length in primed seedlings grown in PEG. The amount of lipid peroxidation is inversely linked to the vegetative growth of seedlings.
The highest amount of ROS production was observed in unprimed seedlings grown in drought (Fig. 1c). Priming treatment decreased ROS in all drought grown seedlings in comparison to the unprimed ones. However the priming with 5 % PEG showed least ROS in drought grown seedlings correlating well with the lowest lipid peroxidation in 5 % PEG primed seedlings.
Effect of seed priming on GPX activity, GSH, catalase and MnSOD levels
GSH was not detected at all in unprimed seedlings (Fig. 2). Substantial amount of GSH was found in hydroprimed (1.49 μg−20 protein) seedlings grown under drought. In drought conditions, moderate amounts of GSH were also detected in 5 % and 10 % NaCl primed seedlings (0.56 and 0.42 μg−20 protein, respectively) and a very little amount in 5 % PEG priming (0.014 μg−20 protein). In other cases, GSH was not detectable. GSH was detected only in primed seedlings grown in drought.
Comparison of glutathione and glutathione peroxidase in unprimed and primed seedlings grown under water and PEG-6000. Graphical representation of glutathione level and glutathione peroxidase activity measured in seedling lysates. CON + H 2 O control seedlings grown in water; CON + PEG control seedlings grown under PEG-6000; HY + H 2 O hydroprimed seedlings grown in water; HY + PEG hydroprimed seedlings grown in PEG-6000; 5 % KH 2 PO 4 + H 2 O 5 % KH2PO4 primed seedlings grown in water; 5 % KH 2 PO 4 + PEG 5 % KH2PO4 primed seedlings grown in PEG-6000; 10 % KH 2 PO 4 + H 2 O 10 % KH2PO4 primed seedlings grown in water; 10 % KH 2 PO 4 + PEG 10 % KH2PO4primed seedlings grown in PEG-6000; 5 % NaCl + H 2 O 5 % NaCl primed seedlings grown in water; 5 % NaCl + PEG 5 % NaCl primed seedlings grown in PEG-6000; 10 % NaCl + H 2 O 10 % NaCl primed seedlings grown in water; 10 % NaCl + PEG 10 % NaCl primed seedlings grown in PEG-6000; 5 % PEG + H 2 O 5 % PEG-6000 primed seedlings grown in water; 5 % PEG + PEG 5 % PEG-6000 primed seedlings grown in PEG-6000; 10 % PEG + H 2 O 10 % PEG-6000 primed seedlings grown in water; 10 % PEG + PEG 10 % PEG-6000 primed seedlings grown in PEG-6000. The data are representative of three experiments. The error bars represent the SD value of three experiments.
GPX was detected in every primed sample under drought, markedly higher than corresponding water grown sets (*p < 0.05 in 5 % KH2PO4, 5 % NaCl, 10 % NaCl, 5 % PEG, 10 % PEG). In contrast, unprimed seeds grown in drought demonstrated absence of GPX activity, even though intermediate levels of enzyme activity were detected in normally grown unprimed seedlings. Under normal growth conditions hydroprimed seedlings and also 10 % KH2PO4 primed seedlings were devoid of either GSH or GPX. In drought, seedlings treated with hydropriming and NaCl priming showed high amount of GPX and moderate GPX activity was detected in PEG priming (Fig. 2). A positive correlation between vegetative growth and GPX activity was observed in primed seedlings (Table 2). However no direct relation was found between GSH levels and GPX activity.
CAT was detected in only unprimed seedlings grown under normal as well as in drought condition. No CAT expression was found (Fig. 3b,c) for all the primed seeds with the exceptions of hydroprimed and 10 % PEG primed seeds for both normal and drought, though at a lower level when compared to controls (Fig. 3a,d).
Expression of catalase in unprimed and primed seedlings grown under water and PEG-6000. a Expression of catalase in unprimed and hydroprimed seedlings grown under water and PEG-6000. Protein expression Western blot is depicted in the left panel. 1 Protein expression in control seedlings grown in water; 2 protein expression in control seedlings grown in PEG-6000; 3 protein expression in hydroprimed seedlings grown in water; 4 protein expression in hydroprimed seedlings grown in PEG-6000. Right panel depicts the graphical representation of band intensities of catalase normalized to actin. 1 Band intensities of catalase normalized to actin in control seedlings grown in water; 2 band intensities of catalase normalized to actin in control seedlings grown in PEG-6000; 3 band intensities of catalase normalized to actin in hydroprimed seedlings grown in water; 4 band intensities of catalase normalized to actin in hydroprimed seedlings grown in PEG-6000. The error bars are the SD value of the band intensity measurement of the same blot. The data are representative of three experiments. b Expression of catalase in unprimed and KH2PO4 primed seedlings grown under water and PEG-6000. Protein expression Western blot is depicted in the left panel. 1 Protein expression in control seedlings grown in water; 2 protein expression in control seedlings grown in PEG-6000; 3 protein expression in 5 % KH2PO4 primed seedlings grown in water; 4 protein expression in 5 % KH2PO4primed seedlings grown in PEG-6000; 5 protein expression in 10 % KH2PO4 primed seedlings grown in water; 6 protein expression in 10 % KH2PO4 primed seedlings grown in PEG-6000. Right panel depicts the graphical representation of band intensities of catalase normalized to actin. 1 Band intensities of catalase normalized to actin in control seedlings grown in water; 2 band intensities of catalase normalized to actin in control seedlings grown in PEG-6000; 3 band intensities of catalase normalized to actin in 5 % KH2PO4 primed seedlings grown in water; 4 band intensities of catalase normalized to actin in 5 % KH2PO4 primed seedlings grown in PEG-6000; 5 band intensities of catalase normalized to actin in 10 % KH2PO4 primed seedlings grown in water; 6 band intensities of catalase normalized to actin in 10 % KH2PO4 primed seedlings grown in PEG-6000. The error bars are the SD value of the band intensity measurement of the same blot. The data are representative of three experiments. c Expression of catalase in unprimed and NaCl primed seedlings grown under water and PEG-6000. Protein expression Western blot is depicted in the left panel. 1 Protein expression in control seedlings grown in water; 2 protein expression in control seedlings grown in PEG-6000; 3 protein expression in 5 % NaCl primed seedlings grown in water; 4 protein expression in 5 % NaCl primed seedlings grown in PEG-6000; 5 protein expression in 10 % NaCl primed seedlings grown in water; 6 protein expression in 10 % NaCl primed seedlings grown in PEG-6000. Right panel depicts graphical representation of band intensities of catalase normalized to actin. 1 Band intensities of catalase normalized to actin in control seedlings grown in water; 2 band intensities of catalase normalized to actin in control seedlings grown in PEG-6000; 3 band intensities of catalase normalized to actin in 5 % NaCl primed seedlings grown in water; 4 band intensities of catalase normalized to actin in 5 % NaCl primed seedlings grown in PEG-6000; 5 band intensities of catalase normalized to actin in 10 % NaCl primed seedlings grown in water; 6 band intensities of catalase normalized to actin in 10 % NaCl primed seedlings grown in PEG-6000. The error bars are the SD value of the band intensity measurement of the same blot. The data are representative of three experiments. d Expression of catalase in unprimed and PEG primed seedlings grown under water and PEG-6000. Protein expression Western blot is depicted in the left panel. 1 Protein expression in control seedlings grown in water; 2 protein expression in control seedlings grown in PEG-6000; 3 protein expression in 5 % PEG-6000 primed seedlings grown in water; 4 protein expression in 5 % PEG-6000 primed seedlings grown in PEG-6000; 5 protein expression in 10 % PEG-6000 primed seedlings grown in water; 6 protein expression in 10 % PEG-6000 primed seedlings grown in PEG-6000. Right panel depicts graphical representation of band intensities of catalase normalized to actin. 1 Band intensities of catalase normalized to actin in control seedlings grown in water; 2 band intensities of catalase normalized to actin in control seedlings grown in PEG-6000; 3 band intensities of catalase normalized to actin in 5 % PEG-6000 primed seedlings grown in water; 4 band intensities of catalase normalized to actin in 5 % PEG-6000 primed seedlings grown in PEG-6000 l; 5 band intensities of catalase normalized to actin in 10 % PEG-6000 primed seedlings grown in water; 6 band intensities of catalase normalized to actin in 10 % PEG-6000 seedlings grown in PEG-6000. The error bars are the SD value of the band intensity measurement of the same blot. The data are representative of three experiments
MnSOD expression was always found in control seedlings grown in water. The MnSOD expression was noticeably diminished to negligible levels when control seedlings were grown under PEG (Fig. 4a–d). Very little MnSOD expression was seen in hydroprimed seedlings and KH2PO4 primed seedlings (Figs. 4a,b and 5). Among other priming treatments (NaCl and PEG), expression of MnSOD was observed in normally growing as well as in stressed plants. The extent of expression of MnSOD under drought in all three chemical priming has been compared in Fig. 5. PEG primed seedlings were found to have the highest amount of MnSOD expression followed by NaCl primed seedlings in PEG grown condition.
Expression of HSP70, MnSOD and actin in unprimed and primed seedlings grown under water and PEG-6000. a Expression of HSP70, MnSOD and actin in unprimed and hydroprimed seedlings grown under water and PEG-6000. Left panel depicts Protein expression Western blot. 1 Protein expression in control seedlings grown under water; 2 protein expression in control seedlings grown under PEG-6000; 3 protein expression in hydroprimed seedlings grown under water; 4 protein expression in hydroprimed seedlings grown under PEG-6000. Right panel depicts the comparison of HSP70 in Control and hydroprimed seedlings grown under water and PEG-6000 as graphical representation of band intensities of HSP70 normalized to actin. Y axis shows HSP 70/actin ratio. 1 HSP 70/actin ratio in control seedlings grown under water; 2 HSP 70/actin ratio in control seedlings grown under PEG-6000; 3 HSP 70/actin ratio in hydroprimed seedlings grown under water; 4 HSP 70/actin ratio in hydroprimed seedlings grown under PEG-6000. The error bars are the SD value of the band intensity measurement of the same blot. The data are representative of three experiments. b Expression of HSP70, MnSOD and actin in unprimed and KH2PO4 primed seedlings grown under water and PEG-6000. Left panel depicts protein expression Western blot. 1 Protein expression in control seedlings grown in water; 2 protein expression in control seedlings grown in PEG-6000; 3 protein expression in 5 % KH2PO4 primed seedlings grown in water; 4 protein expression in 5 % KH2PO4 primed seedlings grown in PEG-6000; 5 protein expression in 10 % KH2PO4 primed seedlings grown in water; 6 protein expression in 10 % KH2PO4 primed seedlings grown in PEG-6000. Right panel depicts the comparison of HSP70 in Control and KH2PO4 primed seedlings grown under water and PEG-6000 as graphical representation of band intensities of HSP70 normalized to actin. Y axis shows HSP 70/actin ratio. 1 HSP 70/actin ratio in control seedlings grown in water; 2 HSP 70/actin ratio in control seedlings grown in PEG-6000; 3 HSP 70/actin ratio in 5 % KH2PO4 primed seedlings grown in water; 4 HSP 70/actin ratio in 5 % KH2PO4 primed seedlings grown in PEG-6000; 5 HSP 70/actin ratio in 10 % KH2PO4 primed seedlings grown in water; 6 HSP 70/actin ratio in 10 % KH2PO4 primed seedlings grown in PEG-6000. The error bars are the SD value of the band intensity measurement of the same blot. The data are representative of three experiments. c Expression of HSP70, MnSOD and actin in unprimed and NaCl primed seedlings grown under water and PEG-6000. Left panel depicts protein expression Western blot. 1 Protein expression in control seedlings grown in water; 2 protein expression in control seedlings grown in PEG-6000; 3 protein expression in 5 % NaCl primed seedlings grown in water; 4 protein expression in 5 % NaCl primed seedlings grown in PEG-6000; 5 protein expression in 10 % NaCl primed seedlings grown in water; 6 protein expression in 10 % NaCl primed seedlings grown in PEG-6000. Right panel depicts the comparison of HSP70 in Control and KH2PO4 primed seedlings grown under water and PEG-6000 as graphical representation of band intensities of HSP70 normalized to actin. Y axis shows HSP 70/actin ratio. 1 HSP 70/actin ratio in control seedlings grown in water; 2 HSP 70/actin ratio in control seedlings grown in PEG-6000; 3 HSP 70/actin ratio in 5 % NaCl primed seedlings grown in water; 4 HSP 70/actin ratio in 5 % NaCl primed seedlings grown in PEG-6000; 5 HSP 70/actin ratio in 10 % NaCl primed seedlings grown in water; 6 HSP 70/actin ratio in 10 % NaCl primed seedlings grown in PEG-6000. The error bars are the SD value of the band intensity measurement of the same blot. The data are representative of three experiments. d Expression of HSP70, MnSOD and actin in unprimed and PEG primed seedlings grown under water and PEG-6000. Left panel depicts protein expression Western blot. 1 Protein expression in control seedlings grown in water; 2 protein expression in control seedlings grown in PEG-6000; 3 protein expression in 5 % PEG-6000 primed seedlings grown in water; 4 protein expression in 5 % PEG-6000 primed seedlings grown in PEG-6000; 5 protein expression in 10 % PEG-6000 primed seedlings grown in water; 6 protein expression in 10 % PEG-6000 primed seedlings grown in PEG-6000. Right panel depicts Comparison of HSP70 in Control and PEG-6000 primed seedlings grown under water and PEG-6000 as graphical representation of band intensities of HSP70 normalized to actin. Y axis shows HSP 70/actin ratio. 1 HSP 70/actin ratio in control seedlings grown in water; 2 HSP 70/actin ratio in control seedlings grown in PEG-6000; 3 HSP 70/actin ratio in 5 % PEG-6000 primed seedlings grown in water; 4 HSP 70/actin ratio in 5 % PEG-6000 primed seedlings grown in PEG-6000; 5 HSP 70/actin ratio in 10 % PEG-6000 primed seedlings grown in water; 6 HSP 70/actin ratio in 10 % PEG-6000 primed seedlings grown in PEG-6000. The error bars are the SD value of the band intensity measurement of the same blot. The data are representative of three experiments
Comparison of MnSOD expression in unprimed and PEG-6000 primed seedlings grown in PEG-6000. Graphical representation of band intensities of MnSOD normalized to actin. The error bars are the SD value of the band intensity measurement of the same blot. CON mean value of control seedlings grown in PEG-6000 from all the MnSOD expression blots; HY protein expression in hydroprimed seedlings grown in PEG-6000; 5 % KH 2 PO 4 protein expression in 5 % KH2PO4 primed seedlings grown in PEG-6000; 10 % KH 2 PO 4 protein expression in 10 % KH2PO4 primed seedlings grown in PEG-6000; 5 % NaCl protein expression in 5 % NaCl primed seedlings grown in PEG-6000; 10 % NaCl protein expression in 10 % KH2PO4 primed seedlings grown in PEG-6000; 5 % PEG protein expression in 5 % PEG-6000 primed seedlings grown in PEG-6000; 10 % PEG protein expression in 10 % PEG-6000 primed seedlings grown in PEG-6000. The data are representative of three experiments. The error bars represent the SD value of three measurements
Effect of priming on Hsp 70 levels of rice seedlings
HSP70 was observed in unprimed control seedlings grown under normal condition. The unprimed control seedlings under drought expressed very little or no HSP70. Hydropriming failed to improve the depressed Hsp70 levels in drought exposed seedlings (Fig. 4a). KH2PO4 and NaCl priming also did not help in noticeable recovery of the Hsp 70 levels. 5 % PEG primed seedlings when exposed to drought recovered Hsp70 expression, whereas 10 % PEG primed seedlings express HSP70 both under normal and drought grown condition (Fig. 4d). However, the expression levels of HSP70 for these conditions are somewhat lower than HSP70 expression levels in control seedlings.
Discussion
Seed–priming of different types have been observed to improve seed germination rate and seedling emergence in a number of studies (Srivastava et al. 2010; Chen and Arora 2011). Priming usually exerts strong influence on germination rate and uniformity in emergence of seedlings under stressful conditions (Wahid et al. 2007). Seed priming also improves field performance of crops (Giri and Schillinger 2003). In our study, the benefits of seed priming on germination did not encompass all priming methods (Table 1). Only priming by PEG and 5 % KH2PO4 resulted in higher germination percentage than unprimed control seeds. In contrast, the growth rate was positively influenced by all of the different priming treatments (Fig. 1a). All the priming conditions were found to be useful for thriving under drought as the shoot and root lengths of all primed seedlings were found to be better than the unprimed control seedlings. However, 5 % PEG priming was found to be the best for growth under drought followed by 5 % NaCl and 5 % KH2PO4 (Fig. 1a). All 10 % priming treatments produced lesser impacts on seedling growth than 5 % priming, indicating perhaps the induction of greater than optimal stress under 10 % priming conditions resulting in lesser amount of protection during continuing stress. Furthermore, the growth advantage by priming was more prominent in primed seeds grown in drought when compared to primed seeds grown normally. It is likely that the impact of a single dose of stress may be reversible and transient, and the survival advantage by one time stress may not be fully discernible in primed seeds grown in water as stress conditions were discontinued during growth.
A very high amount of MDA accumulation in unprimed control plants grown in PEG indicated that (Fig. 1b(ii)) PEG-induced drought stress caused oxidative damage to rice and inhibited growth probably through excessive generation of ROS. Indeed, ROS levels were the highest in unprimed seedlings grown in PEG and the lowest in 5 % PEG primed seeds (Fig. 1c). Lipid peroxidation in both cellular and organelle membranes takes place when ROS levels go beyond threshold not only directly affecting normal cellular functioning, but also aggravating the oxidative stress through production of lipid-derived radicals. It has also been noted that plants exposed to various abiotic stresses exhibit an increase in lipid peroxidation due to the generation of ROS (Gill and Tuteja 2010). In our study, the primed seedlings show especially low Lipid peroxidation in drought condition. All the priming procedures lowered MDA levels significantly (Fig. 1b). The lowest ROS and MDA levels in 5 % PEG primed seedling may justify the highest growth of these seedlings among all priming treatments. The drop in lipid peroxidation is directly correlated with growth of seedlings (Fig. 1b). Therefore, lowering of ROS levels and consequently lipid peroxidation may be a major factor in the drought resistance produced by the priming treatments. Possibly, unprimed seedlings grown under drought condition with increased lipid peroxidation resulting in higher membrane permeability became more susceptible to water stress. But the primed seedlings showed significantly lesser lipid peroxidation and consequently a better moisture stress tolerance. These data agree with the findings of Pandey et al. (2010) comparing drought tolerance in different Avena sp. and with results from Ella et al. (2011) who showed that seed-priming resulted in lower lipid peroxidation under flooding conditions in rice. However, data from the study by Chen and Arora (2011) who demonstrated a higher level of MDA in primed seedlings compared to unprimed seedlings are at variance with our report.
The control unprimed plants grown in PEG experienced severe oxidative stress (high ROS levels and high MDA levels) and their anti-oxidant defense mechanisms possibly were overwhelmed as shown by absence of reduced GSH, disappearance of GPX activity, and lack of expression of MnSOD (Figs. 2 and 5). As GPX removes oxidative stress from plant cells by utilizing GSH to reduce H2O2 and organic and lipid hydroperoxides, presence of GPX activity indicates readiness of the defense mechanisms against moisture stress. Absence of GPX activity in unprimed seeds grown under drought conditions, indicate their lack of ability to defend against oxidative stress. In case of primed seedlings whenever the seeds were placed in drought, GPX activity was detected. GPX activity bears positive correlation with vegetative growth of seedlings grown under drought stress (Table 2). Therefore, priming conferred drought resistance through strengthening of the antioxidant defense enzyme GPX. It is likely that GPX activity is a good indicator of oxidative stress resistance by seed priming in rice seedlings grown in drought conditions.
MnSOD was present in the water grown unprimed seedlings. But it was either not found or was present in low levels in water stressed unprimed seedlings (Fig. 4). Poor growth in unprimed control plants under drought (high ROS and MDA levels) could also be connected to the impairment of antioxidant MnSOD enzyme levels. These results bear similarity to the findings of DaCosta and Huang (2007) in bent grass responding to drought conditions. The depression of SOD activity under increasing magnitude of water stress has also been observed by Zhang and Kirkham (1994) in wheat. The disappearance of MnSOD activity probably impaired the O2 scavenging system of the cells. Accumulation of H2O2 under drought may also impair MnSOD induction. CAT directly reacts with H2O2 to form water and molecular oxygen. In our study, CAT activity was neither markedly influenced by drought stress nor did it improve by priming (Fig. 3). CAT expression seemed not to be necessary for the protection mechanisms activated by seed priming. The depression in the levels of MnSOD during drought stress can be regarded as a weakened ROS scavenging mechanism. On the other hand, high GPX activity (possibly utilizing GSH) observed in most of the primed seedlings could have acted as a deterrent to oxidative damage in primed seedlings. The improved expression of MnSOD in NaCl and PEG primed seedlings could be regarded as another component of the improved ROS removal mechanism (Fig. 5). There are many reports of the overexpression of MnSOD in drought-exposed plants or higher levels of different SODs in other abiotic stress tolerant transgenic plants (Carvalho 2008; Gill and Tuteja 2010). Better adaptability to drought stress has been found by Wang et al. (2005) in a transgenic rice plant expressing pea MnSOD.
Some amount of native HSP70 is present in unprimed normally grown seedlings as has been previously found by Goswami et al. (2010) but very little or no HSP70 expression is found in unprimed control plants under drought. This indicated the impairment of a basic defense system of the plants against stress. Expression of HSP70 was observed in 10 % KH2PO4 primed seedlings and PEG primed seedlings. Perhaps, the expression of HSP70 in 10 % KH2PO4 primed seedlings added support to the protective mechanisms against drought stress as GPX activity is the lowest in these seedlings among primed seedlings even though they demonstrate less membrane damage (LPO = 0. 165 μM MDA μg−1 protein) and better seedling growth than control plants grown in PEG (Figs. 1a,b and 2). The PEG primed seeds with minimum membrane damage, efficient GPX activity, and overexpressed MnSOD also showed HSP70 expression in normal (10 % priming) as well as in drought (5 %, 10 % priming) condition, indicating a superior protective machinery in these seedlings. In a recent study (Srivastava et al. 2010), hydropriming was found to be the best in promoting stress tolerance in seedlings of B. juncea after 15 days of growth under normal conditions in comparison to chemical and hormonal priming. In contrast to the experimental protocol followed in this study, we studied the effects of stress conditions which prevailed through germination to seedling age of 2 weeks which may mimic more closely the conditions in drought-stricken areas. Our results are also in agreement with Chen and Arora (2011) who demonstrated the importance of osmopriming in subsequent seedling growth through strengthening of the antioxidant system.
Conclusion
The exogenous application of various chemicals including water was found to have great implication in growth rate of the seedlings under drought. The use of seed pretreatments was not very useful for the seed germination but these treatments were definitely found to be very effective in the establishment, survival and growth of the seedlings. 5 % PEG and 5 % NaCl priming was found to be the best priming chemical among all the treatments to thrive under drought. GPX activity and lipid peroxidation acted as the marker for survival under drought. Thus our data elucidating the mechanisms underlying the simple economic method of seed priming in an important crop plant like rice are a valuable and noteworthy addition to the present knowledge base on crop improvement.
References
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationships in green cells. Physiolog Planta 100:224–233
Ashraf M, Foolad RM (2005) Pre-sowing seed treatment — a shotgun approach to improve germination, plant growth and crop yield under saline and non-saline conditions. Adv Agron 88:223–271
Bartoli CG, Gómez F, Martínez DE, Guiamet JJ (2004) Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot 55:1663–1669
Bowler C, Van Montagu M, Inzé D (1992) Superoxide dismutases and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Bowler C, Van Camp W, Van Montagu M, Inze D (1994) Superoxide dismutase in plants. Crit Rev Plant Sci 13:199–218
Carvalho MHCD (2008) Drought stress and reactive oxygen species Production, scavenging and signaling. Plant Signal Behav 3(3):156–165
Chen K, Arora R (2011) Dynamics of the antioxidant system during seed osmopriming, post priming germination, and seedling establishment in Spinach (Spinacia oleracea). Plant Sci 180:212–220
DaCosta M, Huang B (2007) Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. J Am Soc Hortic Sci 132(3):319–326
Ella ES, Dionisio-Sese ML, Ismail AM (2011) Seed pre-treatment in rice reduces damage, enhances carbohydrate mobilization and improves emergence and seedling establishment under flooded conditions. AoB PLANTS plr007 doi:10.1093/aobpla/plr007.
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143:8–96
Ghassemi-Golezani K, Aliloo AA, Valizadeh M, Moghaddam M (2008) Effects of hydro and osmo-priming on seed germination and field emergence of lentil (Lens culinaris Medik.). Not Bot Hortic Agrobot Cluj-Napoca 36(1):29–33
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Giri GS, Schillinger WF (2003) Seed priming winter wheat for germination, emergence and yield. Crop Sci 43:2135–2141
Goswami A, Banerjee R, Raha S (2010) Mechanisms of plant adaptation/memory in rice seedlings under arsenic and heat stress: expression of heat-shock protein gene HSP70. AoB PLANTS plq023, doi:10.1093/aobpla/plq023.
Herbette P, Lenne C, Leblanc N, Julien JL, JoeDrevet R, Roeckel-Drevet P (2002) Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur J Biochem 269:2414–2420
Hsiao TC (1982) The soil plant atmosphere continuum in relation to drought and crop production. In Drought resistance in crops with emphasis on rice. IRRI, Los Baños, Philippines, pp 39–52
Jagtap V, Bhargava S (1995) Variation in the antioxidant metabolism of drought tolerant and drought susceptible varieties of Sorghum bicolor (L.) Moench. exposed to high light, low water and high temperature stress. J Plant Physiol 145:195–197
Ji K, Wang Y, Sun W, Lou Q, Mei H, Shen S, Chen H (2010) Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J Plant Physiol 169:336–344
Jung BG, Lee KO, Lee SS, Chi YH, Jang HH, Kang SS, Lee K, Lim D, Yoon SC, Yun D-J, Inoue Y, Cho MJ, Lee SY (2002) A Chinese cabbage cDNA with high sequence identity to phospholipid hydroperoxide glutathione peroxidases encodes a novel isoform of thioredoxin-dependent peroxidase. J Biol Chem 277:1257–12578
Kamoshita A, Rodriguez R, Yamauchi A, Wade L (2004) Genotypic variation in response of rainfed lowland to prolonged drought and rewatering. Plant Prod Sci 7(4):406–420
Khan AA (1992) Preplant physiological conditioning. Hortic Rev 13:131–181
Kramer PJ, Boyer JS (1995) Water relations of plant and soil. Academic Press, San Diego, p 495
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin–phenol reagents. J Biol Chem 193:265–275
Luna CM, Pastori GM, Driscoll S, Groten K, Bernard S, Foyer CH (2004) Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot 56(411):417–423
Mittal D, Madhyastha DA, Grover A (2012) Genome-Wide Transcriptional Profiles during temperature and oxidative stress reveal coordinated expression Patterns and overlapping regulons in rice. PLoS One 7(7):e40899. doi:10.1371/journal.pone.0040899
Mostajeran A, Rahimi-Eichi V (2009) Effects of drought stress on growth and yield of rice (Oryza sativa L.) cultivars and accumulation of proline and soluble sugars in sheath and blades of their different ages leaves. Am-Euras. J. Agric. & Environ. Am-Euras JAgric Environ Sci 5(2):264–272
Murphy TM, Sung WW, Lin CH (2002) H2O2 treatment induces glutathione accumulation and chilling tolerance in mung bean. Funct Plant Biol 29:1081–1087
Nair AS, Abraham TK, Jaya DS (2008) Studies on the changes in lipid peroxidation and antioxidants in drought stress induced cowpea (Vigna unguiculata L.) varieties. J Environ Biol 29(5):689–691
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation, and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53:1283–1304
O’Toole JC (1982) Adaptation of rice to drought prone environments. In Drought resistance in crops with emphasis on rice. IRRI, Los Baños, Philippines, pp 195–213
Oliveira ABD, Alencar NLM, Prisco JT, Gomes-Filho E (2011) Accumulation of organic and inorganic solutes in NaCl-stressed sorghum seedlings from aged and primed seeds. Sci Agric 68(6):632–637
Pandey HC, Baig MJ, Chandra A, Bhatt RK (2010) Drought stress induced changes in lipid peroxidation and antioxidant system in genus Avena. J Environ Biol 31:435–440
Pastore D, Trono D, Laus MN, Fonzo ND, Flagella Z (2007) Possible plant mitochondria involvement in cell adaptation to drought stress a case study: durum wheat mitochondria. J Exp Bot 58(2):195–210
Prasad GSV, Prasadarao U, Shobha Rani N, Rao LVS, Pasalu IC, Muralidharan K (2001) Indian rice varieties released in countries around the world. Curr Sci 80(12):1508–1511
Price AH, Atherton N, Hendry GAF (1989) Plants under drought stress generated activated oxygen. Free Radic Res Commun 8:61–66
Ratnayaka HH, Molin WT, Sterling TM (2003) Physiological and antioxidant responses of cotton and spurred anoda under interference and mild drought. J Exp Bot 54:2293–2305
Sairam RK, Rao KV, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Senaratna T, McKersie BD, Stinson RH (1985a) Simulation of dehydration injury to membranes from soybean axes by free radicals. Plant Physiol 7:472–474
Senaratna T, McKersie BD, Stinson RH (1985b) Antioxidant levels in germinating soybean seed axes in relation to free radical dehydration tolerance. Plant Physiol 78:168–171
Shehab GG, Ahmed OK, El-Beltagi HS (2010) Effects of various chemical agents for alleviation of drought stress in rice plants (Oryza sativa L.). Not Bot Hortic Agrobot Cluj-Napoca 38(1):139–148
Sivritepe N, Sivritepe HO, Eris A (2003) The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci Hortic 97:229–237
Sivritepe HO, Sivritepe N, Eris A, Turhan E (2005) The effects of NaCl pre-treatment on salt tolerance of melons grown under long term salinity. Sci Hortic 106:568–581
Srivastava AK, Lokhande VH, Patade VY, Suprasanna P, Sjahril R, D’Souza SF (2010) Comparative evaluation of hydro-, chemo-, and hormonal-priming methods for imparting salt and PEG stress tolerance in Indian mustard (Brassica juncea L.) Acta Physiolog. Planta 32:1135–1144
Sweetlove LJ, Heazlewood JL, Herald V, Holtzapffel R, Day DA, Leaver CJ, Millar AH (2002) The impact of oxidative stress on Arabidopsis mitochondria. Plant J 32:891–904
Taylor NL, Heazlewood JL, Day DA, Millar AH (2005) Differential impact of environmental stresses on the pea mitochondrial proteome. Mol Cell Proteomics 4:1122–1123
Timperio AM, Egidi MG, Zolla L (2008) Proteomics applied on plant abiotic stresses: Role of heat shock proteins (HSP). J Proteomics 71:391–391
Upadhyaya H, Panda SK (2004) Responses of Camellia sinensis to drought and rehydration. Biol Plant 48:597–600
Wahid A, Shabbir A (2005) Induction of heat stress tolerance in barley seedlings by presowing seed treatment with glycinebetaine. Plant Growth Regul 46:133–141
Wahid A, Perveen M, Gelani S, Basra SMA (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA (2005) Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol 162:465–472
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. EMBO J 10:1723–1732
Wimmer B, Lottspeich F, Van der Klei I, Veenhuis M, Gietl C (1997) The glyoxysomal and plastid molecular chaperones (70-kDa heat shock protein) of watermelon cotyledons are encoded by a single gene. Proc Natl Acad Sci USA 94(25):13624–13629
Yasar F, Uzal O, Ozpay T (2010) Changes of the lipid peroxidation and chlorophyll amount of green bean genotypes under drought stress. Afr J Agric Res 5(19):2705–2709
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35(5):785–791
Acknowledgments
The authors are thankful to Professor Bikas K. Chakrabarti, Centre for Applied Mathematics and Computational Science, Saha Institute of Nuclear Physics, Kolkata, India, for his generous encouragement. They acknowledge Dr. Bijan Adhikari from the Chinsura Rice Research Centre, Hoogly, India, for his cooperation and for providing rice seeds. The authors thank Ms. Riddhi Mahansaria for her help with the morphological measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bhumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Goswami, A., Banerjee, R. & Raha, S. Drought resistance in rice seedlings conferred by seed priming. Protoplasma 250, 1115–1129 (2013). https://doi.org/10.1007/s00709-013-0487-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0487-x