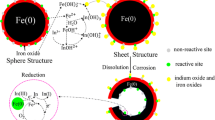
Abstract
Metallic iron (Fe0) is a reactive material that is widely used for industrial water treatment. The course of the metal ion removal process using Fe0 (iron powder) was monitored electrochemically (differential pulse polarography). As probe species, Zn2+, Pb2+, and Cd2+ were selected for their different (1) adsorptive affinity to iron corrosion products (FeCPs), (2) redox properties, (3) precipitation ability at various pH. Batch experiments were carried out with binary (Zn2+/ Pb2+ and Zn2+/ Cd2+) and ternary (Zn2+/Cd2+/Pb2+) systems to reveal the mutual interference of these cations. Detailed time monitoring of iron aging for up to 14 days as well as concentration decay of individual removed cations represent important data for mechanistic discussions. The aqueous concentration of Fe2+ was also monitored. FeCPs were characterized using X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy (SEM). Results showed that the presence of Pb2+ delays the Zn2+ removal whereas the presence of Cd2+ in solution accelerates its removal. The removal of Pb2+ by FeCPs was not affected by the presence of Zn2+ and Cd2+, moreover, the Pb2+ inhibited the effect of Cd2+ on the removal of Zn2+. XPS has proven existence of Fe2O3 and hydrated Fe oxidic phase, whilst the SEM showed that the original Fe grains were partly dissolved into buffered ambient under formation of fine particles of FeCPs. Results confirm that reductive transformation of any contaminant in a Fe0/H2O system is the consequence and not the cause of iron corrosion.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of heavy metals in wastewater generated from industrial activities such as mining and smelting and battery manufacturing [1] is one of the most serious environmental problems worldwide. Heavy metals persist in the environment and pose a high risk to ecosystems and human health [2]. There is a crucial need for low-cost, effective approached to remove heavy metals from wastewater. Metallic iron materials—Fe0 (or zero-valent iron—ZVI) has been used for this purpose for quite a long time [3,4,5] however, on a pure pragmatic approach [6].
Metallic iron materials (Fe0) have been broadly utilized in the water remediation industry for the past three decades [5, 7,8,9,10,11,12,13,14]. The interactions of Fe0 with water (Fe0/H2O systems) involves the in-situ generation of solid iron corrosion products (FeCPs), which in turn act as contaminant scavengers [6, 15,16,17,18,19,20]. The efficiency of freshly generated in-situ FeCPs for the removal of dyes [18, 21], fluoride ion [20, 22], and Zn2+ [19] has been investigated in batch systems. In addition, Touomo-Wouafo et al. [19] monitored the time-dependent changes in a Fe0/Zn2+/H2O system to better characterize the remediation process. The main outcome of these studies is that Fe0 is not the agent reducing directly the cations of contaminants under environmental conditions.
This conclusion is present in the literature since 2007 [23, 24] but has been largely ignored by active researchers [6, 25, 26]. A recent overview article by Hu et al. [27], summarizes the complexity of the Fe0/H2O system and recalls that contaminant removal is an inherent property of aqueous iron corrosion. This implies that upon proper design each polluted water can be treated using Fe0. For this reason, to gain more information about the efficiency of each system, either several Fe0 materials are used for a single-contaminant or multi-contaminant systems are used for the same material, The approach used herein is the latter. Really polluted wastewaters contain more than one heavy metal. Therefore investigation of the effectiveness of FeCPs for the removal of multiple competing cations is very important [28,29,30].
In scientific literature considering Fe0 as a stand-alone reducing agent, the diversity of used experimental conditions renders the comparison of achieved results almost impossible (Table 1). It is evident from the Table 1 that the majority of selected studies deals with defined oxides like hematite [31], goethite [32, 33], or hydrous iron oxides [34]. Those systems are limited by the fact that there is not a continuous production of in-situ FeCPs, the time development is not followed and, therefore, the interpretation of metals removal is limited to adsorption at equilibrium. On the other hand, using Fe0, the removal of contaminants is governed by a synergy of freshly and continuously in situ-generated FeCPs leading to adsorption and precipitation/co-precipitation. In this case, however, detailed time dependence is necessary to understand more deeply the remediation mechanisms.
Recently, Vollprecht et al. [14] investigated the removal of multiple metal cations from wastewater using Fe0. In their batch experiments, substantial differences in reactivity towards FeCPs were observed. These differences influencing effectivity of metals removal are caused among others by different conditions (e.g. pH) and also by the presence of both anions and cations in the tested water. However, in their paper, there is not specified the Fe0 material (size, surface), its aging is not considered and some important metal cations are missing.
For the present work, fresh iron powder -200 mesh (74 µm) with defined size and surface [19] was used as the remediation agent and Zn2+, Cd2+ and Pb2+ ions have been chosen as contaminants because they are commonly present in wastewater and because they have different (1) adsorptive affinity to FeCPs, (2) redox properties, (3) precipitation ability at various pH. The study is performed with binary (Zn2+/Pb2+ and Zn2+/Cd2+) and ternary (Zn2+/Cd2+/Pb2+) mixtures of various proportions of ions, in batch systems at pH 5.5 which is the optimum pH for the removal of Zn2+ obtained from our previous study [19]. The concentration changes of aqueous Fe2+, Zn2+, Cd2+, and Pb2+ are monitored within the time span from minutes to 14 days, using differential pulse polarography (DPP). The DPP technique was chosen because it was possible to follow simultaneously the current (concentration) of several metal ions in aqueous solution. The X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy (SEM) are used for the characterization of FeCPS after the remediation process with the aim to intercept and describe the form of removed metals.
The purpose of the present study is to investigate (1) the efficiency of differently aged FeCPs for the removal of multiple competing heavy metals; (2) the time development of all processes; (3) the iron powder after the process with adsorbed/precipitated/reduced products; (4) the mutual influence of the present metal cations being simultaneously in the mixture because their interference is very probable due to their different adsorptive affinity to FeCPs, and different redox properties. All experiments should contribute to the explanation of all observed effects (for example the reason and relevance of the "induction period" at the beginning of the ZVI aging) and thus to detailed understanding of the mechanism of removal of selected heavy metals.
Results and discussion
Monitoring of Fe0 pre-corrosion
When 0.8 g of Fe0 was added in 0.1 mol/dm3 acetate buffer solution pH 5.5, in the presence of air oxygen (in the absence of Zn2+, Cd2+, and Pb2+), typically only Fe2+ was identified at − 1.4 V vs. SCE and its concentration–time profile is depicted in Fig. 1.
It can be observed at the Fig. 1a that the concentration of Fe2+ gradually increases then reaches a maximum after approx. 3 h, then remains unchanged during few hours (where a plateau is formed). Thereafter, Fe2+ starts to decreases progressively with time. An increase of pH up to 6.7 was noticed during the first 3 h (although the experiment is performed in buffered solution, initial pH 5.5), afterwards the pH diminishes progressively and returns back to its initial value. Similar observation has been reported by Touomo-Wouafo et al. [19]. The authors explained this effect by the corrosion of iron by water with dissolved oxygen leading to the release of Fe2+ and OH− in solution which will progressively transformed to FeCPs [19].
The detailed view of this experiment reveals an "induction period" of the corrosion which occurs during the first 30 min of experiment (Fig. 1b) which could be attributed to the activated time of iron. In that period Fe2+ is not released and its concentration in solution is zero. The same kind of "induction period" was reported in our previous study [19]. In that study, only 0.1 g of Fe0 was used, the "induction period" lasts the first 1–2 h, the pH increased up to 6.0 and then returned back to 5.5. The present investigation points out that, the "induction period" depends on the amount of Fe0 used: higher amount of Fe0 employed in the experiment of the same volume causes shorter "induction period" and higher pH during the maximal concentration of Fe2+. Most probably, these effects are connected with the reaction of Fe0 with dissolved oxygen in the first part of the corrosion process. The detailed interpretation of these induction periods is the subject of our further studies. In the past, analogous kind of induction period but under different conditions was observed (without explanation) by Lavine et al. [35], who "pre-treated" Fe0 by washing it with a variety of aqueous solutions, including HCl.
The preliminary experiments reveal that 0.8 g of Fe0 aged 3 h was suitable, therefore, for all investigations in this study, 0.8 g of Fe0 aged 3 h (time corresponding to maximum of Fe2+ in solution) in 0.1 mol/dm3 acetate buffer solution pH 5.5 was used.
Effect of competitive cations on the removal of Zn2+
Binary systems
For this study, each solution contained only two target metallic cations (besides in-situ Fe2+). The concentration of Zn2+ is set to 0.3 mmol/dm3 whilst the concentration of the competitive cation corresponds to 0.15 mmol/dm3, 0.3 mmol/dm3, and 0.6 mmol/dm3 respectively for the ratio Zn2+:M2+ (M represents Pb or Cd), 1:0.5, 1:1, and 1:2.
Effect of Pb 2+ on the removal of Zn 2+
The time-dependence of Zn2+ decay after addition of 1 cm3 of binary metal mixed stock solutions (Zn2+/Pb2+ of various ratio) in 199 cm3 of acetate buffer containing 0.8 g of Fe0 prior aged 3 h is presented in Fig. 2.
From Fig. 2a, it is evident that in the absence of Pb2+ (ratio Zn2+:Pb2+, 1:0), the majority of Zn2+ ion is removed within the first hours and completely after 3 days, whereas in the increasing presence of Pb2+ the remediation occurs slower and slower and, finally, 97% of Zn2+ is removed after 14 days. When the experiment is evaluated at short reaction time (Fig. 2b), it is obvious that the efficiency of Zn2+ removal using aged Fe0 decreases by increasing concentration of Pb2+ ion in solution. Accordingly, only 88.6, 83, and 68.6% of Zn2+ removal is obtained respectively with 0.15, 0.3, and 0.6 mmol/dm3 of Pb2+ concentration in solution after 3 days. Based on these results, it can be concluded that, the Pb2+ ion delays substantially removal of Zn2+ from the solution and the final percentage of remaining Zn2+ is higher.
Besides the above results, monitoring of the Pb2+ concentration shows that Pb2+ ions completely disappear shortly after their addition to the solution. Hence, interaction of Pb2+ with the aged iron (or with the present solution) is fast regardless to the ratio Zn2+:Pb2+. The same result was obtained in the absence of Zn2+ in solution. Thus, Zn2+ ion does not influence the removal of Pb2+ using ZVI aged 3 h. Similar finding has been reported by Gadde and Laitinen [34], in the binary system Pb2+/Zn2+ using hydrous iron and manganese oxides. The authors mentioned that lead is adsorbed more strongly than any other metal ion studied. However, the role of pH should be also taken into account in the evaluation and interpretation of the data from the Fig. 2a, b. Since the pH of the solution reaches 6.7 after 3 h of aging of iron in the absence of heavy metals, the precipitation of their hydroxides could likewise contribute to the observed rapid removal of Pb2+. This result is consistent with those of Khorshidi and Azadmehr [31]. Using hematite as adsorbent, the authors underlined that at pH more than 6, lead is entirely precipitated into Pb(OH)2. However, those authors did not prove the presence of Pb(OH)2.
All these observations highlight the ion-selective nature of FeCPs including the present solution) towards the present target heavy metals. As a result, after several hours the aged FeCPs mixture reacts preferentially with Pb2+ (or with its precipitate) than with Zn2+. It may cause blocking of the reactive sites at the surface of the aged iron powder and the removal of the remaining Zn2+ occurs substantially slower. This outcome is consistent with Pauling electronegativity values of each investigated cation which are 2.33 and 1.65, respectively, for Pb2+ and Zn2+ [36], hence higher electronegativity causes stronger interaction with FeCPs. The obtained result also matches with the hardness index of target cations, 0.131 and 0.115 for Pb2+ and Zn2+, respectively, and the values of logarithm of the first hydrolysis constant of cations which are − 7.6 and − 9, respectively, for Pb2+ and Zn2+ [36].
In addition of these results, it was also observed a progressive vanishing of Fe2+ concentration in solution with time. This decrease underlines a continuous production of FeCPs and the formation of an oxide layer on Fe0 (“passivation”) [37, 38]. This outcome was expected since the consumption of Fe2+ by dissolved oxygen in water or OH− leads to Fe3+ and Fe(OH)2. Fe3+ thereafter reacts with OH− in solution to form Fe(OH)3. Both Fe(OH)2 and Fe(OH)3 are later transformed to FeOOH, Fe2O3 and Fe3O4 [19].
Effect of Cd 2+ on the removal of Zn 2+
In contrast to the results of above section, the presence of Cd2+ promotes the removal of Zn2+ in solution. As a result, in the binary Zn2+/Cd2+ system the remediation of Zn2+ by aged Fe0 is almost completed after 0.2 days which corresponds to approx. 4 h while in the absence of Cd2+ such result is achieved after 3 days (Fig. 3c). The facilitation of Zn2+ removal by Cd2+ requires detailed studies and will be the subject of our further study.
Time-dependence of the decrease of the a Zn2+, b Cd2+ ions during remediation using ZVI at various Zn2+:Cd2+ ratio. (0.8 g of Fe0 prior aged 3 h in 199 cm3 of 0.1 mol/dm3 acetate buffer pH 5.5, in the presence of air oxygen). c Comparison of the time-dependence of the concentration decay of Zn2+ and Cd2+, both 0.3 mmol/dm3 at the same conditions
The time-dependence of the decrease of the Cd2+ ion concentration (in the absence or in the presence of Zn2+) depicted in Fig. 3b displays a slight decrease of Cd2+ ion concentration during the first day then reaches a pseudo-equilibrium in solution. After 6 days, a new decrease of Cd2+ ion concentration is observed. This behavior might be due to the fact that, the FeCPs formed at the equilibrium (after 3 h of aging) are not suitable for the removal of Cd2+ but appropriate for Zn2+ removal (Fig. 3c). On the other hand, after 6 days, the FeCPs are convenient for the trapping of Cd2+.
It is also evident from the Fig. 3c that the order of effectiveness of metal ions removal by aged Fe0 is as follows: Zn2+ > Cd2+. Like in the previous case, this is consistent with the hardness index of cations, 0.115 and 0.081 for Zn2+ and Cd2+, respectively, and the values of logarithm of the first hydrolysis constant of cations which are -9 and -10.1, respectively, for Zn2+ and Cd2+ [36].
When considering the inverse case (how Zn2+ ions affect the removal of Cd2+), the percentage of removal of Cd2+ obtained after 14 days of reaction is moderately dependent on the Zn2+:Cd2+ ratio in a remarkable manner: Subsequently, 65, 67, 63, and 75% of Cd2+ are removed respectively in the ratio Zn2+:Cd2+, 0:1, 1:0.5, 1:1, 1:2. It is noticed that the Zn2+ slightly diminishes the percentage of the Cd2+removal. Analogous result has been reported by Boparai et al. [39]. In their work, the authors attributed this effect to the chemical similarities between Cd2+ and Zn2+, consequently they may strongly compete for the same adsorption sites. However, in their study, they have not considered the effect of Cd2+ on the removal of Zn2+.
Ternary systems
In the present study, various ratios Zn2+:Cd2+:Pb2+ were investigated namely: 1:0.5:0.5, 1:0.5:1, 1:1:0.5, 1:1:1, 1:1:2, 1:2:1, 1:2:2, where the concentration of Zn2+ was constant and set to 0.3 mmol/dm3 whilst the concentration of competitive cations varied from 0.15 to 0.6 mmol/dm3.
The Fig. 4 shows the concentration evolution of Zn2+ with time during its remediation using Fe0 (aged 3 h in 199 cm3 of 0.1 mol/dm3 acetate buffer pH 5.5) at various ratio Zn2+:Cd2+:Pb2+ (for clarity all eight dependences were divided into two graphs). Although the systems were analyzed in detail for 14 days, the presented dependences are described for the contact time 3.5 days when the data are the most informative.
Time-dependence of the decrease of the Zn2+ ion concentration during remediation using Fe0 (0.8 g of Fe0 prior aged 3 h in 199 cm3 of 0.1 mol/dm3 acetate buffer pH 5.5, in the presence of air oxygen) of various ratio Zn2+:Cd2+:Pb2+ from 0 to 3.5 days (for clarity the set of eight dependences was divided into two graphs)
Generally, it appeared that the percentage of removal of Zn2+ after 14 days is more than 98% from all data of Fig. 4, except in the ratio 1:0:0, where Zn2+ is completely removed after 3 days, hence it is evident that the presence of both competitive cations delays the removal of Zn2+ in solution. This is mainly owing to the already mentioned slowing effect of Pb2+ upon the removal of Zn2+, which inhibited the slightly accelerating effect of Cd2+.
It was also observed a rapid disappearing of Pb2+ at the initial stage of reaction as it was the case in the binary system Zn2+/Pb2+. Accordingly, the presence of Cd2+ does not have any influence on the removal of Pb2+. Moreover, pseudo-equilibrium in the removal of Cd2+ appeared likewise in the ternary system with a short duration in comparison of binary system.
The percentage of removal of Zn2+ ions after approx. 3 days reaction time (taken from the data of Fig. 4) and those of Cd2+ ions at 14 days reaction time are presented in the Table 2. It follows from the data of Table 2 that after 3 days more than 90% removal of Zn2+ is obtained when concentration of Cd2+ ≥ Pb2+. These results confirmed our earlier observation obtained in the binary systems where it was highlighted that the Cd2+ accelerates whilst the Pb2+ reduces the Zn2+ removal percentage (cf. Fig. 2b). It is also evident that for the equal concentration of Cd2+ and Pb2+, a "neutralization" of rather opposing effects of Cd2+ and Pb2+ on the Zn2+removal occurs.
A close comparison of percentages of removal of Cd2+ obtained in Zn2+/Cd2+ system with those presented in Table 2 reveals that any addition of Pb2+ in solution decreases the percentage of removal of Cd2+ which is in agreement with the early observation of Gadde and Laitinen [34].
According to the above-mentioned results, the following order of decreasing efficiency of metal cations removal by aged Fe0 can be deduced: Pb2+ > Zn2+ > Cd2+. This is analogous to the decrease order of selectivity of FeCPs towards the investigated metallic cations reported by several authors [32,33,34, 40], using goethite, iron nanoparticles, hydrous iron, and manganese oxides.
Though no explanation of such order was given by some of those authors, the obtained order correlates well with the hardness index of investigated cations which are respectively for Pb2+, Zn2+, and Cd2+ 0.131, 0.115, and 0.081 as reported Kinraide and Yermiyahu [36] and also with the values of logarithm of first hydrolysis constant of each cations, − 7.6, − 9, and − 10.1, respectively, for Pb2+, Zn2+, and Cd2+ [36]. In any case, one has to keep in mind that it is hardly possible to find one single well-fitting correlation because the remediation mechanism of these three cations is based on different processes: acidobasic at higher pH, (co-)precipitation, adsorption on FeCPs and/or redox. The elucidation of more detailed mechanism distinguishing role of various conditions is in our next plans.
Characterization of the solids by SEM and XPS—mechanistic considerations
The surface of the iron powder exposed to buffered water ambient before and after addition of Zn2+, Cd2+, and Pb2+ ions was characterized by X-ray photoelectron spectroscopy (XPS) and its morphology by scanning electron microscopy (SEM).
The aging of Fe0 powder in the buffer is naturally accompanied by extensive corrosion of Fe0 powder which changes substantially the surface morphology. As follows from SEM results (Fig. 5) the originally smooth grains of the sample 1 (bare Fe powder taken directly from the original flask) after 10 days in the buffer (sample 3), they are partly dissolved and simultaneously/consequently covered by a thick layer of FeCPs fine crystals with high specific surface. Aging is, therefore, a multistep process which manifests itself by the observed "induction period" (cf. Fig. 1b) and by unusual shape of the Fe2+ time dependence (cf. Fig. 1a), and causes curious beginning of all ions removal (cf. Figs. 2, 3).
The survey photoelectron spectra of the samples 4 (top) and 7 (bottom) sputtered by Ar+ ions (t = 5 min) measured at low resolution are depicted in Fig. 6. The spectra are normalized relative to the unit height of O 1 s line for comparison. The low energy regions of spectra (< 520 eV, in red) are expanded to make more visible low intensive photoelectron lines. The survey spectra of the samples 1, 2, 3, and 6 are qualitatively the same as the spectrum of the sample 4, spectrum of the sample 5 qualitatively corresponds to that of the sample 7. The same results were obtained from the spectra of the non-sputtered samples.
The assigned photoelectron and Auger lines detected in investigated samples and presented in the Table 3 show that all samples are composed from Fe, C, and O. Presence of Pb was detected in the samples 5 and 7. On the other hand, the presence of Si in the sample 1, Cd in the sample 6, and Zn in the samples 4 and 7 was not observed on this survey spectrum because their concentration was close to the detection limit. Confirmation of these elements was done using measurements at high resolution (Fig. 7) (Si found in the sample 1 can be due to the presence of trace of Si in the original container of Fe0 powder). Results of quantitative XPS analysis of the samples 1–7 are summarized in Table 4. Concentrations of Fe, C, O, Cd, Pb, and Zn are expressed in atomic %, calculated using Eq. (1). Intensities of photoelectron spectra were calibrated using Wagner sensitivity factors [41].
To discuss the effect of exposition of the sample to buffered water ambient the photoelectron Fe 2p3/2, C 1s, and O 1s spectra of the samples 1 and 3 measured in high resolution before (blue color) and after Ar+ sputtering for 7 min (red color) are compared in Fig. 8.
a Comparison of the Fe 2p3/2 O 1 s and C 1 s spectra of the samples 1 and 3. Blue color: before Ar+ sputtering, red color: after Ar+ sputtering. b The Fe 2p3/2, O 1 s and C 1 s photoelectron spectra before (blue) and after (red) Ar+ sputtering for 7 min (sample 3) and 5 min (samples 4–7). Intensities of the photoelectron spectra are normalized relative to the intensity of Fe 3p spectrum
The Fe 2p3/2 spectra (line I) at binding energy Eb ~ 711.4 eV measured before sputtering correspond to the oxide layer (Fe2O3) on the surfaces of samples 1 and 3, which can be in the case of the sample 1 almost completely eliminated by sputtering by Ar+ ions for 7 min. Asymetric line (II) at Eb = 707.3 eV observed in the sputtered sample 1 belongs to metallic Fe, its asymmetry is affected by presence of very small amounts of residual Fe oxide (cf. intensity of O 1 s line after sputtering).
Much weaker line II is present in the Fe 3p3/2 spectrum of sputtered sample 3 (cf. low energy shoulder in the spectrum) and line I is shifted to lower Eb. These effects reflect much thicker oxide layer of the sample 3 due to long aging of the sample 3 in the buffer. While the oxide layer can be almost quantitatively eliminated from the sample 1, rather thick oxidic layer remained on the sample 3 even after 7 min of sputtering. Obtained findings are in line with observed intensity changes of O 1 s photoelectron spectra (Fig. 8a) and results of quantitative analysis (cf. Table 4). Sputtering further reduced surface hydrocarbon contamination of the samples.
Summary of the photoelectron Fe 2p3/2,1/2, O 1 s, and C 1 s spectra of the samples 3—7 measured at high resolution is depicted in Fig. 8b. To minimize erosion of the samples the sputtering time was reduced to 5 min.
Non-sputtered samples
The samples 4–6 contain various amounts of products of surface corrosion (Fe oxide phase) and contamination (cf. Table 4 above, Fig. 8b, blue lines). The surface Fe oxidic phase is hydroxylated (cf. broadening of O 1 s spectra in the region of high Eb, which is typical for the presence of –OH groups). This Fe oxide phase could be ascribed to FeOOH resulting from surface hydroxylation and subsequent dehydration of the exposed iron in aqueous solution as reported Xi et al. [42].
The line shapes of the photoelectron spectra of the sample 5 are deformed by differential charging. This sample is covered by the phase which is in poor electrical contact with its surface, which induces differential charging, which disables discussion of the line shapes of photoelectron spectra in this case. On non-sputtered samples, Zn (sample 4), Pb (sample 5), and Cd (sample 6) have been observed (Fig. 9a). Hydrocarbon contamination present on the surfaces is composed mostly from aliphatic hydrocabons (C 1 s line at ~ 285 eV, Fig. 8b).
a Photoelectron spectra of non-sputtered sample 4 (Zn 2p3/2), 5 (Pb 4f7/2,5/2), and 6 (Cd 3d5/2,3/2). (red doublet in Pb 4f spectrum: lines shifted by differential charging); b measured and simulated Pb 4f7/2,5/2 spectra of the sample 5 before Ar+ sputtering (top), and samples 5, 7 after Ar+ ion sputtering. Blue doublet I, I*: Pb0, black doublet II, II*: PbO, red doublet III, III*: spectrum of PbO affected by differential charging
Sputtered samples
Rather similar effect of sputtering is observed for the samples 4–7. Much thicker oxide layer remained on the sample surfaces after their short (5 min) time sputtering (cf. absence of low energy shoulder in the Fe 2p3/2 spectrum of the samples 4–7 (Fig. 8b). However, the high energy asymmetry of the O 1 s spectra is reduced, i.e. only small concentration of hydroxylated Fe oxide phase remained after sputtering (Fig. 8b). Hydrocarbon contamination is also reduced (C 1 s spectra in Fig. 8b). No differential charging is observed in the sputtered sample 5.
In agreement with the Table 4, where data for all elements (except Fe) are mostly lower after sputtering, we can conclude that a substantial part of the precipitated phase on the surfaces (together with the adsorbates) of the samples 4–7 was eliminated by sputtering. Thus, Pb is adsorbed on the samples 5, 7, Cd is adsorbed on the sample 6, and Zn on the sample 7 (pertinent spectra cf. Fig. 9a). However, the extent of erosion of the oxidic coating of Fe grains by sputtering is not clear.
The Pb 4f7/2,5/2 photoelectron spectra observed in the samples 5 and 7 are depicted in Fig. 9b. The spectrum was simulated by two doublets (sample 5) and three doublets (sample 7). The doublet I is assigned to Pb0, doublet II to PbO. This result is consistent with those of Xi et al. [42], where their XPS analysis has confirmed that immobilized Pb was present in its zero and bivalent forms. Moreover, the authors proposed that using nano-zero-valent iron, partial reduction of Pb2+ takes place following by its precipitation as PbO.
The XPS study points to the contribution of adsorption in the mechanism of removal of heavy metals using aged Fe0. Furthermore, since it was found lead in the oxidation number 0 (Pb0) we can conclude that partial reduction of Pb2+ by remaining Fe0 contributes in the removal of Pb2+. This mechanism is in straight line with the mechanism reported by Li and Zhang [43], Xi et al. [42]. Moreover, precipitation of lead as Pb(OH)2 (or PbO after drying) also takes place as earlier reported [42].
In the case of Zn2+ and Cd2+, based on the results of characterization, we can suggest that the removal of both cations depends on the affinity of the respective cation towards FeCPs (adsorption onto FeCPs) which could be according to the present study Fe2O3 and FeOOH. Nevertheless, co-precipitation should be the other important reaction pathway, as earlier mentioned Statham et al. [13], Noubactep [23, 44]. It is crucial to point out that the whole discussion has ignored reductive processes but has most rationally discussed the process of Cd2+, Pb2+ and Zn2+ removal in Fe0/H2O systems. Thus, reductive transformation, when they occur are mediated by iron corrosion products, mainly Fe2+ and H2 species [6, 27, 30].
Conclusions
This study assessed the efficiency of aged Fe0 powder for the removal of Zn2+, Cd2+, and Pb2+ in binary and ternary systems by monitoring their removal from aqueous solution under various experimental conditions. Results demonstrated the existence of an "induction period" after immersion of Fe0 powder in buffered solutions. The duration of this lag time depends on the used Fe0 amount. The lag time corresponds to the time necessary for generation of sufficient amount of solid iron corrosion products (FeCPs) for cations removal by adsorption and co-precipitation. SEM results confirmed the formation of high specific surface FeCPs.
The attention was aimed to the mutual interference of multiple cations simultaneously present in the sample during their remediation. Rapid removal of Pb2+ was observed every time at the initial stage of reaction. In the binary system Pb2+/Zn2+ the Pb2+ ion delays substantially removal of Zn2+ whereas Zn2+ ion does not influence the removal of Pb2+. In the system Cd2+/Zn2+ the presence of Cd2+ promotes the removal of Zn2+ in solution while the Zn2+ ions slightly diminish the percentage of the Cd2+ removal. In case of Cd2+/Pb2+ mixture, any addition of Pb2+ in solution decreases the percentage of removal of Cd2+ but the presence of Cd2+ does not have any influence on the removal of Pb2+. In the ternary system the accelerating effect of Cd2+ upon the removal of Zn2+ is competitively inhibited by the presence of Pb2+.
The following order of decreasing efficiency of removal of metal cations by aged Fe0 was found: Pb2+ > Zn2+ > Cd2+. This order positively correlates with the hardness index of investigated cations which are respectively for Pb2+, Zn2+, and Cd2+ 0.131, 0.115, and 0.081 and also with the values of logarithm of their first hydrolysis constants, − 7.6, − 9, and − 10.1, respectively.
XPS analysis has proven the existence of Fe2O3 and FeOOH phases as iron corrosion products. Furthermore, XPS analysis of sputtered and non-sputtered samples reveals that the investigated heavy metals were adsorbed onto FeCPs. In the case of multiple cations present simultaneously in the tested solution, they may strongly compete for the same adsorption sites. Their mutual interference, however, reflects not only different adsorptivity, but also different reaction mechanisms of remediation of Pb2+, Zn2+, and Cd2+ which involves (co)precipitation depending on pH and redox processes where also the dissolved oxygen plays a role.
Experimental
Chemicals
The used chemicals: cadmium acetate dihydrate (Cd(CH3COO)2∙2H2O) and lead acetate trihydrate (Pb(CH3COO)2∙3H2O) were supplied by Aldrich, whilst zinc acetate dihydrate (Zn(CH3COO)2∙2H2O), acetic acid sodium salt trihydrate (CH3COONa∙3H2O), and glacial acetic acid (CH3COOH) were of analytical grade. Concentration of acetate buffer pH 5.5 was 0.1 mol/dm3. Ethanol and acetone of analytical grade were furnished by Lachner Company. Various stock solutions of heavy metals in single (Zn2+, Cd2+, Pb2+), binary (Zn2+/Pb2+ and Zn2+/Cd2+), and ternary (Zn2+/Cd2+/Pb2+) systems were prepared by dissolving the appropriate amount of weighed individual metallic salts in 0.1 mol/dm3 acetate buffer pH 5.5. In the single system, the concentration of stock solution was 0.06 mol/dm3 whereas in the stock solutions of binary as well as in ternary systems the concentration of Zn2+ remains unchanged (0.06 mol/dm3) only the concentrations of Cd2+ and Pb2+ vary from 0.03 to 0.12 mol/dm3. The working solution of Zn2+ ion was 0.3 mmol/dm3 whereas the concentration of Cd2+ and Pb2+ were varying from 0.15 to 0.6 mmol/dm3 depending on the investigated ratios.
Materials
The Fe0 powder utilized in the present study (99.8% Fe0, − 200 mesh, 74 µm, Alfa Aesar) with a specific surface area equal to 13.4 m2 g−1, was identical to that used in our previous study and was employed without any further pre-treatment [19]. Standard filter papers of 55 mm supplied by Fisher scientific and a vacuum pump of 7. 0 mbar were used to dry selected samples for XPS and SEM analysis.
Experimental procedure
All experiments regarding the pre-corrosion and corrosion of iron powder as well as heavy metals ions removal were carried out in duplicates at room temperature in open flasks of 500 cm3 in presence of air oxygen (oxic conditions).
Pre-corrosion
The procedure of pre-corrosion of Fe0 powder as well as that of concentration determination of the Fe2+ ion during the pre-corrosion time was similar to that realized in our previous study [19]. Indeed, 0.8 g of Fe0 powder was pre-equilibrated in 199 cm3 of 0.1 mol/dm3 acetate buffer at pH 5.5 for 0–3 days to generate in-situ FeCPs. Throughout the pre-equilibrated period, the flasks with solution containing Fe0 were gently shaken using a bath shaker with periodic linear motion by 10 cm (speed 10 cm s−1). For concentration determination of Fe2+, amount of 20 cm3 was regularly taken away from the flask at various time intervals and directly subjected to DPP where the concentration of Fe2+ was determined from the cathodic peak at − 1.4 V versus saturated calomel electrode (SCE). After this procedure, the analyzed samples (after separation from mercury by decantation) were always put back to the original flask to keep on the aging.
Effect of competitive cations on the removal of Zn2+
Here, the impacts of Cd2+ and Pb2+ ions on the removal of Zn2+ were investigated in binary and ternary systems. Two binary systems were tested (Zn2+/Pb2+ and Zn2+/Cd2+), where the influence of Pb2+ and Cd2+, respectively, on the Zn2+ removal was studied. In the ternary system, Zn2+/Cd2+/Pb2+, the cumulative effect of Cd2+ and Pb2+ on the removal of Zn2+ was investigated. The time dependences of all effects were followed and evaluated.
The batch experiments for the removal of heavy metal ions were carried out by adding of 1 cm3 of stock solutions of heavy metal ions of various concentration (from 0.03 to 0.12 mol/dm3) to the flasks with 199 cm3 of buffer containing 0.8 g of Fe0 (aged for 3 h). All experiments were then monitored during 14 days. Regularly, at various time intervals, 20 cm3 of the mixture (Fe0-heavy metals ions) was carefully withdrawn and rapidly subjected to DPP analysis without any dilution. The Zn2+, Cd2+, and Pb2+ ions were always identified by DPP at − 1.04, − 0.6, and − 0.43 V vs. SCE, respectively. After analysis the solution was put back to the original flask to keep on the aging.
The percentage of metallic cation removal was calculated by means of equation below where \(C_{{\text{o}}} (M^{2 + } )\) and \(C_{{\text{t}}} (M^{2 + } )\) are the initial concentration of metallic cation (M2+) and that at time t, respectively:
Drying of samples for characterization
Seven samples of the iron powder were selected for characterization: six of them, after 14 days reaction time in the presence of air oxygen with differently aged Fe0 were dried before analysis. As a standard (the seventh sample) fresh Fe0 powder was taken directly from the flask without any treatment. Those samples were denoted and described as follows: (1) Fe0 powder; (2) 0.8 g of Fe0 aged 3 h in 199 cm3 of 0.1 mol/dm3 acetate buffer pH 5.5; (3) 0.8 g of Fe0 aged 10 days in 199 cm3 of 0.1 mol/dm3 acetate buffer pH 5.5; (4) 0.8 g of Fe0 aged 3 h in 199 cm3 of 0.1 mol/dm3 acetate buffer pH 5.5 plus 1 cm3 of stock solution of Zn2+ (0.06 mol/dm3), final concentration of Zn2+ 0.3 mmol/dm3; (5) 0.8 g of Fe0 aged 3 h in 199 cm3 of 0.1 mol/dm3acetate buffer pH 5.5 plus 1 cm3 of stock solution of Pb2+ (0.06 mol/dm3), final concentration of Pb2+ 0.3 mmol/dm3; (6) 0.8 g of Fe0 aged 3 h in 199 cm3 of 0.1 mol/dm3 acetate buffer pH 5.5 plus 1 cm3 of stock solution of Cd2+ (0.06 mol/dm3), final concentration of Cd2+ 0.3 mmol/dm3; (7) 0.8 g of Fe0 aged 3 h in 199 cm3 of 0.1 mol/dm3 acetate buffer pH 5.5 plus 1 cm3 of stock solution of Zn2+:Cd2+:Pb2+ (0.06 mol/dm3), ratio 1:1:1, final concentration of each heavy metal equals to 0.3 mmol/dm3.
Those six samples were filtered under vacuum with standard filter paper size 55 mm, and then washed respectively with ethanol and acetone. The obtained dried powders were carefully withdrawn from the paper and kept in a clear container, ready for XPS and SEM analysis.
Analytical methods
The HI 3220 pH/ORP meter was used for pH determination of the solution.
Differential Pulse Polarography (DPP) was employed for concentration determination of Fe2+ and Zn2+, Cd2+, and Pb2+ ions in solution. Differential pulse polarographic curves were recorded with the 663 VA Stand series 05 connected to a digital computer controlled potentiostat PGSTAT 30 (Autolab-Metrohm). Three electrode system was used with mercury drop working electrode, SCE as reference and Pt wire as auxiliary electrode. DPP parameters: scan rate 10 mV/s, modulation peak amplitude 25 mV, modulation time 50 ms, and drop time 1 s [19].
An Omicron Nanotechnology ESCAProbe P spectrometer (Omicron Nanotechnology GmbH, Taunusstein, DE) was used to measure the photoelectron spectra. XPS analysis was performed at a pressure of ~ 10–8 Pa. The X-ray source was monochromatic at 1486.6 eV. The photoelectron spectra were measured at low resolution (survey spectra in the energy region of 0–1200 eV with a step size of 0.6 eV) and at high resolution in 30 eV scans with a step size of 0.1 eV). The samples were measured before and after sputtering by argon ions (Ar+). The abundance A(x) of the elements in the samples was evaluated in the atomic percents calculated from corrected integral intensities I of the Fe 3p, C 1s, O 1s, Pb 4f, Zn 2p, and Cd 4d spectra:
It can be assumed the presence of the precipitated phase created by dissolution of the powder on the grains surface. To test this possibility the photoelectron spectra were measured as received samples and after their soft argon ion Ar+ sputtering (E = 5 keV, i ~ 5 mA, t = 5 min, 7 min). Possible surface precipitated phase might be reduced by this treatment. Sputtering can erode the samples surface, however. Damped nonlinear least-squares fitting procedure was used to distinguish partially resolved lines in the Pb 4f photoelectron spectra measured in high resolution [45]. Minimal number was used in simulation of the spectra. Assignment of the photoelectron lines was done by comparison of estimated binding energies B.E. (eV) with standard ones [46]. The samples were dried before XPS experiment. The effect of aging of Fe powder in buffered water ambient is investigated first. Then the samples exposed to buffered water ambient which contained Zn2+, Cd2+, and Pb2+ ions are characterized. Metal ions can be present on the Fe powder during aging period (i.e. due to adsorption from water ambient) or during drying of the samples (i.e. as a contamination). We tried to resolve the both of this mechanism by XPS analysis of the samples as received and after cleaning by argon (Ar+) ion sputtering.
For SEM analysis, the S 4800-I scanning electron microscope (Hitachi, Tokyo, Japan) was utilized and acceleration voltage of 5 kV was applied.
References
Gosar M (2004) Geoenviron 51:2097
Xiao R, Wang S, Li R, Wang JJ, Zhang Z (2017) Ecotox Environ Safe 141:17
Oldright GL, Keyes HE, Miller V, Sloan WA (1928) Precipitation of lead and copper from solution on sponge iron. Bulletin 281, Bureau of Mines, p 131
Bojic AL, Bojic D, Andjelkovic T (2009) J Hazard Mater 168:813
Vollprecht D, Plessl K, Neuhold S, Kittinger F, Öfner W, Müller P, Mischitz R, Sedlazeck KP (2020) Processes 8:279
Xiao M, Hu R, Cui X, Gwenzi W, Noubactep C (2020) Processes 8:409
O’Hannesin SF, Gillham RW (1998) Ground Water 36:164
Henderson AD, Demond AH (2011) Environ Eng 137:689
Kishimoto N, Iwano S, Narazaki Y (2011) Water Air Soil Pollut 221:183
Guan X, Sun Y, Qin H, Li J, Lo IMC, He D, Dong H (2015) Water Res 75:224
Noubactep C (2015) Water Res 85:114
Suponik T, Winiarski A, Szade J (2015) Water Air Soil Pollut 226:360
Statham TM, Mumford KA, Stark SC, Gore DB, Stevens GW (2015) Sep Sci Technol 50:2427
Vollprecht D, Krois LM, Sedlazeck KP, Müller P, Mischitz R, Olbrich T, Pomberger R (2018) J Clean Prod 208:1409
Devonshire E (1890) J Frankl Inst 129:449
Lauderdale RA, Emmons AH (1951) J Am Water Works Ass 43:327
Gatcha-Bandjun N, Noubactep C, Loura Mbenguela B (2014) Fresenius Environ Bull 23:2663
Gatcha-Bandjun N, Noubactep C, Loura Mbenguela B (2017) Environ Technol Innov 8:71
Touomo-Wouafo M, Donkeng-Dazie J, Btatkeu-K BD, Tchatchueng JB, Noubactep C, Ludvík J (2018) Chemosphere 209:617
Nde-Tchoupé AI, Nanseu-Njiki CP, Hu R, Nassi A, Noubactep C, Licha T (2019) Chemosphere 219:855
Phukan M, Noubactep C, Licha T (2015) Chem Eng J 259:481
Hildebrant B, Ndé-Tchoupé AI, Lufingo M, Licha T, Noubactep C (2020) Processes 8:265
Noubactep C (2007) Open Environ Sci 1:9
Noubactep C (2008) Environ Technol 29:909
Ghauch A (2015) Freiberg Online Geosci 32:1
Gheju M (2018) Water 10:651
Hu R, Yang H, Tao R, Cui X, Xiao M, Konadu-Amoah B, Cao V, Lufingo M, Soppa-Sangue NP, Ndé-Tchoupé AI, Gatcha-Bandjun N, Sipowo-Tala VR, Gwenzi W, Noubactep C (2020) Water 12:641
Cantrell KJ, Kaplan DI, Wietsma TW (1995) J Hazard Mater 42:201
Qiu SR, Lai HF, Roberson MJ, Hunt ML, Amrhein C, Giancarlo LC, Flynn GW, Yarmoff JA (2000) Langmuir 16:2230
Scott TB, Popescu IC, Crane RA, Noubactep C (2011) J Hazard Mater 186:280
Khorshidi N, Azadmehr AR (2017) Desalin Water Treat 58:106
Christophi CA, Axe L (2000) J Environ Eng 126:66
Forbes EA, Posner AM, Quirk JP (1976) J Soil Sci 27:154
Gadde RR, Laitmen HA (1974) Anal Chem 46:2022
Lavine BK, Auslander G, Ritter J (2001) Microchem J 70:69
Kinraide TB, Yermiyahu U (2007) J Inorg Biochem 101:1201
Nesic S (2007) Corros Sci 49:4308
Lazzari L (2008) General aspects of corrosion. Encyclopedia of hydrocarbons, chapter 9.1, vol V. Istituto Enciclopedia Italiana, Rome
Boparai HK, Joseph M, O’Carroll DM (2013) Environ Sci Pollut Res 20:6210
Pawluk K, Fronczyk J (2015) Pol J Chem Technol 15:7
Wagner CD, Davis LE, Zeller MV, Taylor JA, Raymond RH, Gale LH (1981) Surf Interface Anal 3:211
Xi Y, Mallavarapu M, Naidu R (2010) Mater Res Bull 45:1361
Li XQ, Zhang WX (2007) J Phys Chem C 111:6939
Noubactep C, Btatkeu-K BD, Tchatchueng JB (2011) Chem Eng J 178:78
Kwok RWM (1999) XPSPeak, Version 4.1. Hong Kong, Available online: https://www.phy.cuhk.edu.hk/surface/XPSPeak. Accessed on 21 Sept 2019
Briggs D, Seah MP (1996) Practical surface analysis by Auger and X-ray photoelectron spectroscopy. Wiley, New York
Schultz MF, Benjamin MM, Ferguson JF (1987) Environ Sci Technol 121:863
Benjamin MM (1981) J Coll Inter Sci 79:209
Kinniburgh DG, Jackson ML, Syer JK (1976) Soil Sci Soc Am J 40:796
Acknowledgements
The authors are grateful namely to the institutional support of the J. Heyrovský Institute of Physical Chemistry, Czech Academy of Sciences, RVO 61388955.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Touomo-Wouafo, M., Donkeng-Dazie, J., Jirka, I. et al. Electrochemical monitoring of metal ions removal in Fe0/H2O systems: competitive effects of cations Zn2+, Pb2+, and Cd2+. Monatsh Chem 151, 1511–1523 (2020). https://doi.org/10.1007/s00706-020-02683-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02683-6