Abstract
Isinglass, derived from swim bladders of Caspian Sea fish and consisting predominantly of protein collagen, was found to be an effective, easily accessible heterogeneous biocatalyst for the synthesis of biologically important functionalized spirooxindoles and spiroacenaphthylenes in water. This facile and efficient one-pot, three-component synthesis route involving isatin or acenaphthenequinone, activated methylene reagent, and 1,3-dicarbonyl compounds provides a new strategy giving rise to spiro derivatives in quantitative yields in very short reaction times.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For more than a century, biocatalysts have been used to perform chemical transformations. In recent years, scientists have witnessed dramatic growth of the use of bio-catalysis in the production of fine chemicals, especially for the pharmaceutical industry [1]. Employing this type of catalysis proves efficient and safe and a natural alternative to traditional chemistry. Biocatalysts yield significant advantages in the process of chemical synthesis, particularly now that there is increasing emphasis on ‘green chemistry’ or reducing environmental impact. Biocatalytic processes become an attractive, efficient, and environmentally friendly option for synthetic design [2–4]. Using peptide as a catalyst would allow significant expansion beyond the range of single amino acids yet conserve its advantages as a small molecule catalyst. In this context, we found that Isinglass (IG) as a biopolymer has considerable catalytic efficiency in different organic transformations. Other research studies regarding the use of this catalyst in various organic reactions are ongoing in our research group. IG is derived from the swim bladders of certain tropical fish and consists predominantly of protein collagen, other forms of which are found in skin, tendons, and calcified bone, and which is readily soluble in organic acids. It is a substance that has been used for several 100 years to clarify alcoholic beverages and also, widely used in the pharmaceutical industry and as ingredients or processing aids in food production [5, 6]. IG contains a large amount of protein substances such as alanine, glycine, valine, leucine, isoleucine, proline, phenylalanine, tyrosine, serine, threonine, methionine, arginine, histidine, lysine, aspartic acid, glutamic acid, hydroxyproline, and hydroxylysine. It is a rod-shaped amphoteric molecule of kD molecular weight carrying both negative and positive charges [7]. Consequently it can act as an ideal bifunctional organocatalyst that contains both Lewis base and Lewis acid sites.
The development of multicomponent domino reactions (MDRs) designed to produce elaborate biologically active compounds has become an important area of research in organic, combinatorial, and medicinal chemistry [8]. As one-pot reactions, MCRs satisfy many of the principles of green and sustainable chemistry. Many reactions can be developed in solvent-free conditions, or in water, and in all cases show high atom-economy, high selectivity and procedural simplicity due to the formation of C–C and C-heteroatom bonds [9]. The amounts of solvents, reagents, and energy in domino reactions are dramatically reduced compared to the conventional stepwise approach. Due to growing environmental concerns, there has been an active movement toward developing green chemical processes using more environmentally acceptable chemicals, reagents, solvents and catalysts [10]. In this context, MCRs have played an important role in providing product in a single reaction vessel [11].
The indole nucleus is one of the most well-known heterocycles which is a common and important scaffold in a variety of natural products and medicinal agents [12–15]. It has been reported that sharing the third-carbon atom of indole in the formation of spirooxindole derivatives highly enhances the biological activity [16–19]. Compounds with spiro skeleton have shown both constitute subunits in numerous alkaloids and are templates for drug discovery which have been used as scaffolds for combinatorial libraries [20–22]. The spirooxindole system is the core structure of many pharmacological agents and natural alkaloids [23–26]. Cytostatic alkaloids as spirotryprostatins A [27] (inhibitors of mammalian cell cycle at G2/M phase), elacomine [28], horsfiline [29] (analgesic activity), and mitraphylline, a natural compound containing the spirooxindole framework possessing anti-tumor activity against human brain cancer cell lines and malignant glioma GAMG, are examples which are shown in Fig. 1 [30].
The synthesis of spirooxindoles is of great importance due to their biological activity and applications for pharmaceutical lead discovery. Due to the labor-intensive process of lead discovery and optimization, there has been an ongoing search for simple and efficient methods for the synthesis of spirooxindoles.
A number of methods have been developed for the synthesis of such compounds and the chemistry was comprehensively reviewed by several groups [12, 13, 19, 31–38].
Here, for the first time, this study aims to introduce Caspian IG as a nontoxic, biocompatible, and reusable biocatalyst for which no special handling precautions or storage is required. However, the main purpose of this reusable biocatalyst is the preparation of a heterogeneous and more convenient catalytic system for the application in MCRs and other synthetic reactions. Due to our ongoing interest in MCRs [39–43], the researchers of this study report a versatile method for the synthesis of spirooxindole and spiroacenaphthylenes derivatives via a one-pot three-component reaction of isatin (1) and its derivatives or acenaphthenequinone (5) with active methylene nitriles 2a, 2b (malononitrile or ethylcyanoacetate) and 1,3-dicarbonyl compounds 3a–3f to afford 2-amino-2′,5-dioxo-2′,3′,5,6,7,8-hexahydrospirooxindole-3-carbonitriles 4a–4w or 6a–6e in the presence of Caspian IG as a biocatalyst in water medium (Scheme 1). To the best of our knowledge, no study has heretofore been reported focusing on the use of Caspian IG as catalyst in organic synthesis.

Results and discussion
In the course of our research and evaluation of different catalysts, we have found that IG has an exceptional capability to enhance the rate of the reaction of isatin (1), malononitrile (2a), and dimedone (3a) in aqueous medium. The results are summarized in Table 1. It was observed that when the reaction was carried out without catalyst in water and ethanol, poor yield was obtained (Table 1, entries 1, 2). Other catalysts such as DABCO, Na2CO3, and GABA provided the desired product, but in much lower yields (Table 1, entries 3–5). However, when using IG as the catalyst, the amount of product significantly increased. The present study also evaluated the amount of catalyst required for this synthesis and found that using a mere 5 mg IG in water was sufficient to push the reaction forward. Using a greater amount of the catalyst did not increase the yield. For further optimization of the reaction, it was carried out at different temperatures ranging from room temperature to 100 °C. We found that the yield of product 4a improved and the reaction time was shortened as the reaction temperature was increased to 60 °C. No significant improvement in yield was obtained past that point. Therefore, 60 °C was chosen as the best reaction temperature for all further studies. The increased yields observed at higher temperature may be due to a partial unfolding of the protein and consequently subsequent exposure of acidic and basic residues, which are more readily available for catalysis. In summary, the best results were obtained when the reaction was performed in water at 60 °C in the presence of IG. In previous studies it was reported that a solution of Isinglass prepared at high temperatures would result in a gelatinous solution due to the degradation of collagen, however, no congealing of Isinglass was observed during this reaction.
To determine the optimum reaction conditions, different 2-amino-3-cyano-2′,3′,5,6,7,8-hexahydrospiro[chromene-4,1′-indene]derivatives of 4a–4w were prepared through a one-pot reaction of isatin (1) and its derivatives, malononitrile (2a) or ethylcyanoacetate (2b) and 1,3 dicarbonyl 3a–3f in the presence of IG. The results are summarized in Table 2.

As shown in Table 2, it was found that this method works with an extensive variety of substrates. Series of substituted isatin containing either electron-withdrawing or electron-donating groups and various substituted 1,3-dicarbonyls were used in this reaction.
Furthermore, the reaction with ethyl cyanoacetate instead of malononitrile also worked well; however, the reaction time of ethyl cyanoacetate with isatin and 1,3-dicarbonyl compounds was longer than that of malononitrile, which is undoubtedly due to the lower reactivity of the cyanoacetate. In order to demonstrate the scope of this new efficient methodology, the optimized reaction conditions were adapted for acenaphthoquinone (5) at the next stage. The results are summarized in Table 3.
Taking into account the fact that the pH of reaction mixture is 5.5 therefore at this pH IG, having an isoelectric point (IEP) of around 5.5, exist as a zwitterion having zero net charge. Considering this fact a probable mechanism for the synthesis of spiro derivative is described in Scheme 2. The process represents a typical cascade reaction in which the isatin (1) is first condensed with malononitrile (2a) activated by IG as a heterogeneous acid–base bifunctional organocatalyst through hydrogen bonding and anion formation, respectively, to afford isatylidenemalononitrile I in water. This step is regarded as a rapid Knoevenagel condensation. Subsequently, intermediate I is attacked via Michael addition of enolate derived from dimedone (3a) to provide the intermediate II followed by the cycloaddition of hydroxyl group to the cyano moiety to form the desired product 4a in the presence of IG, which act as an acid–base bifunctional catalysis in all steps (Scheme 2).

Finally, to demonstrate the efficiency and capability of the present protocol in the synthesis of 2-amino-3-cyano-2′,3′,5,6,7,8-hexahydrospiro[chromene-4,1′-indene] the derivatives 4a–4w, it has been compared to some of the previously reported and studies procedures. Summarized results in Table 4 clearly show that the present protocol is indeed superior to several previous catalyst in terms of product yield, reaction time and reaction temperature using IG as a natural, biodegradable and inexpensive published.
For the establishment of the practical applicability of this heterogeneous biocatalyst the level of reusability was also evaluated. For this purpose, the model reaction was used under optimized conditions. After the completion of the reaction, the reaction mixture was dissolved in EtOAc and IG was separated by filtration. Then IG was washed with EtOH and EtOAc, respectively, dried at room temperature and reused in the model reaction. Although some decrease in catalytic activity was observed after four runs (Fig. 2), the catalyst could be used at least four times without significant loss of activity.
Experimental
Melting points were determined in open capillaries using an Electrothermal 9100 instrument. Infrared (IR) spectra were acquired on a Shimadzu FT-IR-8400S spectrometer. The 1H NMR (300 MHz) were obtained on a Bruker Avance DPX-300 instrument. The spectra were obtained in DMSO-d 6 relative to TMS as internal standard. The FT-IR spectra were obtained with a Shimadzu 8400S with spectroscopic grade KBr. Scanning electron microscopy (SEM) was recorded on a VEG//TESCAN with gold coating, and energy dispersive X-ray spectroscopy (EDX) was recorded on a VEG//TESCAN-XMU.
Preparation of IG
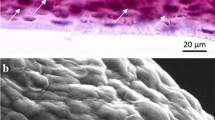
The fish bladders were first washed in hot water to remove extraneous material. The bladders were then cut and soaked in ethanol and stirred for 24 h, collected by filtration, washed with water, and dried at room temperature in the open air for 24 h. Then the dried IG was ground in a ball mill to a fine powder. IG was characterized by FT-IR spectroscopy (Fig. 3a), X-ray diffraction (XRD) techniques (Fig. 3b), and SEM image for both collagenous (Fig. 3c) and powdered IG (Fig. 4). The peak position of the XRD patterns and the absorption bands in IR spectrum confirm amorphous amino acids in the collagenous structure of IG.
General procedure for synthesis of spirooxindoles 4a–4w and spiroacenaphthylene derivatives 6a–6e catalyzed by IG
A mixture of isatin derivatives (1a–1d, 1 mmol) or acenaphthoquinone (5, 1 mmol), methylene nitriles (2a, 2b, 1 mmol), 1,3-dicarbonyl (3, 1 mmol), and 5 mg IG in 3 cm3 H2O was stirred at 60 °C for the mentioned time shown in tables. Rapid conversion of reagents can be clearly confirmed by reaction color change. The progress of the reaction was monitored by TLC using EtOAc/n-hexane (1:3) as an eluent. Upon completion, the reaction mixture was allowed to cool to room temperature and the precipitate was obtained from the reaction mixture by filtration. The product 4a was dissolved in DMSO and the catalyst was separated by simple filtration. Pure products were afforded by evaporation of the solvent under reduced pressure.
References
Zhang W, Cue B (2012) Green techniques for organic synthesis and medicinal chemistry. Wiley, New Jersey, p 217
Koeller KM, Wong CH (2001) Nature 409:232
Gotor V (2002) Org Process Res Dev 6:420
Seoane G (2000) Curr Org Chem 4:283
Hickman D, Sims TJ, Miles CA, Bailey AJ, de Mari M, Koopmans MJ (2000) Biotechnology 79:245
Leach AA (1967) J Inst Brew 73:8
Eastoe JE (1957) Biochem J 65:363
Elinson MN, Ilovaisky AI, Dorofeev AS, Merkulova VM, Stepanov NO, Miloserdov FM, Ogibin YN, Nikishin GI (2007) Tetrahedron 63:10543
Calvo-Flores FG (2009) ChemSusChem 2:905
Majumdar KC, Ponra S, Nandi RK (2012) Tetrahedron Lett 53:1732
Ghahremanzadeh R, Rashid Z, Zarnani AH, Naeimi H (2013) Appl Catal A General 467:270
Zhu SL, Ji SJ, Zhang Y (2007) Tetrahedron 63:9365
Dabiri M, Bahramnejad M, Baghbanzadeh M (2009) Tetrahedron 65:9443
Dandia A, Laxkar AK, Singh R (2012) Tetrahedron Lett 53:3012
Mobinikhaledi A, Foroughifar N, Fard MAB (2011) Synth Commun 41:441
Feng J, Ablajan K, Sali A (2014) Tetrahedron 70:484
Quiroga J, Portillo S, Pérez A, Gálvez J, Abonia R, Insuasty B (2011) Tetrahedron Lett 52:2664
Yu F, Huang R, Ni H, Fan J, Yan S, Lin J (2013) Green Chem 15:453
Rios R (2012) Chem Soc Rev 41:1060
Kuster GJT, van Berkom LWA, Kalmoua M, van Loevezijn A, Sliedregt LAJM, van Steen BJ, Kruse CG, Rutjes FPJT, Scheeren HW (2005) J Comb Chem 8:85
Macleod C, Martinez-Teipel BI, Barker WM, Dolle RE (2005) J Comb Chem 8:132
Maier CA, Wünsch B (2001) J Med Chem 45:438
Dandia A, Singh R, Bhaskaran S, Samant SD (2011) Green Chem 13:1852
Khafagy MM, Abd El-Wahab AHF, Eid FA, El-Agrody AM (2002) Il Farmaco 57:715
Usui T, Kondoh M, Cui CB, Mayumi T, Osada H (1998) Biochem J 333:543
Kang TH, Matsumoto K, Tohda M, Murakami Y, Takayama H, Kitajima M, Aimi N, Watanabe H (2002) Eur J Pharmacol 444:39
Edmondson S, Danishefsky SJ, Sepp-Lorenzino L, Rosen N (1999) J Am Chem Soc 121:2147
James MNG, Williams GJB (1972) J Am Chem Soc 50:2407
Jossang A, Jossang P, Hadi HA, Sevenet T, Bodo B (1991) J Org Chem 56:6527
García Prado E, García Gimenez MD, De la Puerta Vázque R, Espartero Sánchez LL, Sáenz Rodríguez MT (2007) Phytomedicine 14:280
Ball-Jones NR, Badillo JJ, Franz AK (2012) Org Biomol Chem 10:5165
Cheng D, Ishihara Y, Tan BN, Barbas CF (2014) ACS Catal 4:743
Hong L, Wang R (2013) Adv Synth Catal 355:1023
Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, González-Páez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT (2010) Science 329:1175
Kidwai M, Jahan A, Mishra NK (2012) Appl Catal A General 35:425
Pavlovskaya TL, Yaremenko FG, Lipson VV, Shishkina SV, Shishkin OV, Musatov VI, Karpenko AS (2014) Beilstein J Org Chem 10:117
Saluja P, Aggarwal K, Khurana JM (2013) Synth Commun 43:3239
Pavlovska TL, Redkin RG, Lipson VV, Atamanuk DV (2016) Mol Divers 20:299
Javanshir S, Maleki A, Sohrabi B, Kiasadegh M (2014) J Phys Org Chem 27:589
Alinasab-Amiri A, Javanshir S, Dolatkhah Z, Dekamin MG (2015) New J Chem 39:9665
Hosseini-Zare MS, Mahdavi M, Saeedi M, Asadi M, Javanshir S, Shafiee A, Foroumadi A (2012) Tetrahedron Lett 53:3448
Javanshir S, Maleki A, Naimabadi M (2014) RSC Adv 4:30229
Kazemi B, Javanshir S, Maleki A, Safari M, Khavasi HR (2012) Tetrahedron Lett 53:6977
Chai SJ, Lai YF, Xu JC, Zheng H, Zhu Q, Zhang PF (2011) Adv Synth Catal 353:371
Gao S, Tsai CH, Tseng C, Yao CF (2008) Tetrahedron 64:9143
Dandia A, Parewa V, Jain AK, Rathore KS (2011) Green Chem 13:2135
Rad-Moghadam K, Youseftabar-Miri L (2011) Tetrahedron 67:5693
Jalili-Baleh L, Mohammadi N, Khoobi M, Ma’mani L, Foroumadi A, Shafiee A (2013) Helv Chim 96:1601
Li Y, Chen H, Shi C, Shi D, Ji S (2010) J Comb Chem 12:231
Baharfar R, Azimi R (2013) Synth Commun 44:89
Nasseri M, Sadeghzadeh S (2013) J Iran Chem Soc 10:1047
Hari GS, Lee YR (2010) Synthesis 3:453
Oskooie HA, Heravi MM, Karimi N, Hamidi H (2011) Synth Commun 41:3344
Acknowledgments
The authors gratefully acknowledge the support of the Research Council of Iran University of Science and Technology, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Javanshir, S., Saghiran Pourshiri, N., Dolatkhah, Z. et al. Caspian Isinglass, a versatile and sustainable biocatalyst for domino synthesis of spirooxindoles and spiroacenaphthylenes in water. Monatsh Chem 148, 703–710 (2017). https://doi.org/10.1007/s00706-016-1779-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1779-6