Abstract
During sorghum caryopsis development, endosperm epidermal cells near the basal main vascular bundle are specialized by depositing wall ingrowths, differentiating into basal endosperm transfer cells (BETCs). All the BETCs together compose the basal endosperm transfer layer (BETL). BETCs are the first cell type to become histologically differentiated during endosperm development. The initiation and subsequent development of BETCs shows the pattern of temporal and spatial gradient. The developmental process of BETL can be divided into four stages: initiation, differentiation, functional, and apoptosis stage. A placental sac full of nutrient solutions would emerge, enlarge, and eventually disappear between the outmost layer of BETL and nucellar cells during caryopsis development. BETCs have dense cytoplasm rich in mitochondria, lamellar rough endoplasmic reticulum, Golgi bodies, and their secretory vesicles. They show a series of typical characteristics of senescence such as nuclei distortion and subcellular organelle deterioration during their specialization. BETCs probably play an active role in nutrient transfer into the starchy endosperm and embryo. The occurrence, development, and apoptosis of BETCs are in close relation to the caryopsis growth and maturation especially the enrichment of endosperm and the growth of embryo. The timing when BETL is fully developed, composed of three to four layers in radial direction and 70 to 80 rows in tangential direction, consists with the timing when average daily gain of caryopsis dry weight reaches its maximum. It is conceivable that measures that delay the senescence and death of BETCs would help to increase the crop yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In various species of plants, certain strategically located cells are specialized, possessing numerous secondary wall ingrowths invaginating into their protoplasts. This cell type is termed “transfer cells” (Gunning and Pate 1969). The diagnostic morphological feature of transfer cells is their invaginated wall ingrowths ensheathed by plasma membrane forming a unique structure/function unit called “wall-membrane apparatus.” Transfer cells have arisen early in plant evolution (Gottschling and Hilger 2003) and occur ubiquitously in all plant taxonomic groups, from lower plant such as algae and fungi to high plant such as mosses, ferns, gymnosperms, and angiosperms (Offler et al. 2002). Four categories of transfer cells are recognized according to their distribution in plants: (1) transfer cells in the vascular system, (2) transfer cells in reproductive system, (3) transfer cells in the surface of the plants that absorb or secrete solutes to the external environment, and (4) transfer cells in parts of the plant where symbiosis or parasitization occur.
During the endosperm development of a seed, some epidermal cells of the endosperm start to deposit wall ingrowths and differentiate into endosperm transfer cells, which belong to the category of transfer cells in the reproductive system. To date, endosperm transfer cells have been found in several families such as cowpea (Hu et al. 1983) in Leguminosae, Isatis tinctoria L. (Xi 1993) in Cruciferae, buckwheat (Gao et al. 2002) in Polygonaceae, as well as maize (Davis et al. 1990), teosinte (Dermastia et al. 2009), Job’s tears (Xi and Leng 1995), barley (Cochrane and Duffus 1980), wheat (Morrison et al. 1978), and sorghum (Paulson 1969) in Poaceae.
Sorghum is an important grain crop which can be used as human food, livestock forage, and raw materials for the food industry. Sorghum is evolutionarily closely related to maize and teosinte, and their caryopsis structures are similar (Kladnik et al. 2006; Dermastia et al. 2009). In sorghum, maize, and teosinte, the endosperm transfer cells are located at the base of the caryopsis, thus they are termed basal endosperm transfer cells (BETCs), and the whole transfer cell region are termed basal endosperm transfer layer (BETL) (Jain et al. 2008; Hueros et al. 1995). Lots of advances have been achieved towards the investigation of maize BETCs both in morphology (Davis et al. 1990; Gao et al. 1996; Zheng et al. 2009) and molecular biology (Gomez et al. 2002; Muniz et al. 2006; Hueros et al. 1995; Gutierrez-Marcos et al. 2004; Brugière et al. 2008), while little is known about this cell type in sorghum.
Sorghum BETCs owes extremely extensive and abundant wall ingrowths, which makes them a good material for transfer cell study. In this paper, we investigated the structure of sorghum BETCs of different developmental stages systematically by light and transmission electron microscopy, and elucidated the BETL developmental process and its relationship to caryopsis growth and grain filling in detail. Our results could be beneficial to breeders attempting to increase the crop yield of sorghum.
Material and methods
Plant material
High yielding Sorghum plants (Sorghum bicolor (L.) Moench cv. KS-304) were grown in the experimental fields of Yangzhou University. Flowering proceeds basipetally in sorghum panicle and about 4 days are required for all the florets in the panicle to be fertilized. Fertilization occurs 2 to 4 h after pollination (Paulson 1969). Individual florets were marked with a permanent pen to determine the date of flowering. Developing caryopses were harvested at the indicated developmental stages [0–39 days after anthesis (AA)] for further study.
Dry weight determination of developing caryopses
Thirty dehulled caryopses of the same day AA were collected every 3 days and divided into three groups. The caryopses were dried in an air oven at 105°C for 1 h to deactivate the enzymes and then at 80°C for 72 h to attain constant weights for dry weight determination. Average daily gain was calculated according to the dry weights of the caryopses.
Histochemical assays of developing caryopses
Fresh caryopses of different day AA were cut longitudinally from the center and then stained by two different dyes. (1) Staining for starch with I2/KI solution (0.3% iodine and 1% potassium iodide) for 1 min, the position turns black where starch is accumulated. (2) Staining for dehydrogenase activity with 0.5% 2,3,5-Tripheny-2H-tetrazo-lium chloride (TTC) for 30 min. The position turns red where the activity of dehydrogenase in respiration metabolism is high. The darker the red color is, the higher the activity is. All the sections were stained at room temperature and photographed under a Leica MZ6 stereomicroscope with Leica CLS (cold light source) 150XE lighting using Canon digital camera PowerShot S50.
Conventional light and transmission electron microscopy
Longitudinal slices 1-mm thick were cut from the middle of the caryopses with a razor blade and immediately fixed in a fixative containing 2.5% glutaraldehyde plus 1% paraformaldehyde in 50 mM Na cacodylate buffer (pH 7.2) at 4°C for 3 h. Tissue slices were then postfixed in 1% OsO4 in the same buffer at 4°C for 3 h (Browning and Gunning 1977; Harris and Chaffey 1985), dehydrated in a graded ethanol series followed by propylene oxide, and embedded in SPI low-viscosity Spurr’s resin (Spurr 1969).
For light microscopy, semithin sections, 1,000-nm thick, were cut from the resin blocks using a Leica Ultracut rotary microtome with glass knives, and affixed to glass slides. The sections were then stained with 0.5% toluidine blue O (TBO) in 2.5% sodium carbonate for 10 min, rinsed, dried, and examined by a Leica DMLS microscope. Photographs were taken with a Canon digital camera PowerShot S70.
For electron microscopy, ultrathin sections, 70-nm thick, were cut from tissue resin blocks selected on the basis of the light microscope work using the same microtome with a diamond knife. The sections were then picked up on formvar-coated copper mesh grids and doubly stained with saturated uranyl acetate for 30 min followed by lead citrate for 15 min. Ultrathin sections on the grids were examined in a Philips Tecnai 12 transmission electron microscope at 120 kV.
Results
Sorghum caryopsis development
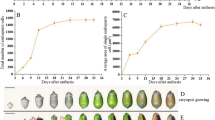
Dry weights and average daily gain (ADG) of caryopses of different day AA were shown in Fig. 1a. The dry weight increases slowly during 0–6 days AA, while there is a steep increase in dry weight during 7–21 days AA with ADG above 1.02 mg/day. The ADG reaches its maximum value of 1.59 mg/day during 16–18 days AA. During 22–30 days AA the dry weight increases slowly again with ADG below 0.8 mg/day. Dry weight reaches its maximum (about 28.17 mg) at 30 days AA.
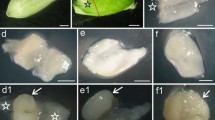
Sorghum BETCs’ development and apoptosis are related to caryopsis growth and maturation. a Line plot shows temporal changes in dry weight of caryopsis of different developmental stage. Ten caryopses were combined into one sample. Data points were means of three replicate samples (30 caryopses total) with the standard error of each shown as a vertical bar. Bar chart shows the average daily gain per caryopsis at different days AA. The horizontal ordinate also indicates the developmental stages of the caryopses in b, d, e, and f. b Caryopsis profile from pollination to maturation. c Schematic diagram illustrating sorghum caryopsis structure of 15 days AA. d I2/KI staining for starch. The black parts are the positions where starch is accumulated. e TTC staining for the activity of dehydrogenase. The red parts show the positions where the activity of dehydrogenase is high. The darker the red color is, the higher the activity is. Caryopses were cut in the center longitudinal plane for histochemical staining, and every caryopsis shown in d and e is the representative of three individual stained caryopses. f Semithin sections of BETCs showing their development and apoptosis. Scale bar, 20 μm. The developmental process of BETL can be divided into four stages: initiation stage (0–4 days AA), differentiation stage (5–18 days AA), functional stage (19–25 days AA), and apoptosis stage (26 days AA to maturation). The duration of each stage may change with the different varieties and their growth environment
Morphological features of caryopsis from pollination to maturity were shown in Fig. 1b. During caryopsis development, the size of caryopsis increases and its color changes from yellow green to green and finally to white. Sorghum caryopsis consists of pericarp, seed coat, endosperm, and embryo. The sorghum endosperm is composed of starchy endosperm, aleurone layer, and BETL (Fig. 1c).
The endosperm tissue expands rapidly during 0–18 days AA and sustains their size thereafter (Fig. 1d). The starchy endosperm continuously accumulates starch during the grain filling process, thus the appearance of the starchy endosperm tissue changes from transparent to milky, and finally to white solid with some parts very hard and transparent. The sequence of starch accumulation is from the top to the base. Besides endosperm, pericarp and embryo also accumulates starch. However, basal main vascular bundle, BETL and its nearby endosperm cells do not accumulate starch, and the activity of respiration metabolism is high in these cells (Fig. 1e). The embryo increases its size rapidly during 6–18 days AA and always shows high respiration metabolism activity during the whole caryopsis development. The respiration metabolism activity of starchy endosperm is high during 0–18 days AA, and then decreases thereafter.
Position of BETCs in sorghum caryopsis
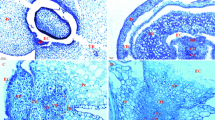
In sorghum caryopsis, the terminal of the main vascular bundle from the rachilla lies in the base–dorsal side of the caryopsis. The epidermal four to five cell layers of the endosperm opposite the vascular terminal differentiate into BETL with wall ingrowths, and the rest of the epidermal cells of the endosperm differentiate into aleurone cells (Fig. 2a). Between the BETL and the vascular terminal are several cell layers of nucellar cells (Fig. 2b).
BETL distribution in sorghum caryopsis. a Central longitudinal section of lower half of an 8-day AA caryopsis showing BETL composed of about three to four layers in the radial direction and 50 to 60 rows in the tangential direction. b Higher magnification view of white box in a. An obvious placental sac has not been observed at this stage. c Central longitudinal section of the lower half of an 18-day AA caryopsis showing BETL. d Higher magnification view of white box in c. BETL are highly developed composed of about three to four layers in the radial direction and 70 to 80 rows in the tangential direction. A large placental sac emerges between the outmost layer of the BETL and the nucellar cells. Some neighboring BETCs bordering the placental sac depart from each other at the bottom (arrowheads). e Higher magnification view of nucellar cells on the other side of the placental sac opposite BETL. f Central longitudinal section of the lower half of a 33-day AA caryopsis showing a dead BETL appressed to the degraded nucellar cells and vascular bundle region. The placental sac disappears. The walls of these dead transfer cells cannot be stained by TBO. All the pictures shown here are light micrographs of semithin sections. AL aleurone layer, BETL basal endosperm transfer layer, E embryo, Nc nucellus, P pericarp, PS placental sac, SAB sym-/apoplasmic boundary, SE starchy endosperm, VBR vascular bundle region. Scale bars: a 100 μm, b 50 μm, c 500 μm, d 100 μm, e 50 μm, f 200 μm
At about 8–9 days AA, an apoplastic cavity termed “placental sac” (Maness and McBee 1986) was observed to emerge between the outmost layer of the BETL and nucellar cells. As the caryopsis grows, the volume of the placental sac increases steadily and reaches its maximum at about 18 days AA (Fig. 1d, e and 2c, d, e). When the caryopsis is about to mature, the placental sac shrinks. The placental sac eventually disappears at about 33 days AA (Fig. 2f). The placental sac originates from the degradation of 1–2 layers of nucellar cells near BETL (Fig. 3). Three factors probably contribute to the enlargement of the placental sac: (1) peripheral cell division increases the number of BETC, (2) BETL distribution changes from being convex to being concave (Fig. 2a, c), and (3) some neighboring BETCs bordering the placental sac depart from each other at the bottom (Fig. 2d). The shrinkage of the placental sac probably results from the degradation of pericarp and caryopsis desiccation.
Placental sac emergence in sorghum caryopsis. a Longitudinal section of a 9-day AA caryopsis. One to two layers of nucellar cells near the BETL degrade. b Higher magnification view of white box in a showing degraded nucellar cells in detail. c Longitudinal section of a 10-day AA caryopsis showing a newly emerged placental sac of small volume. d Higher magnification view of white box in c. All the pictures shown here are light micrographs of semithin sections. BETL basal endosperm transfer layer, Nc nucellus, dNc degraded nucellar cell, nNc normal nucellar cell, P pericarp, PS placental sac, VBR vascular bundle region. Scale bars: a 100 μm, b 20 μm, c 100 μm, d 20 μm
Highly polarized deposition of wall ingrowths in sorghum BETCs
A striking feature of BETCs in sorghum is that their wall ingrowths are highly polarized along the predicted direction of the solute flow. The polarized distribution of the wall ingrowths exhibits in two different levels: both in a single transfer cell and in the whole BETL. In the radial direction, BETCs bordering the placental sac possess the most extensive and complex wall ingrowths with their incapacious cell lumina full of dense cytoplasm. The extent of wall ingrowths development decreases gradually in BETCs further removed from the sym-/apoplasmic boundary and deeper in the endosperm (Fig. 4). In addition to that, these cells change from a column to an irregularly shaped polygon with their size increased. As for a single BETC, wall ingrowth deposition is more extensive in the lower region of the cell compared to that in the upper region (Fig. 4b).
Highly polarized wall ingrowths in sorghum BETCs. a Central longitudinal section of a 20-day AA caryopsis. The outmost one or two layers of BETCs close to the placental sac are nearly full of wall ingrowths. b A higher magnification view of the white box in a showing the BETCs in detail. BETCs with different extent of wall ingrowth development are identified by I, II, and III along the predicted direction of the solute flow (big arrow). BETCs (indicated by asterisks) usually bear long and dense wall ingrowths on the walls in the lower region close to the placental sac, while short and sparse wall ingrowths on the walls in the upper region close to the starchy endosperm. c BETCs close to the starchy endosperm with short and sparse wall ingrowths. d BETCs close to the placental sac with long and dense wall ingrowths, which frequently branch and join with their neighbors. a and b are light micrographs of semithin sections. c and d are transmission electron micrographs. BETC basal endosperm transfer cell, PS placental sac, WI wall ingrowth, SAB sym-/apoplasmic boundary, PCW primary cell wall. Scale bars: a 50 μm, b 20 μm, c 5 μm, d 5 μm
Development of BETL throughout caryopsis growth and maturation
BETCs in the sorghum are the first cell type to become histologically differentiated during endosperm development. These cells undergo initiation, development, and apoptosis throughout the caryopsis growth and maturation.
The endosperm has finished cellularization by 2 days AA. At 3 days AA, the endosperm cells divide frequently, and the epidermal cells near the basal main vascular bundle terminal are marked off from the rest by their elongated columnar shape, thick walls, and dense protoplasmic contents with numerous vacuoles of various sizes (Fig. 5a). These epidermal cells are the first cells which will differentiate into transfer cells later. At 5 days AA, small and sparse wall ingrowths were clearly observed in one or two layers of the endosperm epidermal cells bordering the sym-/apoplasmic boundary near the basal main vascular bundle (Fig. 5b). At 6 days AA, about three to four layers of the epidermal cells of that specific position deposit wall ingrowths (Fig. 5c). The nuclei in some of the early BETCs are irregular in shape (Fig. 5d). During 7–18 days AA, the endosperm cells continue cell division. More and more epidermal cells near the vascular bundle terminal develop wall ingrowths and differentiate into BETCs. As for the cells which have already born wall ingrowths, wall materials continue to deposit, thus wall ingrowths in these cells become larger and more extensive (Fig. 5e∼g). By 18 days AA, the number of BETCs reaches the maximum. BETL is composed of about three to four layers in radial direction and 70 to 80 rows in tangential direction (Fig. 2d). The nuclei in most of the BETCs are irregular in shape. During 19–25 days AA, the division of the epidermal cells ceases, and the number of BETCs is no longer in increment. The wall ingrowths continue to deposit and become more extensive and complex along with the caryopsis filling (Figs. 4a, b and 6a). At 20 days AA, the inner cell space of the outmost one or two layers of BETCs is nearly full of wall ingrowths. At about 26–30 days AA, the cytoplasm of BETCs degrades, and these cells turn into empty dead cell lumina only with cell wall remnants (Fig. 6b∼d). Then along with the caryopsis desiccation, the placental sac disappears, and the cell wall remnants are not stained by TBO (Fig. 2f).
BETL development. a At 3 days AA, the basal epidermal cells of the endosperm near the vascular terminal are columnar in shape with thick walls and dense cytoplasm. b At 5 days AA, one or two layers of the endosperm epidermal cells bordering the sym-/apoplasmic boundary bear small and sparse wall ingrowths. c At 6 days AA, about three to four layers of the epidermal cells deposit wall ingrowths. d BETCs of 6 days AA showing the irregular nuclei (arrowheads). e, f, g showed BETCs of 7, 13, and 15 days AA, respectively with more and more extensive wall ingrowths. Arrows in b–g indicated wall ingrowths. All the pictures shown here are light micrographs of semithin sections. BETC basal endosperm transfer cell, CEC columnar epidermal cell, Es endosperm, N nucleus, Nc nucellus, P pericarp, SAB sym-/apoplasmic boundary. Scale bars: a 100 μm, b 20 μm, c 20 μm, d 10 μm, e 20 μm, f 20 μm, g 20 μm
BETL apoptosis. a BETCs of 25 days AA. b At 27 days AA, the cytoplasm of BETCs degrades. c At 30 days AA, dead BETCs close to the embryo crash. d A higher magnification view of the white box in c. All the pictures shown here are light micrographs of semithin sections. BETC basal endosperm transfer cell, E embryo, PS placental sac, VBR vascular bundle region. Scale bars: a 20 μm, b 20 μm, c 100 μm, d 20 μm
Ultrastructure changes of sorghum BETCs
After the peripheral endosperm cells near the main vascular bundle differentiate into sorghum BETCs, they show a series of typical characteristics of senescence such as nuclei distortion and subcellular organelle deterioration.
During the early stages of BETC development when wall ingrowths just begin to deposit, the nuclei are irregular in shape; and the cytoplasm, just like other types of transfer cells, is characteristically dense and rich in mitochondria and organelles of endomembrane secretory system such as the endoplasmic reticulum (ER), Golgi bodies, and their secretory vesicles (Davis et al. 1990; Offler et al. 2002). Mitochondria are randomly distributed in the cytoplasm (Fig. 7a). The ER observed in sorghum BETCs is extensive lamellar rough ER dividing into many cisternae. These cisternae often arrange regularly to form stacks (Fig. 7b). Tubular smooth ER was hardly seen. Both the ER and the cytoplasm bear numerous ribosomes. Long spiral polysomes were frequently observed (Fig. 7b). Golgi bodies are visible almost everywhere consisting of four to five flattened membrane sacs (cisternae) (Fig. 7a). Abundant small vesicles with the diameter from 0.1 to 0.2 μm, predicted to be derived from their nearby Golgi bodies, were seen around the Golgi bodies. Most of them contain electron-dense materials.
Ultrastructure changes of BETCs. a Portion of a 7-day AA BETC showing five mitochondria and five Golgi bodies. Lots of vesicles with the diameter from 0.1 to 0.2 μm containing electron-dense materials are in the neighborhood of the Golgi bodies (arrows). b Seven-day AA BETCs with extensive stacked cisternae of rough ER and abundant spiral polysomes. c Twenty-day AA BETCs near the placental sac showing quite extensive and complicated wall ingrowths with plasma membrane following their contours. d A portion of a 13-day AA BETC. Note the high density of mitochondria in the interstices of the wall labyrinth and a slight swelling of their cristae. e A portion of a 25-day AA BETC. Large and extensive wall ingrowths occupy most of the cell space. Swollen mitochondria and lamellar rough ER of small length are in the narrow cell lumen. f Twenty-day AA BETCs showing the splitting of rough ER into small lengths. G Golgi body, M mitochondrion, N nucleus, PCW primary cell wall, Pr polysome, RER rough endoplasmic reticulum, V vacuole, WI wall ingrowth. Scale bars: a 1 μm, b 1 μm, c 2 μm, d 2 μm, e 1 μm, f 5 μm
Then along with the specialization of BETCs, the wall ingrowths extend forming very extensive and complicated wall-membrane apparatus, which largely increases the area of their plasma membrane (Fig. 7c). Most of the mitochondria lie in the proximity to the wall ingrowths or in the interstices of the wall labyrinth (Fig. 7d, e), showing polarized distribution (Kang et al. 2009). The cristae of these mitochondria are frequently swollen showing multivesicular profiles. Highly developed lamellar rough ER splits into many small lengths (Fig. 7f). The number of ribosomes both attached to the rough ER and in the cytoplasm decreases and so do the Golgi bodies as well as their nearby vesicles. Finally, the cytoplasm and the nuclei in all BETCs collapse when the caryopsis is about to mature.
Discussion
BETCs in various cereal caryopses are the first cell type to become histologically differentiated during endosperm development. In sorghum, endosperm epidermal cells near the main vascular bundle are marked off from the rest of the endosperm cells by their thickened walls and dense cytoplasms at 3 days AA. In maize, this has occurred at about 6 days AA (Becraft 2001), and in wheat as early as 4 days AA (Evers 1970).
Just like sorghum, all BETCs in various cereal caryopses distribute in the bottlenecks where nutrients are most actively transported. That is just opposite the main vascular bundle if their endosperm develops such a cell type (Fig. 8). In maize and sorghum, BETL is like an oval pad either convex or concave, and BETCs are columnar in shape with highly extensive wall ingrowths (Gao et al. 1996). In wheat and barley, BETL is like a long “S”-shaped strip, and BETCs are composed of two cell types with less extensive wall ingrowths: groove aleurone cells and subaleurone cells. Groove aleurone cells are small in size and oblong or wedge in shape with distinct two wall layers, while groove subaleurone cells are large in size and irregular in shape with many amyloplasts (Cochrane and Duffus 1980; Morrison et al. 1978; Wang et al. 1995). No typical BETCs with wall ingrowths are observed in rice (Gu et al. 2001).
Distribution of BETCs in caryopses of maize, sorghum, wheat, and barley. a Longitudinal section of a 14-day AA maize caryopsis (cv. Suyu 16). b Longitudinal section of a 15-day AA sorghum caryopsis (cv. KS-304) stained by TTC. c Longitudinal section of a 15-day AA wheat caryopsis (cv. Yangmai 5) stained by TTC. d Cross section of a 15-day AA wheat caryopsis (cv. Yangmai 5). e Cross section of a 15-day AA barley caryopsis (cv. Hu 1154). The red curves in a∼e delineate the position of the endosperm transfer cell layer. Em embryo, En endosperm, EC endosperm cavity, NPTC nucellar projection transfer cell, PS placental sac, VBR vascular bundle region
An apoplasmic cavity, termed “placental sac” in sorghum and “endosperm cavity” in wheat and barley, will emerge at the sym-/apoplastic boundary between BETL and their opposite nucellar cells. However, placental sac emergence is not a certain event during maize caryopsis development, which means only some maize caryopses may develop a placental sac. Moreover, no such obvious large apoplastic cavity is observed in rice caryopsis. The formation mechanism of endosperm cavity in wheat and barley is poorly documented. In barley, during caryopsis development, a nucellar projection separates into two lobes, then the two lobes enlarge its size thus inceasing the gaps (endosperm cavity) between BETL and nucellar projection at the center (Cochrane and Duffus 1980). These apoplastic cavities are thought to be full of nutrient solutions which contain high concentrations of nutrients such as sugars and amino acids unloaded from the vascular bundle. Besides, mucopolysaccharides are also detected in the endosperm cavity of barley, and could function in determining the chemical composition and osmolarity of the environment where the endosperm develops (Cochrane and Duffus 1980).
The most remarkable feature of sorghum BETCs is their elaborate wall ingrowths which largely increase the surface area of the plasma membrane, thus increasing the efficiency of the symplastic transport of solutes. Advances in molecular biology experiments indicated that the plasma membrane of seed transfer cells was rich in sugar/H+ transporters and amino acid/H+ transporters both colocalizing with H+-ATPases (Patrick and Offler 2001; Weschke et al. 2000). Abundant mitochondria lie close to these wall ingrowths and presumably provide plentiful metabolic energy for the transmembrane transport of solutes. An endomembrane secretion system including lamellar rough ER, Golgi bodies, and their secretory vesicles is extremely extensive and participates in the metabolic transformation and transportation of substances. Many polysomes, strings of ribosomes attached to the same messenger RNA, are formed both on the rough ER and in the cytoplasm suggesting the high efficiency of protein synthesis. Unlike sorghum, the extensive ER in wheat and barley is divided into many distended cisternae, and may function in the synthesis and/or storage of carbohydrate (Morrison et al. 1978; Cochrane and Duffus 1980).
The initiation and subsequent development of BETCs in sorghum show the pattern of temporal and spatial gradient. In a time scale, the endosperm epidermal cells closest to the basal main vascular bundle bordering the sym-/apoplast boundary are the first cells to differentiate into transfer cells, and then do the cells further remove from the boundary. In a spatial scale, the first differentiated BETCs lying in the outmost layer of the endosperm have dense cytoplasms and the most extensive wall ingrowths, large and dense; the last differentiated BETCs lying in the innermost layer of endosperm have relatively sparse cytoplasms and the least extensive wall ingrowths, short and sparse.
The above position and structural features of sorghum BETCs imply that they probably play an active role in nutrient transfer into the starchy endosperm and embryo both via apoplastic and symplastic pathways.
Most of the literatures documented the distribution and structure of transfer cells in various species of plants as well as in different parts of the plant by light microscopy, transmission microscopy and scanning microscopy. However, few of the literatures were concerned about the whole developmental process of the transfer cells related to the relative tissue development. The development of sorghum BETL can be divided into four stages at the light microscope level: initiation stage, differentiation stage, functional stage, and apoptosis stage. A summary of the duration of each stage and their corresponding developmental characteristics were presented in Table 1. The duration of each stage may change according to different varieties and their growth environment.
During 3–6 days AA, only a layer or two of the peripheral endosperm cells differentiates into transfer cells. Wall ingrowths in these cells are small and sparse, thus the surface to volume ratios of their protoplasts are relatively low. A few transfer cells and their low surface to volume ratios lead to the low capacity of solute transfer of the entire BETL. Therefore, the ADG of the caryopsis dry weight is relatively low, about 0.43 mg/day. The ADG is positively related to the grain filling rate. From 7 days AA to 18 days AA, caryopsis development enters into milk stage. The placental sac emerges and reaches its maximum size at this stage. More and more epidermal cells start to deposit wall ingrowths. As for the cells which have already born wall ingrowths, wall materials continue to deposit, which makes the ingrowths larger and more extensive, thus the capacity of solute transfer of the entire BETL increases sharply during this period. The ADG of the caryopsis increases and reaches its maximum at 16–18 days AA, about 1.59 mg/day. The transported nutrients are supplied to the endosperm and embryo. During 7–18 days AA, the embryo and the endosperm increase its size rapidly, and a large amount of starch is accumulated in both of these two tissues. After 19 days AA, the caryopsis development enters into dough stage, and the ADG decreases. During 19–25 days AA, although wall materials continue to deposit, a decrease in sink viability caused by the enrichment of starchy endosperm cells might be the main reason for the diminishment of the ADG of the caryopsis. Besides, BETCs of this period show a series of characteristics of senescence, and organelles involved in the synthesis and transport of molecules such as the mitochondria and endoplasmic reticulum deteriorate. After 26 days AA, lots of BETCs are degenerated, and the sink viability continues to reduce due to the desiccation of starchy endosperm, thus the ADG continues to decrease. At about 30 days AA, the dry weight of the caryopsis stops increasing, and the caryopsis development enters into full-ripe stage.
In conclusion, the differentiation and development of BETL is in close relation to caryopsis development and grain filling. The occurrence, development, and apoptosis of BETCs proceed along with the caryopsis growth and maturation especially the enrichment of the endosperm and the growth of embryo (Fig. 1). The extent of wall ingrowth development is relative to the grain filling rate. The timing when BETL is fully developed consists with the timing when the grain filling rate reaches its maximum. Thereafter, along with the enrichment of starchy endosperm and the desiccation of the caryopsis, the BETCs undergo apoptosis and eventually lose their function of nutrient transport. The extent of the development of BETCs and their duration of functional stage are essential to the embryo development and endosperm nutrient accumulation. The absence of transfer cell layer in seeds of maize (Hueros et al. 1999), wheat (Wang et al. 1995), and broad bean (McDonald et al. 1995) results in the reduced rates of grain filling and even abortion of the seeds. It is conceivable that measures that delay the senescence and death of BETCs may help to increase the crop yield. Moreover, the degradation of BETCs is speculated to recover a portion of valuable resources such as sugars, amino acids, nucleosides, and mineral elements.
Abbreviations
- ADG:
-
Average daily gain
- BETC:
-
Basal endosperm transfer cell
- BETL:
-
Basal endosperm transfer layer
- AA:
-
After anthesis
- ER:
-
Endoplasmic reticulum
References
Becraft PW (2001) Cell fate specification in the cereal endosperm. Semin Cell Dev Biol 12(5):387–394
Browning AJ, Gunning BES (1977) An ultrastructural and cytochemical study of the wall-membrane apparatus of transfer cells using freeze-substitution. Protoplasma 93(1):7–26
Brugière N, Humbert S, Rizzo N, Bohn J, Habben J (2008) A member of the maize isopentenyl transferase gene family, Zea mays isopentenyl transferase 2 (ZmIPT2), encodes a cytokinin biosynthetic enzyme expressed during kernel development. Plant Mol Biol 67(3):215–229
Cochrane MP, Duffus CM (1980) The nucellar projection and modified aleurone in the crease region of developing caryopses of barley (Hordeum vulgare L. var. distichum). Protoplasma 103(4):361–375
Davis RW, Smith JD, Cobb BG (1990) A light and electron microscope investigation of the transfer cell region of maize caryopses. Can J Bot 68:471–479
Dermastia M, Kladnik A, Koce JD, Chourey PS (2009) A cellular study of teosinte Zea Mays subsp. Parviglumis (Poaceae) caryopsis development showing several processes conserved in maize. Am J Bot 96(10):1798–1807
Evers AD (1970) Development of the endosperm of wheat. Ann Bot 34:547–555
Gao RQ, Dong ST, Hu CH, Wang QY (1996) Studies on the microstructure and ultrastructure of the development endosperm transfer cells in maize. J Shandong Agric Univ 27(3):324–332
Gao XQ, Ren QP, Xi XY (2002) Ultrastructures of aleurone layer transfer cells in buckwheat. J Qufu Norm Univ Nat Sci 28(4):101–104
Gomez E, Royo J, Guo Y, Thompson R, Hueros G (2002) Establishment of cereal endosperm expression domains: identification and properties of a maize transfer cell-specific transcription factor, ZmMRP-1. Plant Cell 14:599–610
Gottschling M, Hilger HH (2003) First fossil record of transfer cells in angiosperms. Am J Bot 90(6):957
Gu YJ, Xiong F, Wang Z, Chen G, Li WF (2001) A contrast of the endosperm development between rice and wheat. J Of Nanjing Norm Univ Nat Sci 24(3):65–74
Gunning BES, Pate JS (1969) “Transfer cells” plant cells with wall ingrowths, specialized in relation to short distance transport of solutes—their occurrence, structure, and development. Protoplasma 68(1):107–133
Gutierrez-Marcos JF, Costa LM, Biderre-Petit C, Khbaya B, O’Sullivan DM, Wormald M, Perez P, Dickinson HG (2004) Maternally expressed gene1 is a novel maize endosperm transfer cell-specific gene with a maternal parent-of-origin pattern of expression. Plant Cell 16(5):1288–1301
Harris N, Chaffey NJ (1985) Plasmatubules in transfer cells of pea (Pisum sativum L.). Planta 165(2):191–196
Hu SY, Zhu C, Zee SY (1983) Transfer cells in suspensor and endosperm during early embryogeny of Vigna sinensis. Acta Bot Sin 25(1):39–52
Hueros G, Varotto S, Salamini F, Thompson RD (1995) Molecular characterization of Bet1, a gene expressed in the endosperm transfer cells of maize. Plant Cell 7:747–757
Hueros G, Royo J, Maitz M, Salamini F, Thompson RD (1999) Evidence for factors regulating transfer cell-specific expression in maize endosperm. Plant Mol Biol 41(3):403–414
Jain M, Chourey PS, Li QB, Pring DR (2008) Expression of cell wall invertase and several other genes of sugar metabolism in relation to seed development in sorghum (Sorghum bicolor). J Plant Physiol 165(3):331–344
Kang B-H, Xiong Y, Williams DS, Pozueta-Romero D, Chourey PS (2009) Miniature1-encoded cell wall invertase Is essential for assembly and function of wall-in-growth in the maize endosperm transfer cell. Plant Physiol 151:1366–1376
Kladnik A, Chourey PS, Pring DR, Dermastia M (2006) Development of the endosperm of Sorghum bicolor during the endoreduplication-associated growth phase. J Cereal Sci 43:209–215
Maness NO, McBee GG (1986) Role of placental sac in endosperm carbohydrate import in sorghum caryopses. Crop Sci 26:1206–1207
McDonald R, Wang HL, Patrick JW, Offler CE (1995) The cellular pathway of sucrose transport in developing cotyledons of Vicia faba L. and Phaseolus vulgaris L.: a physiological assessment. Planta 196:659–667
Morrison IN, O’Brien TP, Kuo J (1978) Initital cellularization and differentiation of the aleurone cells in the ventral region of the developing wheat grain. Planta 140(1):19–30
Muniz LM, Royo J, Gomez E, Barrero C, Bergareche D, Hueros G (2006) The maize transfer cell-specific type-A response regulator ZmTCRR-1 appears to be involved in intercellular signalling. Plant J 48(1):17–27
Offler CE, McCurdy DW, Patrick JW, Talbot MJ (2002) Transfer cells: cells specialized for a special purpose. Annu Rev Plant Biol 54:431–454
Patrick JW, Offler CE (2001) Compartmentation of transport and transfer events in developing seeds. J Exp Bot 52(356):551–564
Paulson IW (1969) Embryogeny and caryopsis development of Sorghum bicolor (L.) Moench. Crop Sci 9:97–102
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Wang HL, Patrick JW, Offler CE, Wang XD (1995) The cellular pathway of photosynthate transfer in the developing wheat grain. III. A structural analysis and physiological studies of the pathway from the endosperm cavity to the starchy endosperm. Plant Cell Environ 18:389–407
Weschke W, Panitz R, Sauer N, Wang Q, Neubohn B, Weber H (2000) Sucrose transport into barley seeds: molecular characterization of two transporters and implications for seed development and starch accumulation. Plant J 21(5):455–467
Xi XY (1993) Embryo and endosperm development in Isatis tinctoria L. and the histochemical study of its storage reserve. Acta Bot Sin 35(1):35–43
Xi XY, Leng M (1995) Ultrastructure of transfer cells in endosperm of Coix lacryma-jobi. Acta Bot Sin 37(3):171–176
Zheng YK, Wang HH, Gu YJ, Kong Y, Wang F, Wang Z (2009) Structure observation and investigation of maize endosperm transfer cells. Acta Bot Boreal Occident Sin 29(12):2464–2467
Acknowledgments
The generous gift of sorghum seeds from Prof. Shu-Zhu Tang is gratefully acknowledged. The authors thank Wei-Dong Zhou, Yi-Fang Chen, and Qing-Li Huang for their excellent technical assistance and the use of the electron microscope facilities at the testing center of Yangzhou University. They also thank Prof. Cun-Xu Wei for the helpful discussion and critical reading of the manuscript. This work is supported jointly by the National Natural Science Foundation of China (grant no. 30670125) and the Research Fund for the Doctoral Program of Higher Education of China (grant no. 20093250110004) to Prof. Zhong Wang, together with the Scientific Research Innovation Program for Graduate Students of Jiangsu Province (grant no. CX08B_026Z) to doctoral candidate Hui-Hui Wang.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Marisa Otegui
Rights and permissions
About this article
Cite this article
Wang, HH., Wang, Z., Wang, F. et al. Development of basal endosperm transfer cells in Sorghum bicolor (L.) Moench and its relationship with caryopsis growth. Protoplasma 249, 309–321 (2012). https://doi.org/10.1007/s00709-011-0281-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-011-0281-6