Abstract
Features of programmed cell death (PCD) and dynamic changes of starch accumulation in developing pericarp cells of wheat (Triticum aestivum L.) were observed and analyzed by periodic acid–Schiff/toluidine blue O double staining, fluorescence staining, terminal deoxynucleotidyl transferase-mediated fluorescein deoxyuridine triphosphate nick-end labeling (TUNEL) and transmission electron microscopy. The results showed that cellular organelles were orderly disintegrated. TUNEL-positive nuclei were detected at 0 day after flowering (DAF), whereas nuclei showed significant features of degradation at 2 DAF, such as chromatin condensation, nuclei condensation, and nuclei deformation. Then, heterochromatin gradually disappeared and the cellular nucleus was completely degraded. The mitochondria degradation and vacuolation also were detected at 15 DAF. These results indicated that the development of pericarp cells was a typical process of PCD. However, the PCD in pericarp cells had their own characteristics: PCD started early and lasted for a considerable time. In the delayed process of PCD, starch granules were synthesized, deposited, and degraded temporarily in amyloplasts or chloroplasts. The delay of PCD in pericarp cells may be due to sufficient photosynthetic assimilates and energy supply. Besides, normal mitochondria were required for pericarp cells to survive. Pericarp cells contained only compound starch granules. Starch was massively synthesized from 0 to 11 DAF, but it was rapidly degraded after 11 DAF. Therefore, apart from protection, pericarp cells played essential roles in starch synthesis, storage, and degradation, as well as nutrient transportation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Programmed cell death (PCD), a genetically regulated process of cell suicide, is central to the development, homeostasis, and integrity of multicellular organisms (Ameisen 2002). The PCD of animal and plant cells have many different features. In plant cells, it is accompanied by increased chromatic agglutination, vacuolation, and orderly degradation of cell inclusions. In some cases, DNA fragmentation and cysteine proteases activities are also detected (Otsuki et al. 2003; Gaffal et al. 2007; Mea et al. 2007). Many examples of PCD exist in the plants, such as root cap abscission, aerenchyma formation, differentiation of tracheary elements, leaf senescence, and hypersensitivity reaction (Pennell and Chris 1997; Jones 2001). However, there is no detailed report about the PCD in developing pericarp of wheat. Recently, a study reports that there is a point in the cell life cycle beyond which PCD cannot be reversed (Van Doorn 2005). In plant cells, the reversal point is associated with the degradation of chloroplasts or mitochondria. A minimum number of remaining chloroplasts or mitochondria can reverse PCD (Van Doorn 2005).
Wheat fruit is called caryopsis in botany because its pericarp and episperm (developed from integument) are tightly integrated. Pericarp is developed from ovary wall and divided into exocarp, mesocarp, and endocarp. In the pericarp of wheat (Triticum aestivum L.; Zhou et al. 2001; Hu 1964; Nakamura et al. 1998; Chevalier and Lingle 1983), barley (Hordeum vulgare L.; Chevalier and Lingle 1983; Rolletschek et al. 2004), rice (Oryza sativa L.; Gu et al. 2002), and sorghum (Sorghum bicolor L. Moench; Earp et al. 2004), starch accumulation has been observed, but most studies focus on wheat. During the development of wheat caryopsis, the starch accumulation shows a dynamical and orderly change in a considerable time. In detail, a starch accumulation peak occurs before fertilization, and the starch content decreases along with fertilization. After fertilization, the starch accumulation increases again, and most of the cells are almost full of starch at the peak at 5 days after flowering (DAF); then, the starch gradually disappears (Chevalier and Lingle 1983). Starch, accumulated in the pericarp, may transform to sugar and then transport to the endosperm as the substrate for starch synthesis (Gu et al. 2002). Therefore, pericarp is not only a structure which can protect ovule and seed but also a place for nutrition transportation and temporary storage (Chevalier and Lingle 1983). However, the relationship between dynamical starch accumulation and PCD process is not clear.
The hydrofugous corneums which lay on each side of the integument form the material exchange barrier between endosperm and pericarp (Gu et al. 2002). As a result, photosynthetic assimilates are first transported to the abdominal vascular bundle of caryopsis and then enter the endosperm by various pathways. Besides, the dynamical changes of starch accumulation mainly occur in the mesocarp; hence, mesocarp in caryopses was selected as an object in this study. In order to make clear the cytological characteristics of developing pericarp and the relationship between the development and starch accumulation in pericarp, periodic acid–Schiff/toluidine blue O double (PAS/TBO) staining, fluorescence staining, terminal deoxynucleotidyl transferase-mediated fluorescein deoxyuridine triphosphate nick-end labeling (TUNEL), and transmission electron microscopy (TEM) were used to study the development process and the dynamical changes of starch accumulation inside the pericarp. Our results may help to clarify the functions of pericarp in caryopsis development.
Materials and methods
Plant growth conditions and sample collection
Wheat (T. aestivum L.) cultivars, Huamai 8, were planted in the experimental field of Huazhong Agricultural University, Hubei Province, China, on November 2, 2006. The line spacing was 25 cm for a total of five lines (on a 2.1 × 4.4-m plot). Fertilizer (30:10:10) was applied at a rate of 0.05 kg m−2 on February 29, 2007, and carbamide was applied at a rate of 0.05 kg m−2 on March 19, 2007; no additional fertilizers were applied. The wheat germinated on November 20, 2006. The arista was observed to be exposed from the spikelet on March 29, 2007, tassel on April 2, 2007, and flower on April 7, 2007.
Two methods were used to mark wheat. First, the ears were tagged with labels at the time of arista emergence from the spikelet [occurred when the outer arista was about 5 mm long; 9 days before flowering (DBF)] and tasseling (5 DBF). Second, at the time of flowering (0 DAF), the aristas of the flowering spikelets were marked with a laboratory marker and the ears were tagged with labels.
Spikelet samples were collected daily from 0 to 24 DAF. In order to obtain the simultaneous development materials, only samples with three labels after the 0 DAF were taken.
Transmission electron microscopy
For TEM, caryopses were dissected, and small pieces of pericarp tissue were immediately prefixed in 2.5% glutaraldehyde (v/v)/0.1 M phosphate buffer (pH 7.2) at 4°C and then vacuum-infiltrated until the samples sank into the bottom of container. More detailed experimental processes have been described in Wang et al. (2008). After fixation, samples were washed three times in 0.1-M phosphate buffer (pH 7.2), 15 min each time, and then postfixed in 1% OsO4 in the same buffer for 2 h. The samples were then washed three times in ddH2O, 15 min each time, and dehydrated through a chilled acetone series [30%, 50%, 70%, 90%, 95%, 100%, and 100% (v/v in ddH2O); 20 min at each concentration] at room temperature. The samples were infiltrated in a graded scale of 3:1, 1:1, and 1:3 (v/v) acetone/SPI-PON 812 resin (SPI Supplies, West Chester, PA, USA) and, as the last step, in 100% (v/v) SPI-PON 812 resin, for 8 h each step. Samples were then embedded in SPI-PON 812 resin and polymerized at 60°C for 24 h. Sections (80–90 nm thick) were cut on an LKB 2088 Ultramicrotome (Bromma, Sweden), stained in lead citrate [1.33 g Pb(NO3)2, 1.76 g Na3C6H5O7•2H2O in 50 ml ddH2O] for 30 min, rinsed three times in ddH2O, 1 min each time, and then stained for 30 min with 2% (w/v) uranyl acetate in ddH2O, followed by three rinses of ddH2O, 1 min each rinse. The sections were viewed using a TEM (H-7650, Hitachi, Japan).
Fluorescence microscopy
To determine changes in the nuclei of the pericarp cells, semithin sections of pericarp sampled from 0 to 15 DAF were stained with acridine orange (AO, Amresco, BA0043) and propidium iodide (PI, Sigma, P4170).
The methods of semithin sections and AO/PI staining for fluorescence microscopy were according to Wang et al. (2008). Semithin sections of 1-μm thickness were obtained using an LKB 2088 Ultramicrotome (Bromma, Sweden). The sections were stained for 5 min in 0.01% (w/v) acridine orange (AO, Amresco, BA0043) in 0.1 M phosphate buffer (pH 7.2), followed by three 2-min rinses in ddH2O. Sections were then stained for 15 min in 1 μg/ml propidium iodide (PI, Sigma, P4170) and rinsed in ddH2O for 2 min. Treatment with ddH2O alone served as the control for the fluorescence. Images were acquired and processed using a fluorescence microscope (Nikon Eclipse 80i, Japan) under blue-light excitation with a B-2A filter (ex 450–490 nm; em >520 nm).
TUNEL
In order to obtain higher-resolution images, TUNEL assays were performed on semithin sections instead of paraffin sections. In this study, green fluorescence denoted the fragmentized site within the DNA. The proportion of positive cell (%) = (number of TUNEL-positive cells / number of total cells) × 100%.
TUNEL experiments were performed via the DeadEnd™ Fluorometric TUNEL System (Promega G3250), according to the methodologies outlined in Ishida-Yamamoto et al. (1999) and Otsuki et al. (2003) and the manufacturer’s instructions, with modifications as mentioned below. Specific steps were done by previous method (Wang et al. 2008).
Samples were prefixed in cold (4°C) 4% formaldehyde and 2.5% glutaraldehyde (v/v) in phosphate-buffered saline (PBS, pH 7.2) and further processed according to the methods used for the TEM samples with the modification that phosphate buffer was replaced by PBS. Semithin sections (1 μm) were mounted on poly-l-lysine-coated glass slides and deresined in the work solution [saturated NaOH in 100% ethanol, which dissolved overnight and mixed with toluene by 3: 1 (v/v)] for 6 min, rinsed three times, 5 min each time, in 100% ethanol, and then rehydrated through a gradient ethanol series (95%, 85%, 70%, 50%, 3 min each concentration). TUNEL assays were performed according to the manufacturer’s instructions with the modification that TUNEL reactions were carried out for 40 min in the dark at 37°C, and the sections without the propidium iodide staining were observed using a fluorescence microscope (Nikon Eclipse 80i, Japan) under blue-light excitation with a B-2A filter (ex 450–490 nm; em >520 nm), and images of cellular structures were made using a phase-contrast microscope (Nikon Eclipse 80i, Japan).
Periodic acid–Schiff/toluidine blue O double staining
The semithin sections (1 μm) were treated with 0.5% periodic acid for 10 min and rinsed in ddH2O for 3 min. Sections were then stained in Schiff’s reagent for 25 min and rinsed three times in rinsing solution (1 mol/l HCl 5 ml + 10% Na2S2O5 5 ml + ddH2O 100 ml), 2 min each time. After that, sections were stained for 2 min in 0.05% TBO (0.1 g TBO + 200 ml antipyrine benzoate buffer) at 45°C and rinsed in ddH2O. Images were acquired and processed using a light microscope (Nikon Eclipse 80i, Japan).
Statistics for starch accumulation in pericarp
At least 20 mesocarp cells on TEM photographs were randomly selected, in which the number of amyloplasts were counted, and at least 40 amyloplasts on photographs were randomly selected, in which the number of starch particles were counted and their length of long axis and minor axis was measured. Images were observed and processed by ACDsee Power Pack software (ACD Systems Ltd.). Statistical analysis was carried through by using Excel (Microsoft Corporation).
On the photographs of 8 DAF, 60 plastids that contained starch particles were randomly selected. To distinguish chloroplast and amyloplast, these plastids were analyzed based on whether the plastids contained stroma lamellae structure. The proportion of chloroplast to amyloplast was also calculated.
Results
The pericarp of wheat can be divided into three parts: exocarp, mesocarp, and endocarp. Exocarp is the outermost layer of the pericarp, formed by the tight connection of vertically elongational cells; endocarp is composed of two-tier cells which are close to integument. Between exocarp and endocarp, there are multilayer and inordinate cells called mesocarp (Fig. 1). The mesocarp near the abdominal vascular bundle of caryopsis was the research object in this study (the following were called pericarp).
Detection of PCD in pericarp cells
Nuclear DNA degradation
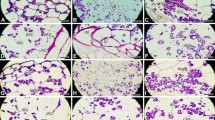
TUNEL assay is a widely applied technique to detect nuclear DNA degradation during PCD process. The emergence of green fluorescence, TUNEL-positive signals, in nuclei predicates that DNA cleavage has occurred. The TUNEL-positive signals could be detected at 0 DAF (Fig. 2 (1)), which indicated that nuclear DNA had disrupted at the moment. The proportion of positive cell (%) gradually decreased from 2 to 7 DAF (Figs. 2 (2 and 3) and 3), which illustrated degradation of some nuclei of pericarp. A negative control failed to produce any detectable signal (Fig. 2 (3), the upper-right corner).
The results of TUNEL staining in pericarp cells. 1, 0 DAF. Large amounts of TUNEL-positive nuclei in pericarp cells. Bar 50 μm. 2, 2 DAF. Decrease of TUNEL-positive nuclei in pericarp cells. Bar 50 μm. 3, 7 DAF. Decrease of TUNEL-positive nuclei in pericarp cells. Bar: 50 μm. The inset and bracket showed control at 4 DAF; cross section showed no fluorescence was detected in pericarp cells. Bar 25 μm. en endosperm; me mesocarp
Nuclear changes
The results from AO/PI double staining suggested that pericarp cell was parenchyma cell at 0 DAF. At this time, the stained cell wall possessed green fluorescence, and the stained nuclei possessed red fluorescence and were integrated (Fig. 4 (1)). After 4 DAF, the number of nuclei gradually decreased (Fig. 4 (2, 3, and 4)); at 15 DAF, there was almost no nucleus in the cells (Fig. 4 (5)). The results were consistent with TUNEL. A negative control failed to produce any detectable signal (Fig. 4 (6), the upper-right corner).
Changes of amyloplasts and nuclei in pericarp cells (AO/PI staining) 1, 0 DAF. Few amyloplasts were detected in pericarp cells. The inset and bracket are amplified from the frame in this figure, showing amyloplasts (arrows) in pericarp cells. Bar 50 μm. 2, 4 DAF. Longitudinal section showed increased quantities of amyloplasts in pericarp cells. The inset and bracket are amplified from the frame in this figure, showing cells were full filled with amyloplasts. Bar 50 μm. 3, 8 DAF. Increased quantities of amyloplasts in pericarp cells. Bar 25 μm. 4, 11 DAF. The maximum of amyloplast accumulation in pericarp cells. Amyloplasts were detected in parenchyma cells of vascular bundle. Bar 50 μm. The inset and bracket showed periodic acid–Schiff/toluidine blue O staining, 12 DAF, large amounts of amyloplasts in pericarp cells (arrows). Bar 500 μm. 5, 15 DAF. Decrease of amyloplasts in pericarp cells. The inset and bracket in the upper-right corner are amplified from the frame in this figure, showing amyloplasts in pericarp cells (arrows). Bar 50 μm. The inset and bracket in the upper-left corner showed PAS/TBO staining, 14 DAF, amyloplasts in pericarp cells. Bar 500 μm. 6, 19 DAF. Amyloplasts disappeared in pericarp cells. Bar 50 μm. The inset and bracket in the upper-left showed PAS/TBO staining, 24 DAF, no amyloplast in pericarp cells. Bar 500 μm. The inset and bracket in the upper-right showed no fluorescence were detected in pericarp cells without stain with AO and PI at 4 DAF. Bar 25 μm. en endosperm; me mesocarp. VB vascular bundle
Ultrastructural analysis
At the early stage of caryopsis development, the heterochromatin was well-distributed in nuclei and eumorphism, and cells contained considerable vacuoles (Fig. 5 (1 and 2)). However, the heterochromatin condensation was observed from 2 to 4 DAF and some nuclei deformed (Fig. 5 (3 and 5)). At 8 DAF, nuclei showed an increasing electron density and started to decline (Fig. 6 (1)). The heterochromatin decreased at 11 DAF. Although the heterochromatin in cell was sparse, still nuclear membrane was integrated (Fig. 6 (3, 4)). At that time, the nuclei were at the later stage of degradation and completely disappeared at 15 DAF (Fig. 6 (7, 8)).
Ultrastructures of pericarp cells. 1, 0 DAF. The electron-dense starch granules (arrows) in pericarp cells. Bar 5 μm. 2, 0 DAF. The electron-dense starch granules (arrows) in pericarp cells. Bar 2 μm. 3, 2 DAF. The cleavage nuclei and few amyloplasts in pericarp cells. Bar 2 μm. 4, 2 DAF. Showed stroma lamella and starch granules in chloroplast. Bar 0.5 μm. 5, 4 DAF. Showed decreased nuclei. Bar 2 μm. The inset and bracket are amplified from the frame in this figure, showing stroma lamella and starch granules in chloroplast. 6, 4 DAF, showing starch granules in chloroplast. Bar 0.5 μm. 7, 6 DAF, showing decreased chromatin in cell. The inset and bracket are amplified from the frame in this figure, showing compound starch granules in chloroplast. Bar 2 μm. 8, 6 DAF, showing plasmodesmata (arrows) in pericarp cell wall. Condensed nuclei and mitochondria in pericarp cells. Bar 0.5 μm. a amyloplast; C chloroplast; me mesocarp; M mitochondria; N nucleus
Ultrastructures of pericarp cells. 1, 8 DAF, showing decreased chromatin and condensed chromatin of periphery nucleus. Bar 1 μm. 2, 8 DAF, showing mitochondria in pericarp cells, stroma lamella, and starch granules in chloroplast. Arrows showed plasmodesmata in pericarp cell wall. Bar 0.5 μm. 3, 11 DAF. Decreased chromatin in cell and chloroplasts were filled full with starch granules. Bar 5 μm. 4, 11 DAF, showing edges and corners appeared at the starch granules in the nuclear envelope. Bar 0.5 μm. 5, 11 DAF. Arrows showed plasmodesmata in pericarp cell wall. Bar 0.2 μm. 6, 11 DAF. Some mitochondria became transparent in appearance. Bar 0.2 μm. 7, 15 DAF. Few amyloplasts and organelles were detected in pericarp cells, and electron-dense amyloplasts were observed. Bar 2 μm. 8, 15 DAF. Most cytoplasm was degraded. Bar 10 μm. a amyloplast; C chloroplast; me mesocarp; M mitochondria; N nucleus
Chloroplast and stroma lamellae structure were obviously observed in cells at 2 DAF (Fig. 5 (4)). After that, the number of mitochondria rapidly increased (Fig. 5 (6, 8)). At 11 DAF, part of mitochondria was degraded and the mitochondrial membrane became indistinct (Fig. 6 (6)). Chloroplasts were filled with starch granules and survival stroma which interspersed among starch showed deep electronic staining (Fig. 6 (1 and 2)). There were many plasmodesmata located on cell wall (Fig. 6 (5)). At 15 DAF, the chloroplasts disappeared with degradation of starch and few organelles in cytoplasm were identifiable (Fig. 6 (7 and 8)).
In conclusion, the degenerative process of pericarp cells took a considerable time (from 0 to 15 DAF). Nuclear DNA was fragmentized at 0 DAF and the obvious degradation of nuclei occurred at 2 DAF, followed by gradual disappearance of heterochromatin. At 15 DAF, nuclei completely degraded. The number of chloroplasts and mitochondrion increased at 2 DAF, but mitochondrion began to show the characteristics of degradation at 11 DAF. Finally, chloroplast and mitochondrion gradually disappeared.
Dynamic state of starchy accumulation in pericarp cells
Light microscope detection
AO/PI double staining showed that amyloplasts possessed green fluorescence. A few amyloplasts existed in pericarp cells at 0 DAF (Fig. 4 (1)) and then gradually increased. At 4 DAF, the number of amyloplasts had an obvious increase, but it was various at different locations of pericarp cells. Some pericarp cells were almost filled with amyloplasts (Fig. 4 (2)). At 8 DAF, all of the endocarp cells contained considerable amyloplasts (Fig. 4 (3)). A large number of amyloplasts were simultaneously detected in vascular bundle parenchyma cells at 11 DAF (Fig. 4 (4)). However, amyloplasts rapidly decreased, especially in the endocarp cells closed to vascular bundle at 15 DAF (Fig. 4 (5)). After 19 DAF, amyloplasts gradually disappeared (Fig. 4 (6)).
PAS/TBO double staining showed that most pericarp cells contained abundant amyloplasts at 12 DAF (Fig. 4 (4), the upper-right corner). At 14 DAF, one or two layers of pericarp cells closed to exocarp contained more amyloplasts (Fig. 4 (5), the upper-left corner), whereas the cells close to vascular bundle contained less amyloplasts. No amyloplasts existed in pericarp cells at 24 DAF (Fig. 4 (6), the upper-left corner). Therefore, the above results were similar to AO/PI double staining.
Ultrastructural analysis
According to the condition of starch accumulation, the developmental process of pericarp cells could be divided into three periods: preparation period (0~3 DAF), synthesis period (4~11 DAF) and resolution period (after 11 DAF).
-
1.
In the preparation period of starch synthesis, cells contained few amyloplasts which were loosely distributed in cytoplasm, and starch granules showed deep electronic staining at 0 DAF (Fig. 5 (1 and 2)). Chloroplasts occurred in cells at 2 DAF, in some of which starch granules could be observed (Fig. 5 (3 and 4)).
-
2.
In the synthesis period of starch, the clear stroma lamellar structure of chloroplast could be observed at 4 DAF (Fig. 5 (5 and 6)), followed by the increase of plastids and starch quantity. At 8 DAF, 95% of starch granules appeared in chloroplasts and others stayed in amyloplasts. The data demonstrated that most of the starch granules were formed in chloroplast in this period. The number and size of amyloplasts in chloroplast reached a peak at 11 DAF (Fig. 6 (3 and 4)).
-
3.
In the resolution period of starch, the surface of some amyloplasts became rough at 4 DAF (Fig. 5 (6)), and the surface of more starch granules became irregular at 6 DAF (Figs. 5 (7 and 8) and 6 (2)). The space among starch granules increased and was detected with deep electronic staining. Besides, angularity appeared on starch granules (Fig. 6 (3 and 4)). The appearance of surface rugosity and angularity on starch granules possibly resulted from the decomposition of starch granules by correlative starch hydrolase. In other words, synthesis and degradation of starch granules were carried through simultaneously from 4 to 11 DAF. The chloroplasts gradually ceased starch synthesis and starch granules were quickly degraded, which led to rapid decrease of starch accumulation in pericarp cells. The chloroplasts completely disappeared at 15 DAF. Meanwhile, the surface of starch granules possessed deep electron staining; it may be some function protein for amylolysis (Fig. 6 (7 and 8)).
Statistical analysis
Statistical results of the numbers of amyloplasts in one pericarp cell (NAC), the numbers of starch granules in one amyloplast (NSGA), and the size of starch granules were shown in Fig. 7. The NAC rapidly increased after flowering and reached a peak at 4 DAF, but it decreased after 4 DAF. The NSGA kept steady state from 4 to 8 DAF, but it quickly increased from 8 to 11 DAF. The NSGA decreased after reaching the peak at 11 DAF. Both the length of long axis and the length of minor axis increased in amyloplasts from 4 to 11 DAF and then sharply decreased.
The statistics of starch accumulation in pericarp cells. The left side of the y-axis represents the length of long axis and short axis of starch granules, and the right side of the y-axis represents the NAC and NSGA. Long axis long axis of starch granules; short axis short axis of starch granules. NSGA numbers of starch granules in one amyloplast. NAC numbers of amyloplasts in one cell. DAF days after flowering. DBF days before flowering. Data represent the average value of at least 20 mesocarp cells or at least 40 amyloplasts. Error bars represent standard deviation
In brief, a mass of starch was synthesized from 0 to 11 DAF. After that, it sharply degraded (as shown in Fig. 7).
Discussion
TUNEL reactivity and TEM have been widely used to detect PCD, and the detection results were reliable. TUNEL-positive nuclei suggest that nuclear DNA degradation preceded the observable cell death morphology. In this study, we observed that pericarp in wheat caryopsis had TUNEL-positive nuclei and obtained direct evidence that these cells underwent increased vacuolation, nuclear degeneration, and chromatin condensation, which coincided with the typical characteristics of PCD in plants (Gaffal et al. 2007; Pennell and Chris 1997). Therefore, the development of pericarp cells was a typical PCD process.
Three essential factors are needed for plant cell maintaining survival, such as materials, energy, and signals. The materials indirectly or directly come from the photosynthate of chloroplast; energy is the adenosine triphosphate which is mainly produced by the oxidation phosphorylation of mitochondria; signals originate from the inside and outside environment of plant cells. Plant cells can transcript varied messenger RNA (mRNA) at different developmental stages, and the mRNA is one of the typical signals of plant cell. Generally, mRNA in plant cells can be translated into protein, by which the cell structure and function formed. However, mRNA in dying cells can be translated to produce hydrolytic enzymes, which execute PCD. Previous studies have pointed out that endosperm cells rapidly enucleated to die, whereas starch was vigorously synthesized during the PCD process of rice endosperm. The reason was predicted that gene selectively expressed before nucleus deterioration, and then long-lived mRNA was transcribed and transported to cytoplasm, which maintained the metabolic activities in cytoplasm development (Lan et al. 2004).
It is a short process from PCD initiation to complete death in plant cell. In in vitro culture, the PCD needs only 96 h to take place to form tracheal tubes in mesophyll cells (Groover et al. 1997), and in endosperm cells PCD needs only 6 h (Lan et al. 2004). However, PCD in pericarp cells has distinct characteristics: the process was slow and lasted for a long time (more than 15 days), accompanied with intense changes of material and energy metabolism. This gives us an interesting question about how pericarp cells simultaneously coordinate two contradictory processes, survival and death, in the same developmental stage. We make the following hypothesis: at the early develop stages of pericarp cells, the gene-related PCDs were transcribed and translated, and the hydrolytic enzyme which caused PCD was produced. However, just before the PCD genes were expressed, pericarp cells also had transcribed some long-life mRNA for synthesizing some enzymes, which were used in photosynthesis of chloroplast or oxidation phosphorylation of mitochondria. Although PCD in pericarp cells had been initiated at 0–8 DAF, pericarp cells could maintain the normal metabolic activities to survival. The death of pericarp cells was delayed. At later developmental stage, the long-life mRNA, nuclei, chloroplast, and mitochondria were degraded. As a result, the pericarp cells could not obtain sufficient energy to maintain survival, and then the cells rapidly died. Therefore, sufficient energy supply was the immediate cause for PCD delay and the long-life mRNAs, normal mitochondrion, and chloroplasts were the prerequisite for cell survival in pericarp of wheat. A similar phenomenon of PCD also appears at sieve development stage (Wang et al. 2008). Mature sieve element has no nuclei but has mitochondrion, mRNAs (possibly come from the companion cell), and sufficient photosynthate, so it can survive for a long time. It illustrates that, if a cell can obtain sufficient energy, it can survive for a long time under the condition that the mitochondrion or other few organelles exist (Van Doorn 2005). The above results are consistent with this opinion.
In animal cells, dying cells are packaged into membrane to form apoptotic bodies and then phagocytized by phagocytes. In contrast, plant cells are surrounded by rigid cell walls and they have no phagocytes, so the system for degrading and recycling dying cells in plants is thought to be different from that in animals. In xylem trachea tube, the degraded cell inclusions are used for secondary cell wall thickening (Wang and Cui 1998). The degraded cellular nucleus are digested and absorbed by developing amyloplasts in starchy endosperm cells of rice (Lan et al. 2004), which illustrates that the degradation of cell inclusions can be reclaimed when the PCD process occurred in plant cells.
The endosperm cells of Huamai 8 start to accumulate starch at 8 DAF, meanwhile starch granules of pericarp cells are disintegrated (Zhou 2000). Then, the productions of degradation may be used for starch synthesis in endosperm cells. Hence, the assimilates for starch synthesis come from two sources: the productions of starch degradation in pericarp cells and photosynthetic assimilates transported from phloem of caryopsis. There are three kinds of amyloplasts in wheat endosperm cells: big starch granule, medium starch granule, and small starch granule (Zhou 2000). The endosperm cells show vigorous activity for starch accumulation at 14 DAF and produce abundant small starch granules (Hu 1964), from which we can conclude that the productions of starch degradation in pericarp cells are used for big starch granule synthesis in endosperm cells from 8 to 14 DAF. These conclusions also indicate that the PCD of pericarp cells are accompanied by the storage of photosynthetic assimilates, which is finally used for forming starch in wheat endosperm.
According to the developmental characteristics and the roles for photosynthetic assimilate transportation of pericarp cells, the developmental process of pericarp cells could be divided into four stages. Stage 1 was before 8 DAF; the major function of pericarp cells was synthesis and temporary storage of starch. Stage 2 was from 8 to 11 DAF; synthesis and amylolysis were simultaneously conducted, but synthesis was in priority. Pericarp cells played roles both in storing starch and transporting the disintegrated material of starch at this stage. Stage 3 was from 11 DAF to starch disappearance; starch synthesis stopped and amylolysis was in priority at this stage. The major function of pericarp cells was transportation of the disintegrated material of starch to endosperm. At final stage, the pericarp cells became dead cells and form protection tissues for the development of wheat kernel.
Abbreviations
- DAF:
-
day after flowering
- DBF:
-
days before flowering
- HR:
-
hypersensitivity reaction
- NSGA:
-
numbers of starch granules in one amyloplast
- NAC:
-
numbers of amyloplasts in one cell
- PCD:
-
programmed cell death
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated fluorescein deoxyuridine triphosphate nick-end labeling
References
Ameisen JC (2002) On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. J Cell Death Differ 9:367–393. doi:10.1038/sj.cdd.4400950
Chevalier P, Lingle SE (1983) Sugar metabolism in developing kernels of wheat and barley. J Crop Sci 23:272–277
Earp CF, McDonough CM, Rooney LW (2004) Microscopy of pericarp development in the caryopsis of Sorghum bicolor (L.) Moench. J Cereal Sci 39:21–27. doi:10.1016/S0733-5210(03)00060-2
Gaffal KP, Friedrichs GJ, El-Gammal S (2007) Ultrastructural evidence for a dual function of the phloem and programmed cell death in the floral nectary of Digitalis purpurea. J Ann Bot (Lond) 99:593–607. doi:10.1093/aob/mcm002
Groover A, DeWitt N, Heidel A, Jones A (1997) Programmed cell death of plant tracheary elements differentiating in vitro. J Protoplasma 196:197–211. doi:10.1007/BF01279568
Gu Y-J, Wang Z, Chen J, Zhao G-Y (2002) The structure and function of pericarp in rice. J Acta Agronomica Sin 28(4):439–444
Hu S-Y (1964) Study on the dynamic state of starch accumulation in the development of wheat caryopsis. J Acta Bot Sin 12(2):139–148
Ishida-Yamamoto A, Yamauchi T, Tanaka H, Nakane H, Takahashi H, Iizuka H (1999) Electron microscopic in situ DNA nick end-labeling in combination with immunoelectron microscopy. J Histochem Cytochem 47:711–717
Jones AM (2001) Programmed cell death in development and defense. Plant Physiol 125:94–97. doi:10.1104/pp. 125.1.94
Lan S-Y, Zhong F-X, Yang Z-M, Jin D-M, Xu Z-X (2004) The starchy endosperm denucleation by a process of programmed cell death during rice grain development. J Acta Biologiae Exp Sin 37(1):34–44
Mea MD, Serafini-Fracassini D, Duca SD (2007) Programmed cell death: similarities and differences in animals and plants. A flower paradigm. J Amino Acids 33:395–404. doi:10.1007/s00726-007-0530-3
Nakamura T, Vrinten P, Hayakawa K, Ikeda J (1998) Characterization of a granule-bound starch synthase isoform found in the pericarp of wheat. J Plant Physiol 118:451–459. doi:10.1104/pp. 118.2.451
Otsuki Y, Li ZL, Shibata MA (2003) Apoptotic detection methods: from morphology to gene. J Prog Histochem Cyto 38:275–339. doi:10.1016/S0079-6336(03)80002-5
Pennell RI, Chris L (1997) Programmed cell death in plants. J Plant Cell 9:1157–1168. doi:10.1105/tpc.9.7.1157
Rolletschek H, Weschke W, Weber H, Wobus U, Borisjuk L (2004) Energy state and its control on seed development: starch accumulation is associated with high ATP and steep oxygen gradients within barley granules. J Exp Bot 55(401):1351–1359. doi:10.1093/jxb/erh130
Van Doorn WG (2005) Plant programmed cell death and the point of no return. J Trends Plant Sci 10:478–483. doi:10.1016/j.tplants.2005.08.003
Wang Y-Q, Cui K-M (1998) Programmed cell death during the vessel element differentiation of the secondary xylem in Eucommia ulmoides shoots. J Acta Bot Sin 40:1102–1107
Wang L-K, Zhou Z-Q, Song X-F, Li J-W, Deng X-Y, Mei F-Z (2008) Evidence of ceased programmed cell death in metaphloem sieve elements in the development caryopsis of Triticum aestivum L. Protoplasma 234:87–96. doi:10.1007/s00709-008-0023-6
Zhou Z-Q (2000) Study on the physiological characteristics of resource, sink and flow and the microstructural or ultrastructural changes of kernels during the process of kernel development in wheat (Triticum aestivum L.) cultivars. Ph.D. dissertation of Huazhong Agricultural University
Zhou Z-Q, Zhu X-T, Wang W-J, Lan S-Y (2001) Observation on the amyloplasts in endosperm of wheat varieties with different kernel types by scanning electron microscope. J Chin Electron Microsc Soc 20(3):178–184
Acknowledgements
Our deepest gratitude goes to first and foremost the National Nature Science Foundation of China (30571101) for their financial support. We also owe our sincere thanks to Mrs. Cui Wang for her revising on the earlier draft.
Conflict of interest
We have a financial relationship with the organization that sponsored the research. We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhuqing Zhou and Likai Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, Z., Wang, L., Li, J. et al. Study on programmed cell death and dynamic changes of starch accumulation in pericarp cells of Triticum aestivum L.*. Protoplasma 236, 49–58 (2009). https://doi.org/10.1007/s00709-009-0046-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-009-0046-7