Abstract
Spirooxindoles are important synthetic targets possessing extended biological activity and drug discovery applications. This review focuses on the various strategies for the enantioselective synthesis of spirocyclic oxindoles relying on reports over the past decade and from earlier work. The spirooxindoles in this review are separated into three structural classes, and then further categorized into the method type from which the spirocycle is generated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spirooxindole systems are of great interest in a modern organic, medicinal, and natural product chemistry. This type of framework has been found as a core structure of many alkaloids with promising pharmacological activity, such as horsfiline, gelsemine, mitraphylline, spirotryprotatins A, B, and others. The indole scaffold of these compounds is a spiro-ring fused with non-planar bicyclic or polycyclic units of saturated or partially saturated heterocycles. Non-planar structures particularly rigid spatial organized spiro heterocyclic systems have a higher affinity to three-dimensional sites of proteins acting as biotargets than flat aromatic compounds. However, in the modern broad range of pharmaceuticals, spiro compounds are not widely used, and spirooxindoles are absent. At the same time, this type of core is prevalent in a number of spiro leader-compounds and drug candidates with different directions of action [1]. For this reason, investigations of the efficient synthetic routes to compounds with spiroheterocycles or spirocarbocycles at C-2 or C-3 positions of the indole system have increasingly appeared in recent publications. Evidently, among the different synthetic strategies, multicomponent reactions (MCRs) are dominating. For the formation of spirooxindole scaffolds, the three-component condensation of isatin, amino acids and 1,3-dipolarophils, Heck reactions, Michael-Michael-aldol cascades, and many others domino reactions have been used [2]. The regio- and stereoselectivity of these processes are the most discussed in the literature. The highly stereoselective construction of the spirooxindole skeletons with unusual regioselectivity suggests a new avenue of great importance to medicinal chemistry and diversity-oriented synthesis. This review is devoted to diverse methods for the synthesis of compounds containing spirooxindole ring systems, including the ones mimicking specific structures of the natural products.
Naturally occurring s spirooxindoles
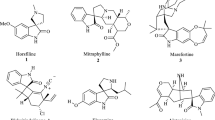
The first isolated spirooxindole alkaloids were spirocycloalkyl-oxindole systems of type 1,2 (gelsemine 1a, gelsevirine 1b, gelsemicine 2a, gelsedine 2b), which were found in the roots of Gelsemium sempervirens and were classified as Gelsemium species [3, 4]. The 3,3\(^\prime \)-spirooxindole skeleton of these compounds is formed by the 2-oxindolic core linked to the cycloalkyl moiety [5] (Fig. 1).
Welwitindolinone A isonitrile 3 is a spirooxindole-containing alkaloid with an antifungal activity derived from the blue-green algae reported by Moore et al. in 1994 [6], which includes a highly functionalized spirocyclobutane oxindole carbon skeleton.
The first hemiterpene spirooxindole alkaloid was isolated in 1968 from the roots of the bush Elaeagnus commutata, Elaegnaceae. X-ray diffraction studies helped to determine the structure of compound 4 named \((\pm )\)-elacomine [7]. The total synthesis of \((\pm )\)-elacomine and establishing of its absolute configuration was achieved by Pellegrini and co-workers [8].
Another simple alkaloid of this group, (-)-horsfiline 5, was originally obtained from the Malaysian medicinal plant Horsfildea Superba (Myristicaceae) by Bodo et al. in 1991 [9]. This compound possesses analgesic properties as well as its synthetic spiro[piperidino-3,3\(^\prime \)-oxindole] analogs [10]. (-)-Horsfiline 5 is closely related to alkaloid (-)-coerulescine 6 which was isolated in 1997 by Anderton et al. from toxic plants of the South Australian Phalaris coerulescens (Poaceae) [11].
Neosurugatoxin 7 contains a spiro[indoline-3,4\(^\prime \)-pyperidine] system. It was extracted by Kosuge et al. from the toxic Japanese Ivory Shell and its structure was determined by X-ray crystallographic analysis [12]. It has about 100 times greater antagonistic nicotinic-receptor activity than relative surugatoxin 8, which contains a piperidone cycle C instead of the cyclopentane ring.
The spiropyrrolidine type of indole alkaloids possessing the same basic terpenoid framework derived from tryptamine and secologanine 9 were discovered in the Mitragyna species and tropical lianas of the genus Uncaria (Ourouparia). They can be further classified into two substructural classes: (1) the tetracyclic secoyohimbane or corynantheidine type (e.g., rhynchophylline 10), and (2) the pentacyclic heteroyohimbane or ajmalicine type (e.g., formosanine 11) [13]. General traditional medicinal uses of Uncaria include treatments for a wide variety of diseases, such as fever, colic, muscular pains, and worm infestations [14–16].
Pteropodine 12 and isopteropodine 13 represent another heteroyohimbine type of oxindole alkaloids with 8-azabicyclo[3.2.1]nonane fragments and act as positive modulators of G protein-coupled muscarinic \(\hbox {M}_{1}\) acetylcholine and \(5-\hbox {HT}_{2}\) (5-hydroxytryptamine) receptors. These compounds can be found in Uncaria tomentosa, a Peruvian medicinal plant known as “cat’s claw” [17].
A number of prenylated indole alkaloids containing a diketopiperazine or a bicyclo[2.2.2]diazaoctane ring were derived from various Aspergillus and Penicillium fungi. The study of their biosynthetic pathways has recently become an area of significant interest. The secondary fungal metabolites spirotryprostatins A 14 and B 15 were found by Osada et al. in Aspergillus fumigatus culture medium [18] and were shown to completely inhibit the G2/M progression of cell division in mammalian tsFT210 cells with \(\hbox {IC}_{50}\) values of 197.5 \(\mu \hbox {M}\) and 14.0 \(\mu \hbox {M}\), respectively [19]. This, in turn, has attracted interest in the synthesis and search of new antitumor agents of this class of compounds [20–22].
Paraherquamide A 16 and notamides 17 contain the unique bicyclo[2.2.2]diazaoctane ring system that is common to this family of natural products and biosynthetically can be the result of an intramolecular Diels-Alder cycloaddition reaction [23].
Spirooxindole systems containing spiro-substituent at position 2 in the 3-oxindole nucleus form a separate group of natural fungal metabolites and are less common than the 3,3\(^{\prime }\)-spirooxindole alkaloids (Fig. 2). Brevianamide A 18 was isolated as the major fluorescent metabolite from culture extracts of the fungus Penicillium brevicompactum in 1969 by A. Birch and J. Wright [24]. These compounds possess modest insecticidal activity [25]. Austamide 20 is a cyclo-L-Trp-L-Pro derivative and contains only one reverse prenyl moiety at position C-2 of the indole ring.
A biological Diels-Alder reaction was proposed as a main route for formation of a unique bicyclo[2.2.2]diazaoctane ring system spirpofused with the oxindole skeleton of brevianamides 18, 19 [26, 27]. Brevianamides 18, 19 can also be attributed to the group spirocycloalkyl-oxindole systems. Structurally and biosynthetically they are related to paraherquamides 16 and notamides 17 that can act as their metabolites (Fig. 1).
Examples of 3-heteroatom-substituted spirooxindoles are also found in nature, e.g., spiroindoline[3,5\(^\prime \)]thiazolidine-type phytoalexins from some plants of the family Cruciferae, cultivated worldwide. Thus, in 1987 the first oxindole phytoalexin \((S)-(^{\_})\)-spirobrassinin 21 was isolated from Japanese radish (Raphanus sativus) by Monde et al. [28, 29]. Later the (R)-(+)-1-methoxyspirobrassinin 22 from kohlrabi (Brassica oleracea var. gongylodes) [30], \((2R,3R)-(^{\_})\)-1-methoxyspirobrassinol methyl ether 23 and N-methoxyspirobrassinol 24 from Japanese radish (R. sativus) have been described as stress metabolites. They possess a heteroatom-rich spirocycle with a sulfur atom in the 3-C position (Fig. 3). N-Methoxyspirobrassinol 24 has an unusual hemi-aminal structure and occurs as a mixture of diastereomers [31].
The appealing molecular diversity of the naturally occurring spirooxindole systems increases interest in the design of novel spirooxindole skeletons. In the present review we report on the different approaches for the synthesis of the spirooxindoles depending on the recent advantages of the natural product synthesis.
Synthesis of the spiro[pyrrolidine-3,3\(^\prime \)-oxindole] and spiro[pyrrolidine-3,2\(^\prime \)-oxindole] systems
Spiropyrrolidinyl-oxindoles can be classified into spiro[pyrrolidine-3,3\(^\prime \)-oxindole] and spiro[pyrrolidine-3,2\(^\prime \)-oxindole] systems. The spiro[pyrrolidine-3,2\(^\prime \)-oxindole] derivatives B are synthetically accessible analogs of the alkaloids with the spiro[pyrrolidine-3,3\(^\prime \)-oxindole] skeleton A (Fig. 4).
The approaches for the design of the spiro[pyrrolidine-3,3\(^\prime \)-oxindole] alkaloids were summarized in some recent reviews. Marti et al. categorized only the spiro[pyrrolidine-3,3\(^\prime \)-oxindoles] construction methods [32], Sh-M. Li presented the report on the prenylated fungal indole derivatives [33], D. Hart classified spiroquinazoline family of alkaloids [34], and G. Singh and Z. Desta focused on the construction of the spirooxindoles derived from isatins [35]. The most recent reviews were devoted to the asymmetric organocatalytic strategies for the synthesis of the spirocyclic oxindoles and to the demonstration of the brief use of spirocyclic scaffolds in drug discovery [36, 37].
Mannich reactions and related transformations
The Mannich reaction successfully found application in the construction of a number of the naturally discovered spirooxindol alkaloids. The biosynthetic pathways of the isomerisation reactions of the oxindol alkaloids are based on the retro-Mannich reaction mechanism that was noted by Wenkert et al. in 1959 [38]. The isomerisation of the spiro center of the alkaloids rhynchophylline 10 and isorychnophylline 25 involves the ring-open form 26 (Scheme 1) [39]. The same situation was observed in the case of hemiterpene spirooxindole alkaloid \((\pm )\)-elacomine 4 [40].
The construction of the spiro[pyrrolidine-3,3\(^\prime \)-oxindole] core via an intramolecular Mannich reaction faces problems mostly in controlling the stereochemistry at the quaternary spiro carbon center and neighboring alkyl groups. Recently, a number of successful attempts were made to solve this problem [32]. The Pictet-Spengler/oxidative rearrangement sequence involving \({\beta }\)-carbolines and the intramolecular Mannich-type condensation of tryptamine- and tryptophan-based oxindoles represent the classical routes of the indole-based natural compound synthesis. Miyake et al. reported the synthesis of elacomine 4 and isoelacomine 29 from 2,6-dibromotryptamine 28 as a new stereoselective method for the spiro[pyrrolidine-3,3\(^\prime \)-oxindoles] formation (Scheme 2) [40].
S. Danishefsky and F. Nussbaum utilized the Mannich condensation for the synthesis of spirotryprostatin B 15 from a readily accessible tryptophan methyl ester 30 and prenyl aldehyde 32. A mixture of diastereoisomeric spiro[pyrrolidine-3,3\(^\prime \)-oxindoles] 33 through several synthetic pathways was then converted into spirotryprostatin B 15 (Scheme 3) [41].
Oxidative rearrangements of tetrahydro-\({\upbeta }\)-carbolines and relative systems
Tetrahydro-\({\upbeta }\)-carbolines are useful starting materials for the construction of the spiro[pyrrolidine-3,3\(^\prime \)-oxindoles]. The first transformation of tetrahydro-\({\upbeta }\)-carboline to spirooxindole alkaloid \((\pm )\)-horsfiline 5 was described by Bodo et al. in 1991 [8]. Thus, the reaction of tetrahydro-\({\beta }\)-carboline 34 with \(\hbox {Pb(OAc)}_{4}\) led to 4 \({\alpha }\)-acetoxyindolenine 35, which was further converted into the intermediates A and B by an acid-catalyzed rearrangement resulting in horsfiline 5 as a racemic mixture (Scheme 4) [42].
A number of authors applied the Pictet–Spengler/oxidative rearrangement method involving a prenyl-substituted tetrahydro-\({\upbeta }\)-carboline [43] and different tryptamines [44] as starting materials that lead to the spirooxindole hemiterpene alkaloids \((\pm )\)-horsfilline 5, \((\pm )\)-elacomine 4 and \((\pm )\)-coerulescine 6.
Other types of the oxidative methods of convertion of indoles into spirocyclic oxindoles include the diastereospecific Sharpless osmylation process, tert-butyl hypochlorite, lead tetraacetate, and sodium tungstate as oxidants. Thus, A. Peterson and J. Cook described a highly diastereoselective synthesis of the spirooxindole diastereomers 41,42 through the conversion of \(\hbox {N}_\mathrm{a}\)-methylated indoloketones 36 by utilizing the Sharpless osmylation process for an asymmetric dihydroxylation (Scheme 5) [45]. The employment of dihydroquinine 4-chlorobenzoate (DHQ-CLB) as the ligand led to the diastereoselective (94 % de) formation of oxindole 42a.
The simple, convenient and stereospecific method of preparation of the spiroketooxindole 44–46 in high yield by utilizing tert-butyl hypochlorite was described by P. Yu and J. Cook [46]. The diastereomers 46 were obtained after treatment of the \(\hbox {N}_\mathrm{b}\)-benzyl tetracyclicketone 43 with t-BuOCl. The same process with \(\hbox {N}_\mathrm{b}\)-H or \(\hbox {N}_\mathrm{b}\)-benzoyl substituted analogs finished the diastereomers 44 and 45, respectively (Scheme 6). These derivatives are of use as templates for the total synthesis of the voachalotine-related oxindole alkaloids.
Somei et al. proposed a method for the selective hydroxylation of the nitrogen atom of indolic cycle by the oxidation of \({\upbeta }\)-carbolines 34 in the presence of hydrogen peroxide and sodium tungstate [47]. The resulting 9-hydroxy-\({\upbeta }\)-carboline 47 was easily converted to (-)-coerulescine 6 (Scheme 7).
Intramolecular heck reactions and similar conversions in spiro[pyrrolidine-3,3\(^\prime \)-oxindoles] synthesis
The highly esteemed Heck reaction is particularly useful for the construction of the asymmetric quaternary carbon centers as well as preparing the 3,3\(^{\prime }\)-disubstituted oxindoles and other complex natural products [48].
Kamisaki et al. performed the synthesis of the spiro[pyrrolidine-3,3\(^\prime \)-oxindole] 49 through the intramolecular domino cyclization of carbamoyl chloride 48 in the presense of catalysts, such as \(\hbox {Pd(OAc)}_{2}\) with \(\hbox {Cs}_{2}\hbox {CO}_{3}\) or \(\hbox {Pd}^{0}\) with \(\hbox {Bi(OTf)}_{3}\), in the absence of any base [49, 50]. The reaction without \(\hbox {Cs}_{2}\hbox {CO}_{3}\) took place smoothly to give the desired spirooxindole 49 as a major product. However, the \(\hbox {Pd}^{0}\)-catalyzed reaction of 48 in the presence of \(\hbox {Bi(OTf)}_{3}\) (10 mol%) and 1,1\(^\prime \)-bis(diphenylphosphino)ferrocene (DPPF) provided spirooxindole 49 in a 52 % yield with no diene 50 contamination (Scheme 8).
L. Overman and M. Rosen achieved the total synthesis of spirotryprostatin B 15 and three stereoisomers through the stereoselective asymmetric Heck cyclization followed by the capture of the resulting \({\upeta }^{3}\)-allylpalladium intermediate that led to the pentacyclic system and the C3-C18 stereorelationship in a single step (Scheme 9) [51]. It was discovered that cyclization of the key intermediate 51 with 10 % \(\hbox {Pd}_{2}\hbox {[DBA]}_{3}\cdot \hbox {CHCl}_{3}\), 40 mol% \(\hbox {(otol)}_{3}\hbox {P}\) and an excess of AcOK in THF at \(70^{\circ }\hbox {C}\) readily led to the formation of a 1:1 mixture of spirooxindole 52 and its isomer. Use of the chiral palladium catalyst (\(\hbox {Pd}_{2}\hbox {[DBA]}_{3}/(S)\)-BINAP-catalyzed) controled the stereochemical outcome of the formation of the first carbon-carbon bond. Cleavege of the SEM protecting group from 52 and chromatographic purification led to spirotryprostatin B 15.
Recently, Zhu et al. developed an oxidative palladium-catalyzed carbo-heterofunctionalization of alkenes through a direct intramolecular aromatic C-H functionalization (Scheme 10) [52]. The conversion of simple N-aryl acrylamides 53 into acetoxylated 3,3\(^{\prime }\)-spiropyrrolidinyloxindoles 54 was performed by utilizing MeCN (c 0.1 M) in the presence of \(\hbox {PdCl}_{2}\) (0.1 equiv) and \(\hbox {PhI(OAc)}_{2}\) (2 equiv) at \(80\,^{\circ }\hbox {C}\) in 37–52 % yield. This domino carboamination process was shown to be applicable to various substrates.
The biologically active spiropyrrolidine-3,3\(^\prime \)-oxindoles 56 were synthesized via the Pd-catalyzed domino spirocyclization process from the linear anilides 55 [53]. The selection of the ligand affects the pathway of the formation of the product from amide 55 through Heck or aminopalladation processes. The 2-di-tert-butylphosphino-2\(^{\prime }\)-methylbiphenyl was used as the most effective ligand in the key step of the trans-aminopalladation of the double bond (Scheme 11).
Jaegli et al. developed the intramolecular domino Heck/cyanation sequence allowing ready access to diversely functionalized 3-alkyl-3-cyanomethyl-2-oxindoles 57, and converted them into described 3,3\(^\prime \)-spiropyrrolidinyloxindoles 58 using methoxymethyl acetal (MOM) protected anilides 55 as common starting materials (Scheme 12) [54].
The Pd-catalyzed intramolecular R-arylation of amides 60 was applied to the synthesis of spirooxindole natural products and its derivatives. Thus, Maison et al. presented a new synthesis of horsfiline 5, giving the natural product in only four steps from commercially available carboxybenzyl (Cbz) protected pyrollidine-2-carboxylic acid 59 (Scheme 13) [55].
1,3-Dipolar cycloaddition reactions in the synthesis of spiropyrrolidinyl-oxindole systems
Methods for the construction of the 3,3\(^{\prime }\)-spirooxindole core
The 1,3-dipolar cycloaddition reactions are regarded as one of the most useful processes in the synthesis of the five membered heterocyclic ring. Among the various dipoles, azomethine ylides were shown to be the most utilized in recent years in the construction of the pyrrolidine derivatives by the reaction with alkens. This method can be applied for the synthesis of spiro[pyrrolidine-3,3\(^\prime \)-oxindoles] and spiro[pyrrolidine-3,2\(^\prime \)-oxindole] systems. Thus, Palmisano et al. represented the synthesis of the (-)-horsfiline 5 based on the the reaction of N-methyl-azomethine ylide 64 prepared in situ from formaldehyde 62 and sarcosine 63 with alken 65 followed by reductive heterocyclization (Scheme 14) [56].
The 1,3-dipolar cycloadditions of azomethine ylides to 2-oxoindolin-3-ylidene derivatives were investigated by a number of authors [57, 58]. Methyl oxindolylidene acetate 67 can be used as a 2-\(\pi \) component in reactions with a wide range of different azomethine ylides generated in situ from sarcosine 63 and the corresponding carbonyl compounds (6-phenyl-4H-pyran-4-one-2-carbaldehyde 68, aldehydes 69–72 and isoquinolinium bromide 73) (Scheme 15). It is noteworthy that when using the anise aldehyde 72 and D, L-proline 74 the resulting product was obtained as a mixture of isomers 79 and 80.
Williams et al. proposed another method of spirotryprostatin B 15 synthesis based on the asymmetric 1,3-dipolar cycloaddition of a generated in situ chiral azomethine ylide 85 to the ethyl oxindolylidene acetate 82 (Scheme 16) [59].
Utilization of the 3-methylideneindolin-2-one 88 and its derivatives as 1,3-dipolyarophiles in the synthesis of the natural spiro[pyrrolidine-3,3\(^\prime \)-oxindoles] allows avoiding the step of the 5-carboxyl group removal [60, 61]. This synthon can be prepared by flash vacuum pyrolysis of the ester 87 in a 60–89 % yield. Cycloaddition reactions of 88 and of the 1-(trimethylsilylmethyl)piperidine-2-carbonitrile 89 gave spirooxindoles 90 in 4–20 % yields (Scheme 17).
3-Nitromethyleneoxindole 93 can be also successfully used as a 1,3-dipolarophile in 1,3-dipolar cycloadditions only under neutral conditions [62]. Various 3,3\(^{\prime }\)-spirooxindol compounds 95 were stereoselectively obtained in one cycloaddition step by treating the mixture of nitroderivatives 92 and isoquinolinium salt 94 with two equivalents of triethylamine in toluene at room temperature (Scheme 18).
Serov et al. synthesized a series of 3,3\(^{\prime }\)-spirooxindoles 98 by the cycloaddition of the phenacyl-quinolinium ylides 97 to the 3-[(E)-2-aryl(hetaryl)-2-oxoethylidene]indolin-2-ones 96. However, completely substituted activated olefin—2-oxo-(3H)-indole-3-ylidine-malononitrile 99 did not react with phenacyl-quinolinium ylides 96 as a dipolarophile (Scheme 19) [63].
Schreiber et al. reported the split-pool synthesis of more than 3000 \(3,3^{\prime }\)-spirooxindoles 102 on the high capacity macrobeads [64]. The key reaction to assemble stereoselectively the \(3,3^{\prime }\)-spirooxindole core is a Williams’ three-component coupling of 83, the allyl ester of 5-iodo-2-oxoindolyl-3-idene acetate 101 and the macrobead-supported aldehydes 100 in the presence of mild Lewis acids \((\hbox {Mg}(\hbox {ClO}_{4})_{2})\) to promote the reaction (Scheme 20).
Wang et al. have used a similar Williams’ approach and synthesized a series of 3,3\(^{\prime }\)-spirooxindoles 107 that could act as potent, specific small-molecule inhibitors of the MDM2-p53 interaction with antitumor activity [21, 65]. The the key step is an asymmetric 1,3-dipolar cycloaddition reaction of 3-arylidene-2-oxindoles 103 with morfolinone 83 and aliphatic aldehyds 104. The amination of 3,3\(^{\prime }\)-spirooxindoles 105 and mild oxidative hydrolysis leads to the target compounds 107 (Scheme 21) [67].
Chen et al. described the asymmetric catalytic three-component 1,3-dipolar cycloaddition of a broad range of methyleneindolinones 110 with amino esters 108 and aldehydes 109 in the presence of chiral phosphoric acids 111, which regioslectively led to the spirooxindols 112 and 113 in high yields under mild conditions (Scheme 22) [68].
The reaction of 1,3-dipolar cycloaddition with azomethine ylides, obtained from isatin and \({\upalpha }\)-amino acids for 3,2\(^{\prime }\)-spirooxindole core building
The domino 1,3-dipolar cycloaddition reactions of azomethine ylides, generated in situ through the decarboxylative condensation of isatins and \({\upalpha }\)-amino acids, with various dipolarophiles, are shown to be the most useful methodology for the regio- and stereoselective formation of a variety of complex 3,2\(^{\prime }\)-spirooxindoles [69]. In 1970, Rizzi reported evidence for the formation of the nonstabilized azomethine ylide intermediate from the decarboxylative condensation between sarcosine and benzophenone. The way of generating the azomethine ylide is believed to proceed through the initial formation of the oxazolidinone, which eliminates carbon dioxide while heating [70]. In the 1990s, Grigg et al. reported on similar reactions by using proline and other \({\upalpha }\)-amino acids as azomethine ylides precursors and methyl acrylate and \({\upalpha },{\upbeta }\)-unsaturated ketones as dipolarophiles forming spiro-pyrrolidine-oxindoles [71–73]. Recently, this substantial method has found many applications in combinatorial chemistry due its simplicity and variability [74, 75].
One of the interests of theoretical investigations of the regioselectivity in 1,3-dipolar cycloadditions is related to high level ab initio methods for the calculation of transition states and activation parameters. Thus, theoretical studies have been carried out to study the stereochemistry of the cycloadducts 119 and 120 from the interaction between the azomethine ylide 116 derived from isatin 114 with L-proline 74 or thiazolidine-4-carboxylic acid 121 and dipolarophiles 117 and 118. Geometry optimization of azomethine ylide 116 points out on its planar structure. The planar proline ring lies in the same plane with the isatin moiety. The authors of this research described the selected products as stereoisomers 115, 119 and 120 but they completely failed to prove their stereochemistry by the relevant methods (Scheme 23) [76].
In the past decades, the understanding of the mechanism in the 1,3-dipolar cycloaddition reactions has grown from an advantageous cooperation between theory and experiment and continues to arouse a real interest. The regio- and stereochemistry of these reactions may be affected by the appropriate dipole and dipolarophile steric and electronic effects or by using a catalyst. Thus, Sarrafi et al. reported the synthesis of spironitropyrrolizines 123 via cycloaddition of isatins 114, proline 74 and \((E)-{\upbeta }\)-phenyl nitroolefins 122 (Scheme 24). The theoretical investigation of all possible regio- and stereocycloaddition pathways of formation of cycloadducts 123 showed that the S-shaped ylide goes through the cycloaddition via an endo-transition state (pathway B) excluding the obtaining of the exo-TS cycloadduct [77]. Later studies had shown that the regioselectivity of the reaction of isatin, L-proline, and \((E)-{\upbeta }\)-phenyl nitroolefins 122 was affected by solvent and temperature, and was independent of the ratio of the reactants [78].
Later, this group of authors synthesized a series of spiropyrrolidine oxindoles 127 via a multicomponent 1,3-dipolar cycloaddition reaction of isatins 114, benzylamine 125 and chalcone derivatives 126 (Scheme 25) [79]. Indeed, this way of synthesis of target spirooxindoles 127 is also attractive because the pool of primary fatty-aromatic amines is much more diverse than the \({\upalpha }\)-amino acids. The possible product 128 was not observed. The calculations of the molecular mechanism of the cycloaddition showed the key role of the [1,5]-H shift in the azomethine ylide generation.
There are many reports in the literature on the formation of spiropyrrolidine oxindoles by the reaction of azomethine ylides, generated from \({\upalpha }\)-aminoacids and isatins, with \({\upalpha },{\upbeta }\)-unsaturated ketones (chalcones). Both N-unsubstituted and N-substituted \({\upalpha }\)-amino acids have been employed in the study [80]. Thus, a series of spiro[pyrrolidine-3,2\(^\prime \)-oxindole] derivatives 131 were synthesized by 1,3-dipolar cycloaddition reaction of isatin 114, \({\upalpha }\)-amino acids, 129 and (E)-ß-substituted-styrenes 130 (chalcones, cinnamic esters, and amide) (Scheme 26). Bioactivity screening conducted by Chen et al. showed that compounds 131 exhibited an antitumor activity in the A549 and P388 cell lines, and several compounds were found to be active under the concentration of \(10^{-4}\hbox {M}\) [81].
Hemamalini et al. presented efficient one-pot synthesis of novel sugar-based spirooxindolopyrrolizidines 133 or -pyrrolidines 134 based on the [3+2] cycloaddition reaction with \({\upalpha },{\upbeta }\)-unsaturated \({\upbeta }-C\)-glycosidic ketones as dipolarophiles (Scheme 27) [82].
The method utilizing azomethine ylides, derived from isatin 114 and sarcosine 63 or L-proline 74, with ether linked \({\upalpha },{\upbeta }\)-unsaturated-\({\upbeta }-C\)-glycosidic ketones 135 (R=\({\upbeta }-C\)-glycosidyl) as a dipolarophiles was proposed by the same authors (Scheme 28) [83].
Recently, Guansheng Wu et al. prepared a series of spirooxindolo-pyrrolidines, pyrrolizidines, and pyrrolothiazoles 140 by regioselective, three-component reactions between \({\upalpha },\,{\upbeta }\)-unsaturated ketones with furanyl substituents 139 and unstable azomethine ylides generated in situ from isatin 114 and different \({\upalpha }\)-amino acids (L-proline 74, thiazolidine-4-carboxylic acid 121, phenylalanine 138) (Scheme 29). The synthesized compounds were screened for their antibacterial activities against a spectrum of pathogens [84].
Another example represents a synthesis of spiro pyrrolidines 142 by cycloaddition reaction of azomethine ylide generated from phenylalanine 138 and isatin 114 with (E)-3-aryl-1-(thiophen-2-yl) prop-2-en-1-ones 141 for a good yield. The reaction proceeded with high regio- and stereoselectivity (Scheme 30). All the synthesized compounds have been evaluated for their anti microbial activity against Echerichia coli, Enterobacter aerogens, Shigella flexneri, Salmonella typhimumium Candida albicans, and Aspergillus niger using the Agar-Agar well diffusion method. The position of the substituent on the phenyl ring significantly influenced anti-microbial activity, with an activity order of p-F \(>\) p-Br \(>\) m-Br \(>\) p-Cl \(>\) m-Cl derivatives [85].
Various applications of the “classical” dipolarophiles, such as 1-aryl-1H-pyrrole-2,5-diones (N-arylmaleimides) were reported by different groups of authors. Most of the synthesized compounds revealed moderate anti-tumor properties against HCT116 (colon), MCF7 (breast), and HEPG2 (liver) human tumor cell lines [86, 87]. The most recent report describes our employment of acyclic \({\upalpha }\)-amino acids in these reactions and the associated stereoselectivity problems of cycloaddition. There was established a stereochemical direction of the cycloaddition of maleimides 144 to azomethine ylides obtained from isatins 114 and acyclic \({\upalpha }\)-amino acids 143, including sulfur-containing ones (cysteine, ethionine). The resulting compounds 144 were obtained in two enantiomeric cyclic forms having a cis configuration of methine protons in the pyrrolo[3,4-c]pyrrole system. The clarification of the mutual disposition of the protons in the pyrrolidine ring of 145a was carried out by using 2D NMR analysis (NOESY, COSY, HSQC, HMBC) (Scheme 31).
The presence of an NH group within the pyrrolidine ring of compounds 145 enabled the study of alkylation, acylation, and nitrosation reactions characteristic of secondary amines. It might be stated that the primary target of electrophilic attack was the N-2’ nitrogen atom of the pyrrolidine ring while employment of \(\hbox {K}_{2}\hbox {CO}_{3}\) enabled the alkylation to be carried out both at the N-1 and N-2’ nitrogen atoms (Scheme 32) [88].
Conversion of N-maleimides 144 into 3-benzylidene-1-alkyl-pyrrolidine-2,5-dions 148 enabled synthesis of series of novel dispiropyrrolidines 149 through 1,3-dipolar cycloaddition of an azomethine ylide generated from sarcosine 63 and isatins 114 (Scheme 33) [89].
There are some publications on the use of esters of acrylic and cinnamic acids as dipolarophiles [72, 90]. In a recent study we put previously unemployed unsymmetrical dipolarophiles acrylamides 150 and methacrylamide 151 in the cycloaddition with azomethine ylides obtained from isatin 114 and sarcosine 63 or from cyclic \({\upalpha }\)-amino acids (proline 74, thiazolidine-4-carboxylic acid 121). The cycloaddition of azomethine ylides to acrylamides may occur along two routes (a and b) and lead to the formation of compounds 152 or 153, respectively (Scheme 34). However, a regioselective formation of spiro[indole- 3,2\(^\prime \)-pyrrolidin]-2-ones 152 was confirmed by \(^{1}\hbox {H NMR}\) spectra [91].
Aroylacrylic acids 153 were for the first time successfully used in this three-component reaction as unsymmetrical dipolarophiles [92]. The domino-reaction of dipolarophiles 153 with isatins 114 and sarcosine 63/proline 74 led to spiropyrrolidines 154 and spiropyrrolizidines 155 in moderate to good yields. All experiments showed the formation of only one type of regioisomer. The higher reactivity of aroylacrylic acids affects the reaction time, which is decreased to 10-15 min by refluxing in a mixture of methanol and water. Interestingly, the long-term heating of isatins 114, aroylacrylic acids 153 , and proline 74 leads to the formation of novel rearranged products 156 (Scheme 35), which have unexpected structures as was confirmed by \(^{1}\hbox {H},\, ^{13}\hbox {C}\) and 2D NMR spectroscopy.
Liu et al. reported a three-component tandem cycloaddition reaction between substituted isatins 114, L-proline 74 and various maleic acid derivatives 157 that led to the racemic spiropyrrolizidine oxindoles 158 (Scheme 36) [93].
Murugan et al. reported the cycloaddition of azomethine ylides generated from the decarboxylative condensation of isatin 114 with octahydro-1H-indole-2-carboxylic acid 159 with triarylideneacetylacetone derivatives 160 to obtain novel spiroheterocycles 162 with high regio- and stereoselectivity. The hypothetical product 163 was not detected (Scheme 37) [94]. Presumably, the anti-ylide 161 is involved in the transition state. The steric repulsion between the carbonyl groups of oxindole and the octahydro-1H-indole-2-carboxylic acid ring disables the formation of syn-ylide. Formation of the cycloadducts was followed by the cleavage of the cinnamoyl group.
3-Acetyl-2H-chromen-2-ones 164 have been used as a cyclic analogs of \({\upalpha },{\upbeta }\)-unsaturated ketones in the synthesis of chromeno[3,4-c]spiropyrrolidine-oxindoles 165 (Scheme 38) [95].
The reactions of 4-hydroxy-6-methyl-3-((E)-3-phenylacryloyl)-2H-pyran-2-ones 166 with isatin 114, sarcosine 63 or thiazolidine-4-carboxylic acid 121 regioselectively gave spiropyrrolidines 167 or spirothiapyrrolizidines 168 (Scheme 39) [96].
Synthesis of pyrrolidinyl-spirooxindoles 171 fused to sugar lactone (Scheme 40) has been achieved by a one-pot 1,3-dipolar cycloaddition of \({\upalpha },{\upbeta }\)-unsaturated lactone 169, isatins 114 and secondary \({\upalpha }\)-amino acids (sarcosine 63/L-proline 74/piperidine-2-carboxylic acid 170). The cycloaddition was found to be highly regio- and diastereoselective [97].
A number of functionalized 3-spiropyrrolidine 173 and 3-spiropyrrolizidine 174 oxindoles has been synthesized with excellent yields utilizing Baylis-Hillman adducts 172 as dipolarophiles (Scheme 41) [98].
The 1,3-dipolar cycloadditions involving 1,4-naphthoquinone 178 as dipolarophile and an azomethine ylide generated from \({\upalpha }\)-amino acids (L-proline 74, L-isoleucine 175, L-phenylalanine 138, L-tryptophan 176, L-valine 177) and isatins 114 have been used to afford the pyrrolidine-2-spiro-3\(^\prime \)-oxindoles 179 with moderate to excellent yields (Scheme 42) [99].
Another example of utilizing the 1,4-naphthoquinone 179 as the dipolarophile describes formation of spirooxindoles 180 followed by spontaneous dehydrogenation (Scheme 43) [100]. Oxydative processes were avoided when the reactions were carried out under nitrogen atmosphere. Synthesized compounds were evaluated for their antimicrobial and antifungal activities.
Taghizadeh et al. described a library of new chiral spirooxindolopyrrolizidines 183 from the isatin derivatives 114, (S)-proline 74, and chiral cinnamoyl oxazolidinone 182 in high to excellent yields followed by the removal of the chiral auxiliary in a non-destructive manner (Scheme 44) [101].
Spirooxindoles 185 containing tri- and tetracyclic fused pyrrolobenzo[b]thiophene-1,1-dioxide were obtained when a benzo[b]thiophene-1,1-dioxide 184 was used as dipolarophile in the three-component reaction with substituted isatins 114 and sarcosine 63 or L-proline 74 (Scheme 45) [102]. The methodology affords high yields of products in a short reaction time.
As has been noted above, 2-oxo-(3H)-indole-3-ylidine-malononitrile99 does not react with phenacyl-quinolinium ylides 97 [63]. Although, spiro- and dispiropyrrolidine oxindoles 188–190 were synthesized using isatylidene malononitrile 99, 2-(1H-indole-3-carbonyl)-3-phenyl-acrylonitrile 186 and 2-(1,3-dioxo-indan-2-ylidene)-malononitrile 187 as dipolarophiles, respectively (Scheme 46) [103]. The observed endo-regioisomers 188–190 are more favorable due to the secondary orbital interaction, which is not possible in the exo-transition state.
2-Oxo-(2H)-acenaphthylen-1-ylidene-malonodinitrile 191 and 2-fluoren-9-ylidene-malonodinitrile 192 have been investigated for the first time as dipolarophiles in the 1,3-dipolar cycloaddition reaction with the azomethine ylides generated in situ from N-substituted isatins 114, and sarcosine 63 to afford novel dispiroheterocycles 193 and 194 (Scheme 47) [104].
Fluorene derivatives, such as 9-arylidine-fluorene 195 can be utilized in the regioselective synthesis of novel dispiro[pyrrolo/pyrrolizidino] ring systems 196 by the cycloaddition to the azomethine ylides generated by a decarboxylative route from sarcosine 63/L-proline 74 and isatin 114 using different methodologies (Scheme 48) [105]. The regioisomers 197 were not observed.
Dispirooxindoles can be obtained when \({\upalpha },{\upbeta }\)-unsaturated ketones, such as 3-aroylmethyleneindol-2-ones 198 are taken as dipolarophiles [106]. Recently, the synthesis of novel dispiropyrrolidine-bisoxindole derivatives 199 has been accomplished by three-component, 1,3-dipolar cycloaddition methodology by stirring the reaction mixture under nitrogen atmosphere at 80 \(^{\circ }\)C in the presence of ionic liquid for the first time (Scheme 49) [107]. The secondary orbital interaction (SOI) of the carbonyl group of dipolarophile 198 with azomethine ylide affects the regiochemistry in the product formation. Hence, only one regioisomer 199 was obtained in the reaction.
In this way, 2-arylmethylideneidene-1,3-indanediones 201 reacted with non-stabilized ylides generated in situ by the decarboxylative condensation of isatins 114 with 1,3-thiazoline-4-carboxylic acid 121 to afford dispiro-oxindolylpyrrolothiazoles 202 (Scheme 50). The obtained compounds possess minimum inhibitory concentration against pathogenic bacteria in the range of 1.4–55.2 \(\mu \hbox {M}\) (near to references of anti-tubercular drugs, such as ethambutol, ciprofloxacin, rifampicin and isoniazid) [108].
2,6-Bis(arylmethylidene)cyclohexanones 203 are of interest in the synthesis of spirooxindole derivatives [109]. Thus, a regioselective 1,3-dipolar cycloaddition reaction with the azomethine ylide derived from isatin 114 and sarcosine 63 by a decarboxylative route afforded a series of spiro[pyrrolidine-2\(^{\prime }\),3-oxindoles] 204 with no traces of the other regioisomers (Scheme 51) [110].
Similar results were obtained by A. Girgis when 3,5-bis(arylmethylene)-1-methyl-4-piperidinones 206 were regioselectively reacted with azomethine ylides, generated in situ via decarboxylative condensation of isatins 114 with sarcosine 63, affording dispiro[3H-indole-3,2\(^{\prime }\)-pyrrolidine-3,3-piperidine]-\(2(1H),4^{\prime \prime }\)-diones 207 (13 examples) (Scheme 52) [111]. It was found that the representative examples of the synthesized compounds reflect mild activity against most of the human tumor cells.
Hazra et al. presented a facile synthesis of novel dispirocompounds 209, 210 via 1,3-dipolar cycloaddition of azomethine ylides generated in situ from isatin derivatives and \({\upalpha }\)-amino acids (sarcosine 63 or L-proline 74) to the conjugated double bond of andrographolide 208 (the major labdane diterpene constituent of Andrographis paniculata) (Scheme 53) [112].
Natural products with steroidal framework have opened so many areas for medicinal and pharmacological chemistry. There was an attempt to apply steroidal dipolarophiles in the synthesis of spirooxindoles [113]. The most recent research is devoted to the facile, atomeconomic synthesis of novel spiro-pyrrolizidino-oxindole adducts 212 of withaferin-A (a polyfunctional steroidal lactone based on an ergostane framework) 211 (10 compounds) via the intermolecular cycloaddition of azomethine ylides generated in situ from proline 74 and isatins 114. The reaction is highly chemo-, regio-, and stereoselective affording the cis-fused products with \({\upbeta }\)-oriented hydrogen (Scheme 54). Bioevaluation of several representatives of adducts 212 against six cancer lines (e.g., CHO, HepG2, HeLa, HEK 293, MDCK-II, and Caco-2) identified them as promising potential anticancer compounds [114].
The ylides generated from isatin 114 and sarcosine 63 or L-thiazolidine-4-carboxylic acid 121 were reacted with arylidene octahydro/decahydro cycloalka[d]thiazolo[3,2-a]pyrimidine-3-ones 213 to yield novel dispiropolycyclic complex heterocycles 214 and 215 (Scheme 55) [115].
The 5-arylidene-1,3-thiazolidine-2,4-dione 216 is described as dipolarophile in a series of research [116, 117]. Recently, dispirooxindolecyclo[pyrrolo[1,2-c]thiazole-6,5\(^\prime \)-thiazolidine] derivatives 217 have been regioselectively synthesized from isatin 114, thiazolidine-4-carboxilic acide 121 and 5-benzylidene-2-thioxothiazolidin-4-one 216 (Scheme 56) [118].
Interestingly, the condensation of 1-allyl (benzyl)-5-haloisatins 114 and L-proline 74 in a molar ratio of 1:1 in ethanol medium under reflux for 1–2 h leads to self-condensation dispiroadducts 218 with carbon dioxide release (Scheme 57) [119].
Enantioselective Michael/Cyclization reaction sequence for the 3,3\(^{\prime }\)- and 3,2\(^{\prime }\)-thiopyrrolidonyl spirooxindole construction
There are two principal different ways to utilize 2-oxindolic reagents in the reaction with \({\upalpha }\)-isothiocyanato compounds, such as the Mannich/cyclizations reactions (discussed below) and the Michael addition/cyclization sequence. However, only the Michael addition leads to the formation of spiro-linked 2\(^\prime \)-thiopyrrolidonyl fragments. Thus 3,3\(^\prime \)- and 3,2\(^\prime \)-thiopyrrolidonyl spirooxindoles can be formed depending on the structure of the 2-oxindolic core [120, 121] (Scheme 58).
The enantioselective Michael addition/cyclization sequence of \({\upalpha }\)-isothiocyanates 219 and methyleneindolinones 220 leads to the 3,3\(^\prime \)-thiopyrrolidonyl spirooxindole scaffolds 222. The methyleneindolinones 220 serve as the perfect electron-deficient olefins because of their high reactivity as Michael acceptors, as well as their unique structural characteristics (Scheme 59).
In particular, Y. Cao group firstly reported the enantioselective Michael addition/cyclization method using an \({\upalpha }\)-isothiocyanato imide and methyleneindolinones [124]. Other authors expanded the usefulness of the \({\upalpha }\)-isothiocyanato nucleophiles in obtaining optically active spirooxindoles. The catalytic asymmetric Michael addition/cyclization of isothiocyanato oxindoles also leads to an enatiomerically enriched bi-spirooxindoles containing three contiguous stereocenters and two spiro-quaternary centers (Scheme 59).
The oxygen-containing heterocycles spiro-fused with the oxindole ring system
Synthesis of spirooxindoles with a spiro-fused pyran fragment
The pyrane/chromene-based heterocycles that fuse with an oxindole ring system represent a potentially promising subset of the tetrahydropyranone and pyrrolidinyl spirooxindole natural products (Fig. 5).
The first group of synthetic strategies are based on cyclization type reactions of 2-indolinone-tethered unsaturated alcohols 224 derived from regioselective addition of stabilized organoindium reagents to isatins 114 in an aqueous environment. The diversely functionalized spiro-dihydropyran-oxindoles 225 have been obtained by using different metal-mediated carbonyl-addition/cyclization reaction sequences under Grubb’s ruthenium-based catalysts (Scheme 60) [125].
S. Hande et al. have developed a concise synthetic route to various spirooxindoles 228 with a tetrahydropyran cycle through a palladium-catalyzed carbosilylation of 1,3-dienes 226 and subsequent Sakurai-type cyclization (Scheme 61) [126].
The highly functionalized spirooxindole 4H-pyran-2-ones 231 with three contiguous stereogenic centers were synthesized through the N-heterocyclic carbenes and catalyzed the three-component reaction of oxindoles 229 with alkynyl aldehydes 230 (Scheme 62) [127].
J. Porco et al., Y. Zhang and J. Panek reported the diversity-oriented stereoselective synthesis of both enantioenriched spirocyclic pyranoxindoles 234 and 235 via Lewis acid mediated Prins cyclizations (Scheme 63) [128, 129]. This strategy is based on the Prins cyclization reaction of isatin dimethyl acetals 232 with enantiopure homoallylic alcohols 233 in the presence of trimethylsilyl trifluoromethane sulfonate (TMSOTf) as a catalyst.
A similar approach based on a Brönsted acid-catalyzed Prins-type cyclization of isatin dimethyl acetal 232 and a \({\upbeta }\)-hydroxy dioxinone fragment 236 leads to the spirooxindole pyrans 237 in high yields and excellent diastereoselectivity (Scheme 64) [130].
Recently, Zh. Lian and M. Shi disclosed a novel nitrogen- and phosphorus-containing Lewis base mediated by [4+2] and [3+2] annulations of N-protected isatins 114 with but-3-yn-2-one 238 to produce the spiro[indoline-3,2\(^{\prime }\)-pyran]-2,4\(^{\prime }(3^{\prime } H)\)-diones 239 and spiro[furan-\(2,3^{\prime }\)-indoline]-\(2^{\prime },4(5H)\)-diones 240, respectively, in good yields under mild conditions [131] (Scheme 65).
In recent years the concept of fast and convenient MCRs has found various applications in the synthesis of spiro-indolones. The main synthetic method for assembling of spiro[4H-pyran-oxindole] compounds is based on the three-component reactions of two (usually different) 1,3-dicarbonyl compounds, or alternatively their synthetic equivalents, with isatin derivatives. We and others investigated three-component reactions of isatins 114, malononitrile 240, phenyl-acetonitrile 241, methyl-, ethyl- and other cyanoacetates 242, and various 1,3-dicarbonyl compounds 243 to afford a series of spiroindolones 244 (Scheme 66) [132–136].
As can be seen from the literature, MCRs procedures use different catalysts, such as tris(2–hydroxyethyl)amine [137], L-proline [138], sodium stearate [139], \(\hbox {[BMIm]BF}_{4}\) [140] as catalysts in an alcoholic or aqueous medium for the activation of these processes, as well as non-catalyst and solvent-free conditions [141–143]. The most recent research described a silica-bonded 5-n-propyloctahydro-pyrimido[1,2-a]azepinium chloride (SB-DBU)Cl as the heterogeneous silica-supported ionic liquid catalyst used for the efficient synthesis of spiro[4H-pyran-oxindoles] 244 [144].
Several approaches were made for the synthesis of spiro[indoline-3,4\(^{\prime }\)-pyrano[2,3-c]pyrazoles] 246 in the presence of basic catalysts in an alcoholic medium as well as solvent-free reaction of isatins 114, 3-Methyl-5-pyrazolone 245 and methylene active nitriles 240 and 242 in the presence of \(\hbox {NaHCO}_{3}\) under grinding [141–143, 145]. A plausible mechanism for this process may probably involve the formation of arylidenemalononitriles A via Knoevenagel condensation reaction of isatins methylene active nitriles 240 using various bases. The following Michael addition of the nucleophile 1-aryl-3-methyl-5-pyrazolone 245 to arylidenemalononitriles A gives compound B. After that, the intramolecular nucleophilic addition reaction between the hydroxyl group and the cyano group in compound C leads to the imine D followed by formation of the spiro compounds 246 (Scheme 67).
Recently, D. Shi et al. described the one-pot synthesis of spiro[indoline-3,4\(^{\prime }\)-pyrano[2,3-c]pyrazole] derivatives 246 by the four-component reaction of hydrazine 247, \({\upbeta }\)-keto ester 248, isatins 114, and methylene active nitriles 240 and 242 catalyzed by piperidine under ultrasound irradiation (Scheme 68) [146].
Our experience in this reactions has shown that the utilization of the 1-allyl-4-hydroxy-2-oxo-1,2-dihydroquinolines 249 (aza-analogs of 4-hydroxycoumarin) leads to similar spirocompounds 250 (Scheme 69) [147].
3-Hydroxy-1H-phenalen-1-one 251 is a very interesting enol-neuclophilic component in similar three-component reactions. Thus, A. Bazgir et al. described the synthesis of spiro[benzo[g]chromene-4,3\(^{\prime }\)-indoline]-3-carbonitriles 252 from 3-hydroxy-1H-phenalen-1-one 251, malononitrile 240 and isatin 114 in aqueous media in the presence of p-TSA (Scheme 70) [148].
The Michael addition of isatinilidenemalonodinitriles 99 with ketones 253 with a cinchona-based chiral primary amine A and L-camphorsulfonic acid B as catalysts gave the optically active adducts 254 in high yields with excellent enantioselectivity (95 to \(>\)99 % ee). Lately, the Michael adducts 254 were used in a cascade reduction/cyclization process for the synthesis of the spiro[2H-pyran-3,4\(^{\prime }\)-indoline] derivatives 255 in moderate to good yields with 90–99 % ee (Scheme 71) [149].
The selective Rh(I)-catalyzed condensation of N-methylisatin 114 with two molecules of 1,3-cyclohexanedione 256 or 4-hydroxy-6-methyl-2-pyrone 257 gives spirooxindoles 258 and 259 with 46 and 36 % yield (dr \(=\) 5:1), respectively (Scheme 72) [150]).
The cyclocondensation reaction of isatins 114, 1,3-cyclohexadiones 256, and 2-methylpyrimidine-4,6-diol 260 or 3-methyl-1-phenyl-1H-pyrazol-5-ol 261 in aqueous media under p-TSA catalysis gives the spiro[chromeno[2,3-d]pyrimidine-5,3\(^\prime \)-indoline]-2\(^\prime \),6(7H)-diones 262 and spiro[chromeno[2,3-c]pyrazole-4,3\(^\prime \)-indoline]-2\(^\prime \),5(6H)-diones 263 (Scheme 73) [151].
In recent years there has been considerable interest in the utilization of barbituric acid 264 in the construction of spiro compounds. Several approaches have been made for developing of new selective and environmentally friendly methodologies in the synthesis of spirooxindole heterocycles containing chromenopyrimidine ring fragments 265. Thus, the procedures used water as a solvent in the presence of p-TSA as a catalyst [152]. Recently, two groups of authors identified dodecyl benzenesulfonic acid (DDBSA) functionalized by silica-coated magnetic nanoparticles (\(\gamma -\hbox {Fe}_{2}\hbox {O}_{3}@\hbox {SiO}_{2}\)-DDBSA) [153] and \(\hbox {KAl}(\hbox {SO}_{4})_{2}\cdot 12\hbox {H}_{2}\hbox {O}\) in [Bmim]\(\hbox {PF}_{6}\) [154] as an efficient catalysts for the synthesis of a library of similar spirooxindole-chromeno[2,3-d]pyrimidine derivatives 265 by reaction of isatins 114, cyclohexane-1,3-diones 256, and barbituric acids 264 (Scheme 74).
It was shown that refluxing of a mixture of barbituric acid 264, \({\upbeta }\)-naphthol 266, and isatin 114 in water in the presence of catalytic p-TSA afforded the spironaphthopyranopyrimidine-indolines 267 in good yields (Scheme 75) [155].
Recently, A.Bazgir et al. presented a practical, simple, and efficient method for the synthesis of pyrano-fused spirooxindoles 269 and 270 via an organocatalytic reaction of isatins 114, malononitrile 240, and dialkyl acetylenedicarboxylate 268 in the presence of 3,4-dimethylaniline as a catalyst in ethanol (Scheme 76) [156].
Synthesis of 3-spiroindolinones spiro-fused with piperidine moieties
Organic compounds incorporating the spiro[indoline-3,4\(^\prime \)-piperidine] scaffold have been considered as “privileged structures” for drug research [157]. For example, Ibutamoren (MK-677, L-163,191) is a potent, orally active growth hormone secretagogue that mimics the stimulating action of the endogenous hormone ghrelin [158, 159]. Some spiro[indoline-3,4\(^\prime \)-piperidines] have been identified as vesicular acetylcholine transporters (Fig. 6) and as novel targets for insecticide action against major agricultural pest species with low mammalian toxicity [160].
The synthetic strategies for the formation of the spiro[indoline-3,4\(^\prime \)-piperidine] skeleton are based on a large variety of classical synthetic methods [161, 162]. Thus, an intramolecular Heck reaction of a tetrasubstituted alkene 271 was used in the total synthesis of the marine fungal alkaloid \((\pm )\)-communesin F 273 [163] (Scheme 77).
A novel synthetic strategy was realized for the formation of the chiral spiropiperidineoxindole system 276 from a ring closing metathesis of an enantiopure quaternary 3-aminooxindole 274 in the presence of a \(2^\mathrm{nd}\) generation Grubbs catalyst (Scheme 78) [164].
Later, the synthesis of spirooxindoles 281 and 282 with the same stereochemistry as the core structure of tabernoxidine was accomplished by a Sakurai type reaction (Scheme 79) [130]. In the first step, a Mitsunobu reaction of alcohol 227 with glutarimide 277 or succinimide 278 was followed by reduction to give compounds 279 or 280, respectively. The followed Sakurai-type cyclization with \(\hbox {BF}_{3}\cdot 3\hbox {OEt}_{2}\) proceeded diastereoselectively and gave compounds 281 or 282 in excellent yields.
Recently, an efficient \(\hbox {FeCl}_{3}\)-catalyzed stereoselective intramolecular tandem 1,5-hydride transfer/ring closure reaction was developed by Han et al. (Scheme 80) [165]. This method allows obtaining structurally diverse spirooxindole tetrahydroquinolines 284 in high yields (up to 98 %) with good to excellent levels of diastereoselectivity (up to 99:1 dr).
MCRs have been also widely used in the synthesis of spiro[indoline-3,4\(^\prime \)-piperidines]. A novel efficient route for the synthesis of spiro dihydropyridines 287 was developed through a four-component reaction of isatin 114, malononitrile 240, primary amines 285 and acetylenic esters 286 with high yields and a simple experimental procedure (Scheme 81) [166].
Another four-component reaction of isatin 114, 1,3-dicarbonyl compounds 288, 1-phenyl-2-(1,1,1-triphenyl- \(\lambda ^{5}\)-phosphanylidene)-1-ethanone 289 and amine 285 under refluxing in dry methanol afforded a series of spirooxindole derivatives 290 containing indoline-3,4\(^{\prime }\)-pyridine-3\(^{\prime }\)-carboxylate fragments in 74–85 % yields (Scheme 82) [167].
The reactions of isatin with aromatic amines and suitable CH-acids proceed very easily and can be done even in the absence of solvents, for example, under mechanical activation. For instance, it was suggested a simple synthesis of spiro[diindenopyridine-indoline]triones could be done 292 via the reaction of 1,3-indandione 291, aromatic amines 285 and isatins 114 based on a “Grindstone Chemistry” method in the presence of a catalytic amount of p-TSA (Scheme 83) [168]. Compounds 292 are potent anticancer agents, which have cytotoxic and apoptosis inducing potencies that compare favorably with the clinical anticancer agent etoposide [169].
Among the first, S. Ahadi et al. reported the synthesis of spiro[benzo[h]pyrazolo[3,4-b][1,6]naphthyridine-7,3\(^{\prime }\)-indoline]-2\(^{\prime }\),6(5H)-diones 296 and spiro[chromeno[4,3-b]pyrazolo[4,3-e]pyridine-7,3\(^{\prime }\)-indoline]-2\(^{\prime }\),6(6aH,10H)-diones 297 from the reactions of CH-acids (4-hydroxy-1-methylquinolin-2(1H)-one 294 or 4-hydroxycoumarin 295) with isatins 114 and 1H-pyrazol-5-amines 293 in water catalysed by p-TSA [170]. It is noteworthy that the product 297 does not eliminate water even under prolonged refluxing (Scheme 84).
A few years later, it was described the interactions between 5-amino-3-methyl-1-phenylpyrazoles 293, \({\upbeta }\)-diketones 243 and isatin 114 in aqueous media with p-TSA as a catalyst, leading to the formation of several spiro-pyrazolo[3,4-b]pyridine derivatives 298 (Scheme 85). The alternative products 299 were not observed [171, 172].
At the same time, G. Shakibaei et al. presented a catalyst-free synthesis of 2-amino-1H-spiro[indeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3\(^{\prime }\)-indoline]-2\(^{\prime }\),4,6(11H)-triones 301 by the similar MCRs of isatins 114 with 1,3-indandione 291 and 2,6-diaminopyrimidin-4(3H)-one 300 in refluxing ethanol with 73–82 % yields (Scheme 86) [173].
Isatins with various substituents react differently with 2,6-diaminopyrimidin-4(3H)-one 300 [174]. It was found that a mixture of 2,6-diaminopyrimidin-4(3H)-one 300 and N-unsubstituted isatins 114 in the presence of a catalytic amount of p-TSA afforded the spiro[pyrimido[4,5-b]quinoline-5,5\(^\prime \)-pyrrolo[2,3-d]pyrimidine]-triones 302 in a 85 % yield after refluxing in ethanol for 8 h. This reaction may have proceeded through the intermediate A, formed in situ by interreaction of isatins 114 with 2,6-diaminopyrimidin-4(3H)-one 300, and converted into the intermediate B followed by formation of cyclized product 302 and ammonia. Although, when using N-alkylisatins 114 under similar conditions, different products \(2^{\prime },8^{\prime }\)-diamino-spiro[indoline-3,5\(^{\prime }\)-pyrido[2,3-d:6,5-\(d^{\prime }\)]dipyrimidine]-\(2,4^{\prime },6^{\prime }(3^{\prime } H,7^{\prime } H,\,10^{\prime } H\))-triones 303 were formed in 78–87 % yields (Scheme 87).
Aminouraciles have also found application in the synthesis of spirooxindoles. Thus, the refluxing of 1,3-indandione 291 with amino uracils 304, and isatins 114 without any catalyst in ethanol for 3 h afforded spiroindeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3\(^\prime \)-indolines 305 in good yields (Scheme 88). It should be noted, that when the reaction of aminouraciles 304 and isatin 140 was carried out with other cyclic diketones, such as dimedone 256 or barbituric acid 264 in the same conditions, the reaction mixture showed a combination of starting materials and other numerous products [175].
When isoxazole 306 was used instead of 2,6-diaminopyrimidin-4(3H)-one 300 or 1,3-diaryl-pyrazol-5-amines 293 in the reactions with isatins 114 and barbituric acids 304, the spiro[indoline-isoxazolo[4\(^\prime \),3\(^\prime \):5,6] pyrido[2,3-d] pyrimidine] derivatives 307 were obtained in high yields (Scheme 89) [176].
Synthesis of 3-spirooxindoles fused with cycloalkyl radicals
Methods of spiro[cyclohexane-1\(^{\prime }\),3-indoline]-2-one framework construction
The synthesis of the spiro[cyclohexane-1\(^{\prime }\),3-indoline]-2-one scaffold is of general interest due to its connection with a gelsemine group of alkaloids (Fig. 1). For example, this nucleus is presented in the highly potent and selective vasopressin \(\hbox {V}_{2}\)-receptor antagonist SR121463A [177]. The spiro-oxindole 308 [178] is a potent inhibitor of the MDM2–p53 interaction in the discovery of anticancer agents. The novel bichromophoric spirocyclic indolones 309 possess bright fluorescence and high quantum yield (Fig. 7) [179].
The spiro[cyclohexane-1,3\(^\prime \)-indolin]-2\(^\prime \),4-dione ring can be prepared either from a preexisting 4-oxo protected cyclohexyl derivative or from an oxindole. Thus, methyleneindolinones 310 were used as starting materials in the synthesis of spirocyclohexenindolone derivatives 312 via the Diels-Alder cycloaddition with several dienes, for example, Danishefsky’s diene 311 (Scheme 90) [180, 181].
Variousortho-iodo anilides were successfully used in the synthesis of spiro[cyclohexane-1\(^{\prime }\),3-indoline]-2-ones [182]. Th. Müller et al. applied another approach based on the insertion-coupling-isomerization-Diels-Alder domino reaction for a search of new luminescent bichromophoric spirocyclic indolones 309 [178]. The reaction with alkynyl ortho-iodo anilides 313 and 1-phenylpropargyl prenyl ethers 314 as substrates and \([\hbox {PdCl}_{2}(\hbox {PPh}_{3})_{2}]\) and CuI as a catalytic system under \(130^{\,\circ }\hbox {C}\) for 16 h led to formation of spirocyclic indolones 315 in moderate yields (Scheme 91).
Y-C. Chen and co-workers found that interaction of 2,4-hexadienal 317 with the diphenylprolinolsilyl ether 318 and o-fluorobenzoic acid (OFBA) leads to reactive trienamine intermediates A which undergo Diels-Alder reactions with 3-olefinic oxindoles 316 (Scheme 92). This method offers a facile entry to highly complex molecular frameworks with excellent stereocontrol [183].
An example of a highly efficient organocatalytic Diels-Alder reaction is presented by the synthesis of carbazolespirooxindole derivatives 322 from methyleneindolininones 316 and 3-vinylindoles 320 (Scheme 93). A simple bis-thiourea 321 was used as the organocatalyst, that provided the products in excellent yields and stereoselectivity (\(>\)99:1 dr, up to 99 % ee) [184].
Methyleneindolinones have also been used in bifunctional organocatalytic asymmetric [4+2] cycloaddition reactions for the construction of spiro[4-cyclohexanone-1,3\(^{\prime }\)-oxindoline] derivatives [185]. Recently, cyclobutenones 323 have been used in the asymmetric intermolecular 1,4-dipolar spiroannulation with isatylidenemalononitrile 99 in the presence catalyst 324 followed by formation of 3-spirocyclohexenone 2-oxindoles 325 in good yield with up to 87 % ee (Scheme 94) [186].
Recently, chiral N-arylnitrones 327 and 328 were used with carbocyclic alkylarylketenes 326 in a pericyclic cascade comprising [3+2]-cycloaddition followed by a [3,3]-sigmatropic rearrangement process to generate spirocyclic oxindoles 329 and 330 in good yields and with excellent levels of enantioselectivity (90–99 % ee) (Scheme 95) [187].
The organo-catalyzed Michael/Michael/aldol condensation sequences allow the direct, one-step synthesis of complex spirooxindolic cyclohexane derivatives starting from simple precursors [188]. In 2010, Y-C. Chen’s group offered the one-pot method for the synthesis of methyleneoxindoles 331 with two molecules of \({\upalpha },{\upbeta }\)-unsaturated aldehyde 332 and 333 under quadruple iminium/enamine/iminium/enamine catalysis that led to spirooxindoles 334 bearing six contiguous stereocenters in excellent stereoselectivities (96 to 99 % ee, \(>\)99 % dr). A chiral amine \({\upalpha },{\upalpha }\)-diphenylprolinol O-TMS ether 318 served as a catalyst in this unique tripleMichael/aldol process (Scheme 96) [189]. Subsequently, a tandem reaction of aliphatic aldehydes with electron-deficient olefinic oxindoles could be supplemented with various activated olefins or imines to afford spirocyclic oxindoles with miscellaneous molecular complexity [190, 191].
Later, it was disclosed that oxindoles 335 could react with unsaturated aldehydes 336 via a Michael–Michael-aldol reaction to give the desired spirocyclic compounds 337 in the presence of the catalyst 318 (20 %) and benzoic acid (20 %) in toluene. The final products were obtained in good yields and in a total stereocontrolled fashion in most of the examples (Scheme 97) [192].
The optically pure spiro[cyclohexane-1,3\(^\prime \)-indoline]-2\(^\prime \),3-diones 341 could be efficiently synthesized in high yields (88–99%) with excellent diastereo- and enantioselectivity (94:6–99:1 dr, 95–99 % ee) through the cascade Michael additions of isatylidene malononitriles 99 with (R)-\({\upbeta }\)-unsaturated ketones 338 via the catalysis of a cinchona alkaloidderived primary amine 339 together with an BINOL-phosphoric acid 340 (Scheme 98) [193].
The most recent report devoted to a Michael–Michael–aldol cascade sequence represents an interaction between 1,3-dicarbonyl compounds 342, nitroalkenes 343, and methyleneindolinones 316 in the presence of 5 mol% chiral squaramide 344. The reactions led to a series of enantioenriched spirocyclohexane oxindoles 345 bearing six contiguous stereocenters in good yields (up to 85 %) and with excellent stereoselectivity (\(>\)20:1 dr, \(>\)99 % ee) (Scheme 99) [194].
There have been described interesting examples of the intramolecular Friedel-Crafts reaction for the synthesis of diversely functionalized spirooxindoles [195, 196]. In the latter case, compounds 347 can be derived from readily accessible \({\upalpha }\)-keto-N-arylacetamides 346 bearing alkyl side chain residues in the presence of trifluoroacetic acid (TFA) at room temperature or at \(45^{\,\circ }\hbox {C}\). This method could be applied for the synthesis of spirooxindoles fused with cyclopentyl-, cyclohexyl and cycloheptyl rings (Scheme 100).
Spirocyclopentaneoxindoles synthesis
A number of natural alkaloids (Fig. 8) and synthetic drug candidates include the 3-spirocyclopentane-2-oxindoles as a main motif of their scaffolds [197, 198]. The direct catalytic enantioselective synthesis of these compounds is fraught with challenges in chiral substrate-controlled methods.
Zhiguo Bian et al. have achieved the first total synthesis of (–)-citrinadin A 348 through this methodology, which takes 20 stages. The key step has a vinylogous Mannich reaction of the dienolate derived from 348 with the chiral pyridinium salt 349 followed by formation of the first stereogenic center of compound 350. The chirality at this center served as a control point when introducing other stereocenters in the pentacyclic core (Scheme 101). Citrinadin A has shown to exhibit cytotoxicity against murine leukemia L1210 (\(\hbox {IC}_{50}\) 6.2 mg/mL) and human epidermoid carcinoma KB cells \((\hbox {IC}_{50}\,10 \hbox {mg/mL})\) [199, 200].
Several attempts were made to provide the stereocontrolled synthesis of the citrinadin B core 354 [201]. Recently, Li et al. have developed a convergent synthetic strategy that employs enone 352, which was serving as the dipolarophile in the stereoselective intermolecular nitrone 353 cyloaddition reactions as a key step (Scheme 102) [202].
The asymmetric organocatalytic multistep one-pot reactions have appeared as a powerful tool for efficient construction of complex molecules from readily available simple starting materials. Li et al. have described organocatalyzed Michael addition/intramolecular silyl nitronate-olefin cycloaddition (ISOC)/fragmentation reaction of 3-allyl-substituted oxindoles 356 and nitroolefines 343, which gave diastereoselective (up to \(>\) 30:1 dr) and enantioselective (up to \(>\) 99 % ee) spirocyclopentaneoxindoles 358 with the oxime functional group and including one spiroquaternary stereocenter in good yields (Scheme 103) [203].
Later, the Michael-Henry cascade reactions provided spirooxindoles 361 in high yields and excellent enantioselectivity in a single step from various oxindole derivatives 359 and nitroolefines 343 as starting materials in the presence of a chiral tertiary amine catalyst 360 in DCM at \(0^{\,\circ }\hbox {C}\) for 2 h (Scheme 104) [204].
In recent years, organocatalytic enantioselective domino/cascade reactions have been employed for the synthesis of spirocyclopenteneoxindoles by various groups of authors [205–208]. Later, the cinchona-based primary amine 363 organocascade catalysis was used to access a variety of complex highly optically pure spirocompounds 365 with four contiguous stereocenters when reacting with the cyclic dienones 362 and the 3-substituted oxindoles 359 (Scheme 105) [209].
Recently, a novel iminium–enamine tandem process was established to construct densely substituted spirocyclopentaneoxindole core units 367 from 3-substituted bifunctional oxindoles 366 and readily available \({\upalpha },{\upbeta }\)-unsaturated aldehydes 336 catalyzed by a chiral secondary amine 318 with excellent stereoselectivity (up to 99 % ee) (Scheme 106) [210].
Several approaches were made for the construction of spirocyclic oxindolic cyclopentanes via [3+2] cycloaddition reactions. Asymmetric variants of these reactions have been implemented by using chiral catalysts [211, 212]. High interest is represented in the annulation reactions of Morita-Baylis-Hillman carbonates and olefins with phosphine catalysts. Thus, a novel organocatalytic asymmetric [3+2] cycloaddition reaction between methyleneindolinones 368 and allylic compounds 369 leads to complex spirocyclopentaneoxindoles 371 with a chiral phosphine 370 as a nucleophilic organocatalyst (Scheme 107) [213].
Another example of a chemo- and enantioselective [3+2] annulation of Morita–Baylis–Hillman carbonates of isatins 372 by propargyl sulfone 373 and catalyzed by \({\upbeta }\)-isocupreidine (\({\upbeta }\)-ICD) O-MOM ether 374, describes a synthesis of spirocyclic 2-oxindoles 375 bearing an unusual cyclopentadiene motif in outstanding ee values (up to \(>\)99 %) (Scheme 108) [214].
Electrophiles, such as N-phenylmaleimide, have been also utilized to deliver complex spirocyclic 2-oxindoles with good results. Thus, an efficient asymmetric [3+2] cycloaddition reaction between Morita–Baylis–Hillman carbonates of isatins 372 and N-phenylmaleimide 144 catalyzed by Me-DuPhos 376 afforded spirocyclopentaneoxindoles 377 in good yields (up to 84 %) with excellent diastereo- and enantioselectivity (up to 99 % ee) (Scheme 109) [215].
Synthesis of spiro[indoline-3,1\(^\prime \)-cyclopropan]-2-ones
The Synthesis of spirocyclopropanes is of great interest and especially challenging due to the presence of three consecutive stereogenic centers in the highly strained three-membered ring of their molecules. The spiro[indoline-3,1\(^\prime \)-cyclopropan]-2-ones are important semi-products for the alternate bond construction strategy for spiro[pyrrolidine-3,3\(^\prime \)-oxindole] ring systems, relying on a cyclopropane-opening/ring-expansion reaction [32]. Spirocyclopropane-1,3-oxindole 378 acts as a kinase inhibitor and 379 is a potent HIV-1 non-nucleoside reverse transcriptase inhibitor (Fig. 9) [216, 217].
Xiaowei Dou et al. developed the first direct organocatalytic asymmetric cyclopropanation reaction of oxindoles. In this strategy, oxindoles 380 were employed as a dinucleophilic \(\hbox {C}_{1}\) synthons and bromonitroolefins 381 with a dielectrophilic center were used as a \(\hbox {C}_{2}\) synthon (Scheme 110). An amino acid-based multifunctional catalyst 383 promoted the [2+1] reaction, gave the products 384 and 385 in high yields and excellent enantioselectivity. By using 1,4-diazabicyclo[2.2.2]octane (DABCO) as a nucleophilic catalyst, a stereochemically retentive conversion of different diastereomers of cyclopropyl spirooxindoles was discovered [218].
The cyclopropanation formation spirooxindoles has been usually performed in the presence of toxic metal catalysts [219]. Further, spirocyclopropanes have also been reported from diazo compounds and alkenes in the presence of expensive transition metals, such as \(\hbox {Rh}_{2}\hbox {(OAc)}_{4}\), CuOTf, \(\hbox {Hg(OTf)}_{2}\) or Au(I)-complexes [220, 221]. The disadvantage of the metal-catalyzed process is that heteroatom containing alkenes could bind tightly to a transition metal present in the catalyst, resulting in loss of their catalytic activity. Recently, there was discovered a highly efficient diastereoselective method to synthesize spiro[cyclopropane-1,3-oxindoles] 389 and 390 from thermal decomposition of 3-diazooxindoles 386 and mono-substituted 387 or 1,2-disubstituted 388 alkenes under solvent- and transition metal-free conditions in excellent yields (Scheme 111) [222].
A new asymmetric organocatalytic synthesis of trans-substituted spirocyclopropane oxindoles 393 based on the Michael addition of N-Boc-protected 3-chlorooxindole 390 to unsaturated 1,4-dicarbonyl compounds 391 running with an amino acid-based multifunctional catalyst 392 has been developed by Oseka et al. This methodology provides products 393 with two identically substituted tertiary stereocenters in moderate yields and with very high diastereo- and enantioselectivity (Scheme 112) [223].
Synthesis of 3-spirooxindoles containing different two heteroatoms-substituted hetherocycles
The 3-heteroatom-substituted spirooxindoles, especially sulfur-containing phytoalexins, were firstly isolated from the plants of the family Cruciferae (syn. Brassicaceae) [224, 225]. As it was pointed out earlier, spirobrassinin 21 and its related analogues (Figs. 4, 10) posess a potent antimicrobial, antitumor, and oviposition stimulant for biological activities and are of great interest in the applying of novel methodologies for their synthesis [226–228].
The Mannich/cyclizations reactions represent one of the ways of utilisation of 2-oxindols in the synthesis of the spiroindoline[3,4’]oxazolines. Thus, Yuan et al. firstly reported the organocatalytic direct asymmetric synthesis of a library of enantioenriched spirocyclic oxindoles 396 through the aldol reactions of 3-isothiocyanato oxindoles 219 with ketones 394 and bifunctional thiourea-tertiary amines 395 as catalysts (Scheme 113) [228].
Later, Han et al. developed a method for highly efficient and diastereoselective construction of structurally diverse dispiro[oxazolidine-2-thione]bisoxindoles 396 in excellent yields (up to 97 %) and diastereoselectivity (up to 99:1) by the reaction of 3-isothiocyanato oxindoles 219 with isatins 114 in the presence of 1 mol% \(\hbox {Et}_{3}\hbox {N}\) under mild reaction conditions (Scheme 114). The following methylation of 396 led to spirobrassinin’s spiroindoline[3,4\(^\prime \)]oxasoline analogs 397 [230].
Jiang et al. developed a highly efficient and convenient strategy of the construction of unique spiroindoline[3,5\(^\prime \)]oxasolines 400 and 401 through the organocatalyzed asymmetric aldol reaction of the N-substituted isatins 114 with isothiocyanates 398 and 399 (Scheme 115). Preliminary biological evaluation of several representatives of spirooxazolines revealed promising antipyretic activity [231].
Badillo et al. described a method for the synthesis of a new class of spirocyclic oxindole oxazolines 403 and 404 by the addition of 5-alkoxy-2-aryloxazoles 402 to isatin 114 by adding the catalytic amounts of titanium (IV) chloride (10 or 20 mol%). Utilizing the substitution at the 4-position of the oxazole enabled access to either the 2- oxazoline 403 or 3-oxazoline 404 spirocycles with excellent regiocontrol (dr \(>\)99:1) (Scheme 116) [232].
The spirocyclic isoxazolines represent another type of 3-heterocyclic spirooxindoles. The most common way for their synthesis is the 1,3-dipolar cycloaddition of alkene dipolarophiles and nitrile oxides [233]. Thus, the nitrile oxide 406 was generated in situ by dehydrochlorination of hydroximoyl chloride 405. The following cycloaddition of 3-methylene oxindoles 407 gave the product 408 as a single regioisomer, albeit in low yield (Scheme 117) [234].
A similar approach to the synthesis of spiro[indole-dioxazoline-1,3,4] compounds 410 was applied by 1,3-dipolar cycloaddition reaction of isatins 114 with the aryl nitrile oxide generated in situ from 4-methoxybenzaldoxime 409 and sodium hypochloride (Scheme 118) [235].
Carlos et al. described utilization of zinc as a dehydrochlorinating agent for chlorooximes 405 with an aryl or ester side chains in the 1,3-dipolar cycloaddition reactions with 3-methylene indolin-2-ones 410. This method can proceed without an addition of base and leads to spiroisoxazoline oxindoles 411 containing ester groups at position 4\(^\prime \) and aromatic or ester groups at position 3\(^\prime \) of the isoxazoline ring (Scheme 119) [236].
Gomez-Monterrey et al. reported the direct spirocondensation of isatins 114 and cysteine ethyl ester that led to spiro(oxoindolethiazolidine) ethyl esters 412. The following intramolecular cyclization of these derivatives was performed in refluxing methanol in the presence of TEA and gave the novel highly antitumor potential spiro[imidazo[1,5-c]thiazole-3,3\(^\prime \)-indoline]-2\(^\prime \),5,7-trione derivatives 413 with 39–56 % overall yields as simple isomer (Scheme 120) [237].
Vintonyak et al. established the synthesis of spirothiazolidinones 416 through the cyclisation of the isatin-3-imines 414 with mercaptoacetic acid 415. The following oxidation of sulfides 416 with meta-chloroperbenzoic acid (mCPBA) led to a library of 200 indolin-2-on-3-spirothiazolidinones 417 (Scheme 121). All tested compounds of 417 are potent inhibitors of the pathophysiologically relevant title protein MptpB (Mycobacterium tuberculosis protein tyrosine phosphatases B) [238].
The similar approach to spiro[indole-thiazolidinones] 419 was made by R. Lesyk et al. through the one-pot three-component reaction of isatins 114, primary aromatic amines 418, and a mercaptoacetic acid 415 in anhydrous benzene. The following synthesis of 5-ylidene-4-thiazolidinones 421 was realized in a Knoevenagel reaction of 419 with aldehydes 420 in 2-propanol with potassium tert-butylate as catalyst. The reaction of isatins 114 with mercaptoacetic acid 415 and amino acids esters 422 under microwave assistance led to compounds 423 with significant antitumor activity (Scheme 122) [239].
However, novel heptacyclic spiro[indoline-3,4\(^\prime \)-pyrazolo[3,4-e][1,4]thiazepine]diones 425 were obtained when amines 418 were replaced by 5-amino-3-methylpyrazoles 293 in a facile one-pot reaction with isatins 114 and mercaptoacetic acid 415 due to the formation of of 3-(5-aminopyrazol-3-yl)-3-hydroxy 2-oxindolines 424 as intermediates (Scheme 123) [240].
Conclusion
This review is devoted to the recent advances in the strategies of the enantioselective synthesis of various spirooxindoles that can possess significant biological activity. However, the evolution of the methodologies for the construction of spirooxindoles has increased through the past decade and is expected to have important employment for the development of complex natural compounds as well as in drug design.
References
Lipson VV, Zamigajlo LL, Petrova ON (2011) Development of 11\({\beta }\)-HSD1 inhibitors for the treatment of metabolic syndrome. Ukrainica Bioorganica Acta 2:3–13. http://www.bioorganica.org.ua/UBAdenovo/vol_9_2_ukr.htm
Stuart L (2000) Schreiber target-oriented and diversity-oriented organic synthesis in drug discovery. Science 17:1964–1969. doi:10.1126/science.287.5460.1964
Bindra JS (1973) Chapter 2 oxindole alkaloids. Alkaloids: Chem Physiol 14:83–121. doi:10.1016/S1876-0813(08)60219-5
Schun Y, Cordell GA (1985) 14\({\beta }\)-Hydroxygelsedine, a new oxindole alkaloid from Gelsemium sempervirens. J Nat Prod 48:788–791. doi:10.1021/np50041a012
Kitajima M (2007) Chemical studies on monoterpenoid indole alkaloids from medicinal plant resources Gelsemium and Ophiorrhiza. J Nat Med 61:14–23. doi:10.1007/s11418-006-0101-z
Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA (1994) Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricata. Relationship to fischerindoles and hapalinodoles. J Am Chem Soc 116:9935–9942. doi:10.1021/ja00101a015
James MNG, Williams GJB (1972) The molecular and crystal structure of an oxindole alkaloid (6-Hydroxy-2’-(2-methylpropyl)-3,3’ spirotetrahydropyrrolidino-oxindole). Can J Chem 50:2407–2412. doi:10.1139/v72-386
Pellegrini C, Weher M, Borschberg H-J (1996) Total synthesis of (+)-elacomine and (-)-isoelacomine, two hitherto unnamed oxindole alkaloids from Elueagnus cornrnutata. Helv Chim Acta 79:151–168. doi:10.1002/hlca.19960790116
Jossang A, Jossang P, Hadi HA, Sévenet T, Bodo B (1991) Horsfiline, an oxindole alkaloid from Horsfieldia superba. J Org Chem 56:6527–6530. doi:10.1021/jo00023a016
Kornet MJ, Thio AP (1976) Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J Med Chem 19:892–898. doi:10.1021/jm00229a007
Anderton N, Cockrum PA, Colegate SM, Edgar JA, Flower K, Vit I, Willing RI (1998) Oxindoles from Phalaris coerulescens. Phytochem 48:437–439. doi:10.1016/S0031-9422(97)00946-1
Kosuge T, Tsuj K, Hirai K, Yamaguchi K, Okamoto T, Iitaka Y (1981) Isolation and structure determination of a new marine toxin, neosurugatoxin, from the Japanese Ivory Shell. Tetrahedron Lett 2:3417–3420. doi:10.1016/S0040-4039(01)81920-1
Lerchner A, Carreira EM (2006) Synthesis of \((\pm )\)-strychnofoline via a highly convergent selective annulation reaction. Chem Eur J 12:8208–8219. doi:10.1002/chem.200600957
Beecram AF, Hart K, John SR (1968) Lambert the stereochemistry of oxindole alkaloids: uncarines A, B (formosanine), C (pteropodine), D (speciophylline), E (isopteropodine), and F. Aust J Chem 21:491–504. doi:10.1071/CH9680491
Pandey R, Singh SC, Gupta MM (2006) Heteroyohimbinoid type oxindole alkaloids from Mitragyna parvifolia. Phytochem 67:2164–2169. doi:10.1016/j.phytochem.2006.06.017
Heitzman ME, Neto CC, Winiarz E, Vaisberg AJ, Hammond GB (2005) Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochem 66:5–29. doi:10.1016/j.phytochem.2004.10.022
Kang TH, Matsumoto K, Tohda M, Murakami Y, Takayama H, Kitajima M, Aimi N, Watanabe H (2002) Pteropodine and isopteropodine positively modulate the function of rat muscarinic M\(_{1}\) and 5-HT\(_{2}\) receptors expressed in Xenopus oocyte. Eur J Pharmacol 444:39–45. doi:10.1016/S0014-2999(02)01608-4
Cui C-B, Kakeya H, Osada H (1996) Novel mammalian cell cycle lnhibitors, spirotryprostatins a and b, produced by Aspergillusfumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 52:12651–12666. doi:10.1016/0040-4020(96)00737-5
Cui C-B, Kakeya H, Osada H (1996) Spirotryprostatin B, a novel mammalian cell cycle inhibitor produced by Aspergillus fumigatus. J Antibiot (Tokyo) 49:832–835. doi:10.7164/antibiotics.49.832
Ket D, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, Roller PP, Wang S (2006) Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem 49:3432–3435. doi:10.1021/jm051122a
Yu S, Qin D, Shangary S, Chen J, Wang G, Ding K, McEachern D, Qiu S, Nikolovska-Coleska Z, Miller R, Kang S, Yang D, Wang S (2009) Potent and orally active small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem 52:7970–7973. doi:10.1021/jm901400z
Williams RM, Cox RJ (2003) Paraherquamides, brevianamides, and asperparalines: laboratory synthesis and biosynthesis. An Interim Rep. Acc Chem Res 36:127–139. doi:10.1021/ar020229e
Sunderhaus JD, Sherman DH, Williams RM (2011) Studies on the biosynthesis of the stephacidin and notoamide natural products: a stereochemical and genetic conundrum. Israel J Chem 51:442–452. doi:10.1002/ijch.201100016
Birch AJ, Wright JJ (1969) The brevianamides: a new class of fungal alkaloid. J Chem Soc Chem Commun 12:644–645. doi:10.1039/C2969000644B
Paterson RRM, Simmonds MJS, Kemmelmeier C, Blaney WM (1990) Effects of brevianamide A, its photolysis product brevianamide D, and ochratoxin A from two Penicillium strains on the insect pests Spodoptera frugiperda and Heliothis virescens. Mycol Res 94:538–542. doi:10.1016/S0953-7562(10)80017-6
Auclair K, Sutherland A, Kennedy J, Witter DJ, Van den Heever JP, Hutchinson CR, Vederas JC (2000) Lovastatin nonaketide synthase catalyzes an intramolecular diels-alder reaction of a substrate analogue. J Am Chem Soc 122:11519–11520. doi:10.1021/ja003216+
Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM (2008) Isolation, structure elucidation, and biomimetic total synthesis of versicolamide B, and the isolation of antipodal (-)-stephacidin A and (+)-notoamide B from aspergillus versicolor NRRL 35600. Angew Chem Int Ed 47:3573–3577. doi:10.1002/anie.200800106
Takasugi M, Monde K, Katsui N, Shirata A (1987) Spirobrassinin, a novel sulfur-contaning phytoalexin from the daikon Raphanus sati6us L. var. hortensis (Cruciferae). Chem Lett 16:1631–1632. doi:10.1246/cl.1987.1631
Suchý M, Kutschy P, Monde K, Goto H, Harada N, Takasugi M, Dzurilla M, Balentová E (2001) Synthesis, absolute configuration, and enantiomeric enrichment of a cruciferous oxindole phytoalexin, (s)-(-)-spirobrassinin, and its oxazoline analog. J Org Chem 66:3940–3947. doi:10.1021/jo0155052
Monde K, Taniguchi T, Miura N, Kutschy P, Curillová Z, Pilátová M, Mojzis J (2005) Chiral cruciferous phytoalexins: preparation, absolute configuration, and biological activity. Bioorg Med Chem 13:5206–5212. doi:10.1016/j.bmc.2005.06.001
Monde K, Takasugi M, Shirata A (1995) Three sulphur-containing stress metabolites from Japanese radish. Phytochem 39:581–586. doi:10.1016/0031-9422(95)00011-U
Marti C, Erick M, Carreira EM (2003) Construction of spiro[pyrrolidine-3,3’-oxindoles] recent applications to the synthesis of oxindole alkaloids. Eur J Org Chem 2003:2209–2219. doi:10.1002/ejoc.200300050
Li Sh-M (2010) Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep 27:57–78. doi:10.1039/b909987p
Hart DJ (2010) The spiroquinazoline family of alkaloids: a review. ARKIVOC IV: 32-65.doi:10.3998/ark.5550190.0011.405
Singh GS, Desta ZY (2012) Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem Rev 112:6104–6155. doi:10.1021/cr300135y
Cheng D, Ishihara Y, Tan B, Barbas CF III (2014) Organocatalytic asymmetric assembly reactions: synthesis of spirooxindoles via organocascade strategies. ACS Catal 4:743–762. doi:10.1021/cs401172r
Zhen Yajun, Colin MT, Singh Suresh B (2014) The use of spirocyclic scaffolds in drug discovery. Bioorg Med Chem Lett 24:3673–3682. doi:10.1016/j.bmcl.2014.06.081
Wenkert E, Delhofen JHU, Bhaitacharyya NK (1959) 3-Hydroxymethyleneoxindole and its derivatives. J Am Chem Soc 81:3763–3768. doi:10.1021/ja01523a068
Laus G (1998) Kinetics of isomerization of tetracyclic spiro oxindole alkaloids. J Chem Soc, Perkin Trans 2:315–317. doi:10.1039/A705871C
Miyake FY, Yakushijin K, Horne DA (2004) Preparation and synthetic applications of 2-halotryptamines: synthesis of elacomine and isoelacomine. Org Lett 6:711–713. doi:10.1021/ol030138x
Nussbaum F, Danishefsky SJ (2000) A rapid total synthesis of spirotryprostatin B: proof of its relative and absolute stereochemistry. Angew Chem Int Ed 29:2175–2178. doi:10.1002/15213773(20000616)39:12<2175::AID-ANIE2175>3.0.CO;2-J
Finch N, Taylor WI (1962) Oxidative transformations of indole alkaloids. I. The preparation of oxindoles from yohimbine; the structures and partial syntheses of mitraphylline, rhyncophylline and corynoxeine. J Am Chem Soc 84:1318–1320. doi:10.1021/ja00866a062
White JD, Li Y, Ihle DC (2010) Tandem intramolecular photocycloaddition-retro-mannich fragmentation as a route to spiro[pyrrolidine-3,3’-oxindoles]. total synthesis of \((\pm )\)-coerulescine, \((\pm )\)-horsfiline, \((\pm )\)-elacomine, and \((\pm )\)-6-deoxyelacomine. J Org Chem 75:3569–3577. doi:10.1021/jo1002714
Wang H, Ganesan A (2000) A biomimetic total synthesis of (-)-spirotryprostatin B and related studies. J Org Chem 65:4685–4693. doi:10.1021/jo000306o
Peterson AC, Cook JM (1995) Studies directed toward the enantiospecific synthesis of Gardneria, Voacanga, and Alstonia oxindole alkaloids. J Org Chem 60:120–129. doi:10.1021/jo00106a024
Yu P, Cook JM (1997) Diastereospecific synthesis of ketooxindoles. Potential intermediates for the synthesis of alstonisine as well as for Voachalotine related oxindole alkaloids. Tetrahedron Lett 38:8799–8802. doi:10.1016/S0040-4039(97)10420-8
Somei M, Noguchi K, Yamagami R, Kawada Y, Yamada K, Yamada F (2000) Preparation and a novel rearrangement reaction of 1,2,3,4-tetrahydro-9-hydroxy-\({\beta }\)-carboline, and their applications for the total synthesis of \((\pm )\)-coerulescine. Heterocycl 53:7–10. doi:10.3987/COM-99-8743
Dounay AB, Overman LE (2003) The asymmetric intramolecular heck reaction in natural product total synthesis. Chem Rev 103:2945–2963. doi:10.1021/cr020039h
Kamisaki H, Nanjo T, Tsukano C, Takemoto Y (2011) Domino Pd-catalyzed heck cyclization and bismuth-catalyzed hydroamination: formal synthesis of elacomine and isoelacomine. Chem Eur J 17:626–633. doi:10.1002/chem.201002287
Kamisaki H, Yasui Y, Takemoto Y (2009) Pd-catalyzed intramolecular amidation of 2-(buta-1,3-dienyl)phenylcarbamoyl chloride: a concise synthesis of spiro[indoline-3,3\(^\prime \)-pyrrolidine]. Tetrahedron Lett 50:2589–2592. doi:10.1016/j.tetlet.2009.03.100
Overman LE, Rosen MD (2000) Total synthesis of (-)-spirotryprostatin B and three stereoisomers. Angew Chem Int Ed 39:4596–4599. doi:10.1002/15213773(20001215)39:24<4596::AID-ANIE4596>3.0.CO;2-F
Jaegli S, Dufou J, Wei H, Piou T, Duan X, Vors J, Neuville L, Zhu J (2010) Palladium-catalyzed carbo-heterofunctionalization of alkenes for the synthesis of oxindoles and spirooxindoles. Org Lett 12:4498–4501. doi:10.1021/ol101778c
Jaegli S, Erb W, Retailleau P, Vors J-P, Neuville L, Zhu J (2010) Palladium-catalyzed domino process to spirooxindoles: ligand effect on aminopalladation versus carbopalladation. Chem Eur J 16:5863–5867. doi:10.1002/chem.201000312
Jaegli S, Vors JP, Neuville L, Zhu J (2010) Palladium-catalyzed domino Heck/cyanation: synthesis of 3-cyanomethyloxindoles and their conversion to spirooxoindoles. Tetrahedron 66:8911–8921. doi:10.1016/j.tet.2010.09.056
Deppermann N, Thomanek H, Prenzel A, Maison W (2010) Pd-catalyzed assembly of spirooxindole natural products: a short synthesis of horsfiline. J Org Chem 75:5994–6000. doi:10.1021/jo101401z
Cravotto G, Giovenzana GB, Pilati T, Sisti M, Palmisano G (2001) Azomethine ylide cycloaddition/reductive heterocyclization approach to oxindole alkaloids: asymmetric synthesis of (-)-horsfiline. J Org Chem 66:8447–8453. doi:10.1021/jo015854w
Grigg R, Basanagoudar LD, Kennedy DA, Malone JF, Thianpatanagul S (1982) X=Y-ZH systems as potential 1,3-dipoles. Cycloadditions of thiominoethers and thioiminocarbonates. Tetrahedron Lett 23:2803–2806. doi:10.1016/S0040-4039(00)87463-8
Fejes I, Nyerges M, Nyerges M, Szöllõsy Á, Blaskó G, Tõke L (2001) 2-Oxoindolin-3-ylidene derivatives as 2-\(\pi \) components in 1,3-dipolar cycloadditions of azomethine ylides. Tetrahedron 57:1129–1137. doi:10.1016/S0040-4020(00)01085-1
Sebahar PR, Osada H, Usuib T, Williams RM (2002) Asymmetric, stereocontrolled total synthesis of (+) and (-)-spirotryprostatin B via a diastereoselective azomethine ylide [1,3]-dipolar cycloaddition reaction. Tetrahedron 58:6311–6322. doi:10.1016/S0040-4020(02)00630-0
Bell SEV, Brown RFC, FrW Eastwood, Horvath JM (2000) An approach to some spiro oxindole alkaloids through cycloaddition reactions of 3-methylideneindolin-2-one. Aust J Chem 53:183–190. doi:10.1002/chin.200043209
Onishi T, Sebahar PR, Williams RM (2004) Concise, asymmetric total synthesis of spirotryprostatin A. Tetrahedron 60:9503–9515. doi:10.1016/j.tet.2004.07.047
Fejes I, To’ke L, Nyerges M, Pak ChS (2000) Tandem in situ generation of azomethine ylides and base sensitive nitroethylene dipolarophiles. Tetrahedron 56:639–644. doi:10.1016/S0040-4020(99)01028-5
Serov AB, Kartsev VG, Aleksandrov YuA, Dolgushin FM (2005) 1,3-Dipolar cycloaddition reaction of heteroaromatic N-ylides with 3-[(E)-2-aryl(hetaryl)-2-oxoethylidene]indolin-2-ones. Russ Chem Bull 54:2432–2436. doi:10.1007/s11172-006-0133-2
Lo M, Neumann CS, Nagayama S, Perlstein EO, Schreiber SL (2004) A library of spirooxindoles based on a stereoselective three-component coupling reaction. J Am Chem Soc 126:16077–16086. doi:10.1021/ja045089d
Ding K, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, Roller PP, Wang S (2006) Structure-based design of spiro-oxindoles as potent, specific small- molecule inhibitors of the MDM2-p53 interaction. J Med Chem 49:3432–3435. doi:10.1021/jm051122a
Ding K, Lu Y, Nikolovska-Coleska Z, Qiu S, Ding Y, Gao W, Stuckey J, Krajewski K, Roller P, Tomita Y, Parrish D, Deschamps J,Wang S (2005) Structure-based design of potent non-peptide MDM2 inhibitors. J Am Chem Soc 127:10130–10131. doi:10.1021/ja051147z
Ding K, Wang G, Deschamps JR, Parrish DA, Wang Sh (2005) Synthesis of spirooxindoles via asymmetric 1,3-dipolar cycloaddition. Tetrahedron Lett 46:5949–5951. doi:10.1016/j.tetlet.2005.06.114
Chen Xiao-Hua, Wei Qiang, Luo Shi-Wei, Xiao Han, Gong Liu-Zhu (2009) Organocatalytic synthesis of spiro[pyrrolidin-3,3\(^{\prime }\)-oxindoles] with high enantiopurity and structural diversity. J Am Chem Soc 131:13819–13825. doi:10.1021/ja905302f
Lashgari N, Ziarani GM (2012) Synthesis of heterocyclic compounds based on isatin through 1,3-dipolar cycloaddition reactions. ARKIVOC I :277–320. doi:10.3998/ark.5550190.0013.108
Rizzi GP (1970) Evidence for an azomethine ylide intermediate in the carbonyl-assisted decarboxylation dl-phenylephrine hydrochloride of sarcosine. A novel synthesis of dl-phenylephrine hydrochloride. J Org Chem 35:2069–2072. doi:10.1021/jo00831a098
Da Silva JFM, Garden SJ, Pinto AC (2001) The chemistry of isatins: a review from 1975 to 1999. J Braz Chem Soc 12:273–324. doi:10.1590/S0103-50532001000300002
Coulter T, Grigg R, Maloneb JF, Sridharan V (1991) Chiral induction in cycloaddition reactions of azomethine ylides derived from secondary \({\alpha }\)-amino acids by the decarboxylative route. Tetrahedron Lett 32:5417–5420. doi:10.1016/S0040-4039(00)92401-8
Grigg R (1987) Prototropic routes to 1,3- and 1,5-dipoles, and 1,2-ylides: applications to the synthesis of heterocyclic compounds. Chem Soc Rev 16:89–121. doi:10.1039/CS9871600089
Fokas D, Ryan WJ, Casebier DS, Coffen DL (1998) Solution phase synthesis of a spiro[pyrrolidine-2,3\(^{\prime }\)-oxindole] library via a three component 1,3-dipolar cycloaddition reaction. Tetrahedron Lett 39:2235–2238. doi:10.1016/S0040-4039(98)00234-2
Powers DG, Casebier DS, Fokas D, Ryan WJ, Troth JR, Coffen DL (1998) Automated parallel synthesis of chalcone-based screening libraries. Tetrahedron 54:4085–4096. doi:10.1016/S0040-4020(98)00137-9
Pardasani RT, Pardasani P, Chaturvedi V, Yadav SK, Saxena A, Sharma I (2003) Theoretical and synthetic approach to novel spiroheterocycles derived from isatin derivatives and L-proline via 1,3-dipolar cycloaddition. Heteroatom Chem 14:36–41. doi:10.1002/hc.10063
Sarrafi Y, Hamzehloueian M, Alimohammadi K, Yeganegi S (2011) An experimental and theoretical investigation of the regio- and stereoselectivity of the polar [3+2] cycloaddition of azomethine ylides to nitrostyrene. Tetrahedron 67:1589–1597. doi:10.1016/j.tet.2010.12.034
Chen G, Miao Y, Zhou R, Zhang L, Zhang Y, Hao X (2013) Investigation of regioselectivity in the synthesis of spiro[pyrrolidine-2,30-oxindoles] by use of the Huisgen reaction. Res Chem Intermed 39:2445–2450. doi:10.1007/s11164-012-0770-z
Sarrafi Y, Hamzehloueian M, Alimohammadi K, Yeganegi S (2012) Experimental and theoretical approaches to [1,5]-prototropic generation of an azomethine ylide and a 1,3-dipolar cycloaddition for novel spiropyrrolidine oxindoles synthesis. J Mol Struct 1030:168–176. doi:10.1016/j.molstruc.2012.04.013
Rehn S, Bergman J, Stainsland B (2004) The three-component reaction between isatin, \({\alpha }\)-amino acids, and dipolarophiles. Eur J Org Chem 2:413–418. doi:10.1002/ejoc.200300621
Chen G, He H, Ding J, Hao X (2009) Synthesis and antitumor activity evaluation of regioselective spiro[pyrrolidine-2,3’-oxindole] compounds. Heterocycl Commun 15:355–360. doi:10.1515/HC.2009.15.5.355
Hemamalini A, Nagarajan S, Ravinder P, Subramanian V, Thangamuthu B, Das M (2011) An easy access to novel sugar-based spirooxindole-pyrrolidines or -pyrrolizidines through [3+2] cycloaddition of azomethine ylides. Synth 15:2495–2504. doi:10.1055/s-0030-1260111
Hemamalini A, Nagarajan S, Das ThM (2012) A novel class of sugar-based ether-linked-dispirooxindolo-pyrrolidines/pyrrolizidines through [3+2]-cycloaddition of azomethine ylides. Carbohydr Res 352:12–17. doi:10.1016/j.carres.2012.01.023
Wu G, Ouyang L, Liu J, Zeng S, Huang W, Han B, Wu F, He G, Xiang M (2013) Synthesis of novel spirooxindolo-pyrrolidines, pyrrolizidines, and pyrrolothiazoles via a regioselective three-component [3+2] cycloaddition and their preliminary antimicrobial evaluation. Mol Divers 17:271–283. doi:10.1007/s11030-013-9432-3
Kanagaraju G, Thangamani A (2014) Design and synthesis of spiro derivatives containing a thiophene ring and evaluation of their anti-microbial activity. Orient J Chem 30:1619–1630. doi:10.13005/ojc/300421
Azizian J, Asadi A, Jadidi Kh (2001) One-pot highly diastereo-selective synthesis of new 2-substituted 8-(sprio-3\(^\prime \)-indolino-2\(^\prime \)-one)-pyrrolo[3,4-a]-pyrrolizine-1,3-diones mediated by azomethine ylide induced by microwave irradiation. Synth Commun 31:2727–2733. doi:10.1081/SCC-100105318
Girgis AS, Stawinski J, Ismail NSM, Fara H (2012) Synthesis and QSAR study of novel cytotoxic spiro[3H-indole-3,2’(1\(^{\prime }\)H)-pyrrolo[3,4-c]pyrrole]-2,3\(^{\prime }\),5\(^{\prime }\)(1H,2\(^{\prime }\)H,4\(^{\prime }\)H)-triones. Eur J Med Chem 47:312–322. doi:10.1016/j.ejmech.2011.10.058
Pavlovskaya TL, Red’kin RG, Yaremenko FG, Shishkina SV, Shishkin OV, Musatov VI, Lipson VV (2013) Synthesis and chemical properties of new derivatives of 3a\(^\prime \),6a\(^\prime \)-dihydro-2\(^\prime \)H-spiro- [indole-3,1\(^\prime \)-pyrrolo[3,4-c]pyrrole]- 2,4\(^\prime \),6\(^\prime \)(1H,3\(^\prime \)H,5\(^\prime \)H)-trione. Chem Heterocycl Comp 49: 882–896 (Russian Original 49: 945–960). doi:10.1007/s10593-013-1322-1
Karthikeyan K, Sivakumar PM, Doble M, Perumal PT (2010) Synthesis, antibacterial activity evaluation and QSAR studies of novel dispiropyrrolidines. Eur J Med Chem 45:3446–3452. doi:10.1016/j.ejmech.2010.04.035
Faraji L, Arvinnezhad H, Alikami N, Jadidi K (2010) Synthesis of pyrrolizidine derivatives in ionic liquid [bmim]Br. Lett Org Chem 7:472–415. doi:10.2174/157017810791824946
Pavlovskaya TL, Lipson VV, Yaremenko FG, Musatov VI (2013) Acryl- and methacrylamides. New dipolarophiles in reactions of [2+3]-dipolar cycloaddition to 2-oxindolazomethine ylides. Russ J Org Chem 49(11):1712–1714 (Russian Original 49:1728–1730). doi:10.1134/S1070428013110274
Pavlovskaya TL, Yaremenko FG, Lipson VV, Shishkina SV, Shishkin OV, Musatov VI, Karpenko AS (2014) The regioselective synthesis of spirooxindolo pyrrolidines and pyrrolizidines via three-component reactions of acrylamides and aroylacrylic acids with isatins and \({\alpha }\)-amino acids. Beilstein J Org Chem 10:117–126. doi:10.3762/bjoc.10.8
Xie Yong-Mei, Yao Yu-Qin, Sun Hong-Bao, Yan Ting-Ting, Liu Jie, Kang Tai-Ran (2011) Facile Synthesis of Functionalized Spiropyrrolizidine Oxindoles via a Three-Component Tandem Cycloaddition Reaction. Molecules 16:8745–8757. doi:10.3390/molecules16108745
Murugan R, Raghunathan R, Narayanan SS (2010) Synthesis of novel spiroheterocycles through 1,3-dipolar cycloaddition of azomethine ylides with triarylideneacetylacetone through decarboxylation. Synth Commun 40:3135–3151. doi:10.1080/00397910903341189
Ghandi M, Taheri A, Abbasi A (2010) A facile synthesis of chromeno[3,4-c]spiropyrrolidine-oxindoles via 1,3-dipolar cycloadditions. Tetrahedron 66:6744–6748. doi:10.1016/j.tet.2010.06.078
Liu H, Dou G, Shi D (2010) Regioselective synthesis of novel spiropyrrolidines and spirothiapyrrolizidines through multicomponent 1,3-dipolar cycloaddition reaction of azomethine ylides. J Comb Chem 12:633–637. doi:10.1021/cc100035q
Rao JNS, Raghunathan R (2012) An expedient diastereoselective synthesis of pyrrolidinyl spirooxindoles fused to sugar lactone via [3+2] cycloaddition of azomethine ylides. Tetrahedron Lett 53:854–858. doi:10.1016/j.tetlet.2011.12.025
Shanmugam P, Viswambharan B, Madhavan S (2007) Synthesis of novel functionalized 3-spiropyrrolizidine and 3-spiropyrrolidine oxindoles from baylis-hillman adducts of isatin and heteroaldehydes with azomethine ylides via [3+2]-cycloaddition. Org Lett 9:4095–4098. doi:10.1021/ol701533d
Chen H, Wang S, Xu X, Ji SJ (2011) Facile three-component synthesis of spirooxindolepyrrololine ring systems via 1,3-dipolar cycloaddition with 1,4-naphthoquinone. Synth Commun 41:3280–3288. doi:10.1080/00397911.2010.517413
Bhaskar G, Arun Y, Balachandran C, Paramasivan CS, Perumal T (2012) Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur J Med Chem 51:79–91. doi:10.1016/j.ejmech.2012.02.024
Taghizadeh MJ, Arvinnezhad H, Samadi S, Jadidi K, Javidan A, Notash B (2012) Synthesis of new enantiomerically pure spirooxindolopyrrolizidines via a three-component asymmetric 1,3-dipolar cycloaddition reaction of azomethine ylides derived from isatin. Tetrahedron Lett 53:5148–5150. doi:10.1016/j.tetlet.2012.07.066
Lakshmi NV, Thirumurugan P, Jayakumar C, Paramasivan T (2010) An easy access to novel spiro-fused pyrrolo benzo[b]thiophene 1,1-dioxide derivatives via 1,3-dipolar cycloaddition using benzo[b]thiophene 1,1-dioxide. Synlett 6:955–961. doi:10.1055/s-0029-1219550
Lakshmi NV, Thirumurugan P, Perumal PT (2010) An expedient approach for the synthesis of dispiropyrrolidine bisoxindoles, spiropyrrolidine oxindoles and spiroindane-1,3-diones through 1,3-dipolar cycloaddition reactions. Tetrahedron Lett 51:1064–1068. doi:10.1016/j.tetlet.2009.12.079
Jain AK, Bhati DS (2011) Direct construction of novel dispiro heterocycles through 1,3-dipolar cycloaddition of azomethine ylides. Tetrahedron Lett 52:5333–5337. doi:10.1016/j.tetlet.2011.08.014
Jayashankaran J, Manian DRS, Raghunathan R (2004) A facile synthesis of novel dispiroheterocycles through solvent-free microwave-assisted [3+2] cycloaddition of azomethine ylides. Tetrahedron Lett 45:7303–7305. doi:10.1016/j.tetlet.2004.08.015
Babu S, Raghunathan R (2007) Ultrasonic assisted-silica mediated [3+2] cycloaddition of azomethine ylides - a facile multicomponent one-pot synthesis of novel dispiroheterocycles. Tetrahedron Lett 48:6809–6813. doi:10.1016/j.tetlet.2007.07.085
Jain R, Sharma K, Kumar D (2012) Ionic liquid mediated 1,3-dipolar cycloaddition of azomethine ylides: a facile and green synthesis of novel dispiro heterocycles. Tetrahedron Lett 53:1993–1997. doi:10.1016/j.tetlet.2012.02.029
Maheswari SU, Balamurugan K, Perumal S, Yogeeswari P, Sriram D (2010) A facile 1,3-dipolar cycloaddition of azomethine ylides to 2-arylidene-1,3-indanediones: synthesis of dispiro-oxindolylpyrrolothiazoles and their antimycobacterial evaluation. Bioorg Med Chem Lett 20:7278–7282. doi:10.1016/j.bmcl.2010.10.080
El-Ahl Abdel-Aziz S (2002) Three-component 1,3-dipolar cycloaddition reactions in synthesis of spiro[pyrrolidine-2,30-oxindoline] derivatives. Heteroat Chem 13:324–329. doi:10.1002/hc.10038
Raj AA, Raghunathan R (2001) A novel entry into a new class of spiroheterocyclic framework: regioselective synthesis of dispiro[oxindole-cyclohexanone]pyrrolidines and dispiro[oxindole-hexahydroindazole]pyrrolidines. Tetrahedron 57:10293–10298. doi:10.1016/S0040-4020(01)01042-0
Girgis AS (2009) Regioselective synthesis and stereochemical structure of anti-tumor active dispiro[3H-indole-3,2\(^{\prime }\)-pyrrolidine-3\(^{\prime },3^{\prime \prime }\)-piperidine]-2(1H), 4\(^{\prime \prime }\)-diones. Eur J Med Chem 44:1257–1264. doi:10.1016/j.ejmech.2008.09.007
Hazra A, Paira P, Sahu KB, Naskar S, Saha P, Paira R, Mondal S, Maity A, Luger P, Weber M, Mondal NB, Banerjee S (2010) Chemistry of andrographolide: formation of novel di-spiropyrrolidino and di-spiropyrrolizidino-oxindole adducts via one-pot three-component [3+2] azomethine ylide cycloaddition. Tetrahedron Lett 51:1585–1588. doi:10.1016/j.tetlet.2010.01.052
Babu SR, Raghunathan R (2008) An easy access to novel steroidal dispiropyrrolidines through 1,3-dipolar cycloaddition of azomethine ylides. Tetrahedron Lett 49:4618–4620. doi:10.1016/j.tetlet.2008.05.089
Bharitkar YP, Kanhar S, Suneel N, Mondal SK, Hazra A, Mondal NB (2015) Chemistry of withaferin-A: chemo, regio, and stereoselective synthesis of novel spiro-pyrrolizidino-oxindole adducts of withaferin-A via one-pot three-component [3+2] azomethine ylide cycloaddition and their cytotoxicity evaluation. Mol Divers 19:251–261. doi:10.1007/s11030-015-9574-6
Poornachandran M, Raghunathan R (2006) Synthesis of dispirooxindolecycloalka[d]pyrimidino[2,3-b]-thiazole pyrrolidine/thiapyrrolizidine ring systems. Tetrahedron 62:11274–11281. doi:10.1016/j.tet.2006.09.008
Murugan R, Anbazhagan S, Narayanan SS (2009) Synthesis and in vivo antidiabetic activity of novel dispiropyrrolidines through [3+2] cycloaddition reactions with thiazolidinedione and rhodanine derivatives. Eur J Med Chem 44:3272–3279. doi:10.1016/j.ejmech.2009.03.035
Liu H, Zou Y, Hu Y, Shi DQ (2011) An efficient one-pot synthesis of dispiropyrrolidine derivatives through 1,3-dipolar cycloaddition reactions under ultrasound irradiation. J Heterocycl Chem 48:877–881. doi:10.1002/jhet.654
Hu Y, Zou Y, Wu H, Shi D (2012) A facile and efficient ultrasound-assisted synthesis of novel dispiroheterocycles through 1,3-dipolar cycloaddition reactions. Ultrason Sonochem 19:264–269. doi:10.1016/j.ultsonch.2011.07.006
Mamari KA, Ennajih H, Zouihri H, Bouhfid R, Ng SW, Essassi EM (2012) Synthesis of novel dispiro-oxindoles via 1,3-dipolar cycloaddition reactions of azomethine ylides. Tetrahedron Lett 53:2328–2331. doi:10.1016/j.tetlet.2012.02.097
Dalpozzo R, Bartoli G, Bencivenni G (2012) Recent advances in organocatalytic methods for the synthesis of disubstituted 2- and 3-indolinones. Chem Soc Rev 41:7247–7290. doi:10.1039/C2CS35100E
Tan B, Zeng X, Leong WWY, Shi Barbas III CF, Zhong G (2012) Core structure-based design of organocatalytic [3+2]-cycloaddition reactions: highly efficient and stereocontrolled syntheses of 3,3\(^{\prime }\)-pyrrolidonyl spirooxindoles. Chem Eur J 18:63–67. doi:10.1002/chem.201103449
Cao Y, Jiang X, Liu L, Shen FF, Zhang F, Wang R (2011) Enantioselective michael/cyclization reaction sequence: scaffold-inspired synthesis of spirooxindoles with multiple stereocenters. Angew Chem 123:9290–9293. doi:10.1002/ange.201104216
Hande SM, Nakajima M, Kamisaki H, Tsukano C, Takemoto Y (2011) Flexible strategy for syntheses of spirooxindoles using palladium- catalyzed carbosilylation and sakurai-type cyclization. Org Lett 13:1828–1831. doi:10.1021/ol2003447
Alcaide B, Almendros P, Rodriguez-Acebes R (2006) Efficient entry to diversely functionalized spirocyclic oxindoles from isatins through carbonyl-addition/cyclization reaction sequences. J Org Chem 71:2346–2351. doi:10.1021/jo0525027
Du D, Hu Z, Jin J, Lu Y, Tang W, Wang B, Lu T (2012) N-Heterocyclic carbene-catalyzed three-component domino reaction of alkynyl aldehydes with oxindoles. Org Lett 14:1274–1277. doi:10.1021/ol300148f
Castaldi MP, Troast DM, Porco JA (2009) Stereoselective synthesis of spirocyclic oxindoles via Prins cyclizations. J Org Lett 11:3362–3365. doi:10.1021/ol901201k
Zhang Y, Panek JS (2009) Stereocontrolled Synthesis of spirooxindoles through lewis acid-promoted [5+2]-annulation of chiral silyl alcohols. Org Lett 11:3366–3369. doi:10.1021/ol901202t
Wang J, Crane EA, Scheidt KA (2011) Highly stereoselective Brønsted acid catalyzed synthesis of spirooxindole pyrans. Org Lett 13:3086–3089. doi:10.1021/ol200987c
Zh L, Shi M (2012) Nitrogen- and phosphorus-containing Lewis base catalyzed [4+2] and [3+2] annulation reactions of isatins with but-3-yn-2-one. Eur J Org Chem 3:581–586. doi:10.1002/ejoc.201101338
Zhu SL, Ji SJ, Zhang Y (2007) A simple and clean procedure for three-component synthesis of spirooxindoles in aqueous medium. Tetrahedron 63:9365–9372. doi:10.1016/j.tet.2007.06.113
Chen WB, Wu ZJ, Pei QL, Cun LF, Zhang XM, Yuan WC (2010) Highly enantioselective construction of spiro[4h-pyran-3,3’-oxindoles] through a domino Knoevenagel/Michael/cyclization sequence catalyzed by cupreine. Org Lett 12:3132–3135. doi:10.1021/ol1009224
Shishkina SV, Shishkin OV, Redkin RG, Shemchuk LA, Chernykh VP (2007) Crystal structure of 3,4-spiro-[(5-acetyl-2-amino-3-carbethoxy-6-methyl-4H-pyrano)-1H,3H-indol-2-on]. Acta Cryst 63:3193–3196. doi:10.1107/S1600536807027547
Shemchuk LA, Chernykh VP, Redkin RG (2008) Synthesis of fused 2\(^{\prime }\)-amino-3\(^{\prime }\)-R-spiro-[indole-3,4\(^{\prime }\)-pyran]-2(1H)-ones. Russ J Org Chem 44:1789–1791. doi:10.1134/S1070428008120117
Litvinov YM, Mortikov VY, Shestopalov AM (2008) Versatile three-component procedure for combinatorial synthesis of 2-aminospiro[(3\(^{\prime }\)h)-indol-3\(^{\prime }\),4-(4h)-pyrans]. J Comb Chem 10:741–745. doi:10.1021/cc800093q
Redkin RG, Shemchuk LA, Chernykh VP, Shishkin OV, Shishkina SV (2007) Synthesis and molecular structure of spirocyclic 2-oxindole derivatives of 2-amino-4H-pyran condensed with the pyrazolic nucleus. Tetrahedron 63:11444. doi:10.1016/j.tet.2007.08.050
Heravi MH, Zakeri M, Moharami A (2012) Versatile three-component procedure for combinatorial synthesis of spiro-oxindoles with fused chromenes catalysed by L-proline. J Chem Sci 124:865–869. doi:10.1007/s12039-012-0284-7
Wang L-M, Jiao N, Qiu J, Yu J-J, Liu J-Q, Guo F-L, Liu Y (2010) Sodium stearate-catalyzed multicomponent reactions for efficient synthesis of spirooxindoles in aqueous micellar media. Tetrahedron 66:339–343. doi:10.1016/j.tet.2009.10.091
Rad-Moghadam K, Youseftabar-Miri L (2011) Ambient synthesis of spiro[4H-pyran-oxindole] derivatives under [BMIm]BF\(_{4}\) catalysis. Tetrahedron 67:5693–5699. doi:10.1016/j.tet.2011.05.077
Mobinikhaledi A, Foroughifar N, Fard MAB (2011) Simple and efficient method for three-component synthesis of spirooxindoles in aqueous and solvent-free media. Synth Commun: Int J Rap Commun Synth Org Chem 41:441–450. doi:10.1080/00397911003587507
Zhao L-Q, Zhou B, Li Y-Q (2011) An efficient one-pot three-component reaction for synthesis of spirooxindole derivatives in water media under catalyst-free condition. Heteroat Chem 22:673–677. doi:10.1002/hc.20723
Elinson MN, Ilovaisky AI, Merkulova VM, Zaimovskaya TA, Nikishin GI (2012) Non-catalytic thermal multicomponent assembling of isatin, cyclic CH-acids and malononitrile: an efficient approach to spirooxindole scaffold. Mendeleev Commun 22:143–144. doi:10.1016/j.mencom.2012.05.010
Hasaninejada A, Golzar N, Beyrati M, Zare A, Doroodmand MM (2013) Silica-bonded 5-n-propyl-octahydro-pyrimido[1,2-a]azepinium chloride (SB-DBU)Cl as a highly efficient, heterogeneous and recyclable silica-supported ionic liquid catalyst for the synthesis of benzo[b]pyran, bis(benzo[b]pyran) and spiro-pyran derivatives. J Mol Catal A: Chem 372:137–150. doi:10.1016/j.molcata.2013.02.022
Liu Y, Ren Z, Cao W, Chen J, Deng H, Shao M (2011) Solvent-free one-pot synthesis of spiro[indoline-3,4\(^{\prime }(1\text{ H }^{\prime })\)-pyrano[2,3-c]pyrazol]-2-one derivatives by grinding. Synth Commun 41:3620–3626. doi:10.1080/00397911.2010.519449
Zou Y, Hu Y, Liu H, Shi D (2012) Rapid and efficient ultrasound-assisted method for the combinatorial synthesis of spiro[indoline-3,4\(^{\prime }\)-pyrano[2,3-c]pyrazole] derivatives. ACS Comb Sci 14:38–43. doi:10.1021/co200128k
Ukrainets IV, Redkin RG, Sidorenko LV, Turov AV (2009) 4-Hydroxy-2-quinolones 172. Synthesis and structure of 4,3\(^\prime \)-spiro[(6-allyl-2-amino-5-oxo-5,6-dihydro-4h-pyrano-[3,2-c]quinoline-3-carbo-nitrile)-2\(^\prime \)-oxindole]. Chem Heterocycl Comp 45:1478–1484. doi:10.1007/s10593-010-0454-9
Ghahremanzadeh R, Amanpour T, Bazgir A (2009) An efficient, three-component synthesis of spiro[benzo[g]chromene-4,3\(^{\prime }\)-indoline]-3-carbonitrile and spiro[indoline-3,5\(^{\prime }\)-pyrano[2,3-d]pyrimidine]-6\(^{\prime }\)-carbonitrile derivatives. J Heterocycl Chem 46:1266–1270. doi:10.1002/jhet.240
Zhao H, Lan Y-B, Liu Z-M, Wang Y, Wang X-W, Tao J-C (2012) Enantioselective construction of spiro[2H-pyran-3,4’-indoline] by a systematic Michael/Reduction/Cyclization sequence triggered by the asymmetric conjugate addition of ketones to isatylidenemalononitriles. Eur J Org Chem 10:1935–1944. doi:10.1002/ejoc.201101810
Liang B, Kalidindi S, Porco JA, Stephenson CRJ (2010) Multicomponent reaction discovery: three-component synthesis of spirooxindoles. Org Lett 12:572–575. doi:10.1021/ol902764k
Ghahremanzadeh R, Fereshtehnejad F, Yasaei Z, Amanpour T, Bazgir A (2010) One-pot and three-component synthesis of spiro[chromeno[2,3-d]pyrimidine-5,30-indoline]-diones and spiro[chromeno[2,3-c]pyrazole-4,30-indoline]-diones. J Heterocycl Chem 47:967–972. doi:10.1002/jhet.399
Jadidi K, Ghahremanzadeh R, Bazgir A (2009) Efficient synthesis of spiro[chromeno[2,3-d]pyrimidine-5,3\(^{\prime }\)-indoline]-tetraones by a one-pot and three-component reaction. J Comb Chem 11:341–344. doi:10.1021/cc800167h
Deng J, Mo L-P, Zhao F-Y, Zhang Z-H, Liu S-X (2012) One-pot, three-component synthesis of a library of spirooxindole-pyrimidines catalyzed by magnetic nanoparticle supported dodecyl benzenesulfonic acid in aqueous media. ACS Comb Sci 14:335–341. doi:10.1021/co3000264
Moghaddam MM, Bazgir A, Mehdi AM, Ghahremanzadeh R (2012) Alum (KAl\(({\rm SO}_{4})_{2}\cdot 12{\rm H}_{2}{\rm O}\)) catalyzed multicomponent transformation: simple, efficient, and green route to synthesis of functionalized spiro[chromeno[2,3-d]pyrimidine-5,3\(^{\prime }\)-indoline]-tetraones in ionic liquid media. Chinese J Chem 30:709–714. doi:10.1002/cjoc.201280014
Ghahremanzadeh R, Amanpour T, Sayyafi M, Bazgir A (2010) One-pot, three-component synthesis of spironaphthopyrano[2,3-d]pyrimidine-5,3\(^{\prime }\)-indolines in water. J Heterocycl Chem 47:421–424. doi:10.1002/jhet.331
Tisseh ZN, Ahmadi F, Dabiri M, Khavasi HR, Bazgir A (2012) A novel organocatalytic multi-component reaction: an efficient synthesis of polysubstituted pyrano-fused spirooxin. Tetrahedron Lett 53:3603–3606. doi:10.1016/j.tetlet.2012.05.019
Bondensgaard K, Ankersen M, Thogersen H, Hansen BS, Wulff BS et al (2004) Recognition of privileged structures by g-protein coupled receptors. J Med Chem 47:888–899. doi:10.1021/jm0309452
Patchett AA, Nargund RP, Tata JR, Chen MH, Barakat KJ, Johnston DB, Cheng K, Chan WW, Butler B, Hickey G (1995) Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. PNAS 92:7001–7005. doi:10.1073/pnas.92.15.7001
Hickey G, Jacks T, Judith F, Taylor J, Schoen WR, Krupa D, Cunningham P, Clark J, Smith RG (1994) Efficacy and specificity of L-692,429, a novel nonpeptidyl growth hormone secretagogue, in beagles. Endocrinology 134:695–701. doi:10.1210/en.134.2.695
Sluder A, Shah S, Cassayre J, Clover R, Maienfisch P et al (2012) Spiroindolines identify the vesicular acetylcholine transporter as a novel target for insecticide action. PLoS One 7:e34712. doi:10.1371/journal.pone.0034712
Inoue S, Okada K, Tanino H, Hashizume K, Kakoi H (1994) Total synthesis of (\(\pm \))-surugatoxin. Heterocycl 50:2729–2752. doi:10.1016/S0040-4020(01)86989-1
Inoue S, Okada K, Tanino H, Kakoi H (1988) Synthesis of (+)-prosurugatoxin and ring transformation of prosurugatoxin into surugatoxin. Tetrahedron Lett 29:1547–1550. doi:10.1016/S0040-4039(00)80348-2
Seo JH, Liu P, Weinreb SM (2010) Evolution of a strategy for total synthesis of the marine fungal alkaloid \((\pm )\)-communesin F. J Org Chem 75:2667–2680. doi:10.1021/jo100339k
Lesma G, Landoni N, Sacchetti A, Silvani A (2010) The spiropiperidine-3,3\(^\prime \)-oxindoles caffold: a type II \({\beta }\)-turn peptideisostere. Tetrahedron 66:4474–4478. doi:10.1016/j.tet.2010.04.077
Han Y-Y, Han W-Y, Hou X, Zhang X-M, Yuan W-C (2012) \(\text{ FeCl }_{3}\)-catalyzed stereoselective construction of spirooxindole tetrahydroquinolines via tandem 1,5-hydride transfer/ring closure. Org Lett 14:4054–4057. doi:10.1021/ol301559k
Kiruthika SE, Lakshmi NV, Banu BR, Perumal PT (2011) A facile strategy for the one pot multicomponent synthesis of spiro dihydropyridines from amines and activated alkynes. Tetrahedron Lett 52:6508–6511. doi:10.1016/j.tetlet.2011.09.119
Alizadeh A, Mokhtari J (2011) Novel four-component route to the synthesis of spiro[indoline-3,4\(^{\prime }\)-pyridine]-3\(^{\prime }\)-carboxylate derivatives. Tetrahedron 67:3519–3523. doi:10.1016/j.tet.2011.03.032
Ghahremanzadeh R, Ahadi S, Shakibaei GI, Bazgir A (2010) Grindstone chemistry: one-pot synthesis of spiro[diindenopyridine-indoline]triones and spiro[acenaphthylene-diindenopyridine]triones. Tetrahedron Lett 51:499–502. doi:10.1016/j.tetlet.2009.11.041
Manpadi M et al (2007) Three-component synthesis and anticancer evaluation of polycyclic indenopyridines lead to the discovery of a novel indenoheterocycle with potent apoptosis inducing properties. Org Biomol Chem 5:3865–3872. doi:10.1039/B713820B
Ahadi S, Ghahremanzadeh R, Mirzaei P, Bazgir A (2009) Synthesis of spiro[benzopyrazolonaphthyridine-indoline]-diones and spiro[chromenopyrazolopyridine-indoline]-diones by one-pot, three-component methods in water. Tetrahedron 65:9316–9321. doi:10.1016/j.tet.2009.09.009
Quiroga J, Portillo S, Pérez A, Gálvez J, Abonia R, Insuasty B (2011) An efficient synthesis of pyrazolo[3,4-b]pyridine-4-spiroindolinones by a three-component reaction of 5-aminopyrazoles, isatin, and cyclic \({\beta }\)-diketones. Tetrahedron Lett 52:2664–2666. doi:10.1016/j.tetlet.2011.03.067
Ghahremanzadeh R, Sayyafi M, Ahadi S, Bazgir A (2009) Novel one-pot, three-component synthesis of spiro[indoline-pyrazolo[4\(^{\prime }\),3\(^{\prime }\):5,6]pyrido[2,3-d]pyrimidine]trione library. J Comb Chem 11:393–396. doi:10.1021/cc8001958
Shakibaei GI, Feiz A, Bazgir A (2011) A simple and catalyst-free three-component method for the synthesis of spiro[indenopyrazolopyridine indoline]diones and spiro[indenopyridopyrimidine indoline]triones. Comptes Rendus Chimie 14:556–562. doi:10.1016/j.crci.2010.10.001
Jadidi K, Ghahremanzadeh R, Bazgir A (2009) Spirooxindoles: reaction of 2,6-diaminopyrimidin-4(3H)-one and isatins. Tetrahedron 65:2005–2009. doi:10.1016/j.tet.2009.01.013
Shakibaei GI, Feiz A, Khavasi HR, Soorki AA, Bazgir A (2011) Simple three-component method for the synthesis of spiroindeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3\(^\prime \)-indolines. ACS Comb Sci 13:96–99. doi:10.1021/co1000053
Rahmati A, Khalesi Z (2012) A one-pot, three-component synthesis of spiro[indolineisoxazolo[4\(^{\prime }\),3\(^{\prime }\):5,6]pyrido[2,3-d]pyrimidine]triones in water. Tetrahedron 68:8472–8479. doi:10.1016/j.tet.2012.07.073
Venkatesan H, Davis MC, Altas Y, Snyder JP, Liotta DC (2001) Total synthesis of SR 121463 A, a highly potent and selective vasopressin \(\text{ V }_{2}\) receptor antagonist. J Org Chem 66:3653–3661. doi:10.1021/jo0004658
Liu J-J, Zhang Z (2008) Spiroindolinone derivatives (Hoffmann-La Roche AG). PCT Int Appl. WO2008(055812)
Sconhaber J, Mueller ThJJ (2011) Luminescent bichromophoric spiroindolones—synthesis and electronic properties. Org Biomol Chem 9:6196–6199. doi:10.1039/c1ob05703k
Beccalli EM, Clerici F, Gelmi ML (1999) 6-Chloro-spiroeyelohexenindol-2-ones: an unusual ring transformation to ethyl 2-(cyelohexa-1,4-dlenyl)phenylearbamates. Tetrahedron 55:8579–8586. doi:10.1016/S0040-4020(99)00475-5
Beccalli EM, Clerici F, Gelmi ML (2003) A new synthetic procedure to spiro[cyclohexane-1,3\(^{\prime }\)-indoline]-2\(^{\prime }\),4-diones. Tetrahedron 59:4615–4622. doi:10.1016/S0040-4020(03)00627-6
Ashimori’ A, Overman LE (1992) Catalytic asymmetric synthesis of quarternary carbon centers. Palladium-catalyzed formation of either enantiomer of spirooxindoles and related spirocyclics using a single enantiomer of a chiral diphosphine ligand. J Org Chem 57(4571–4572):4671. doi:10.1021/jo00043a005
Jia ZJ, Jiang H, Li JL, Gschwend B, Li QZ, Yin X, Grouleff J, Chen YC, Jørgensen KA (2011) Trienamines in asymmetric organocatalysis: Diels-Alder and tandem reactions. J Am Chem Soc 133:5053–5061. doi:10.1021/ja1112194
Tan B, Hernández-Torres G, Barbas CF (2011) Highly efficient hydrogen-bonding catalysis of the Diels-Alder reaction of 3-vinylindoles and methyleneindolinones provides carbazolespirooxindole skeletons. J Am Chem Soc 133:12354–12357. doi:10.1021/ja203812h
Wei Q, Gong L-Z (2010) Organocatalytic asymmetric formal [4+2] cycloaddition for the synthesis of spiro[4-cyclohexanone-1,3\(^{\prime }\)-oxindoline] derivatives in high optical purity. Org Lett 12:1008–1011. doi:10.1021/ol100020v
Li Y, Su X, Zhou W, Li W, Zhang J (2015) Amino-acid derived phosphine-catalyzed enantioselective 1,4- dipolar spiroannulation of cyclobutenones and isatylidenemalononitrile. Chem Eur J 21:1–6. doi:10.1002/chem.201406475
Richmond E, Duguet N, Slawin AMZ, Lébl T, Smith AD (2012) Asymmetric pericyclic cascade approach to spirocyclic oxindoles. Org Lett 14:2762–2765. doi:10.1021/ol300982f
Bencivenni G, Wu LY, Mazzanti A, Giannichi B, Pesciaioli F, Song MP, Bartoli G, Melchiorre P (2009) Targeting structural and stereochemical complexity by organocascade catalysis: construction of spirocyclic oxindoles having multiple stereocenters. Angew Chem Int Ed 48:7200–7203. doi:10.1002/anie.200903192
Jiang K, Jia Z-J, Yin X, Wu L, Chen Y-C (2010) Asymmetric quadruple aminocatalytic domino reactions to fused carbocycles incorporating a spirooxindole motif. Org Lett 12:2766–2769. doi:10.1021/ol100857s
Jiang K, Jia Z-J, Chen S, Li Wu, Chen Y-C (2010) Organocatalytic tandem reaction to construct six-membered spirocyclic oxindoles with multiple chiral centres through a formal [2+2+2] annulation. Chem Eur J 16:2852–2856. doi:10.1002/chem.200903009
Ghosh AK, Zhou B (2013) Enantioselective synthesis of spiro[cyclohexane-1,3\(^\prime \)-indolin]-2\(^\prime \)-ones containing multiple stereocenters via organocatalytic Michael/aldol cascade reactions. Tetrahedron Lett 54:2311–2314. doi:10.1016/j.tetlet.2013.02.030
Companyo X, Zea A, Alba A-NR, Mazzanti A, Moyano A, Rios R (2010) Organocatalytic synthesis of spiro compounds via a cascade Michael-Michael-aldol reaction. Chem Commun 46:6953–6955. doi:10.1039/C0CC01522A
Lan YB, Zhao H, Liu ZM, Liu GG, Tao JC, Wang XW (2011) Chiral counteranion synergistic organocatalysis under high temperature: efficient construction of optically pure spiro[cyclohexanone-oxindole] backbone. Org Lett 13:4866–4869. doi:10.1021/ol201943g
Sun QS, Chen X-Y, Zhu H, Lin H, Sun X-W, Lin G-Q (2015) Asymmetric synthesis of poly-substituted spirocyclohexane oxindole via a squaramide catalyzed cascade Michael-Michael-aldol sequence. Org Chem Front 2:110–113. doi:10.1039/C4QO00299G
Basavaiah D, Reddy KR (2007) Simple and one-pot protocol for synthesis of indene-spiro-oxindoles involving tandem prins and friedel-crafts reactions. Org Lett 9:57–60. doi:10.1021/ol062561m
Gorokhovik I, Neuville L, Zhu J (2011) Trifluoroacetic acid-promoted synthesis of 3-hydroxy, 3-amino and spirooxindoles from \({\alpha }\)-keto-N-anilides. Org Lett 13:5536–5539. doi:10.1021/ol202263a
Bond RF, Boeyens JCA, Holzapfel CW, Steyn PS (1979) Cyclopiamines A and B, novel oxindole metabolites of Penicillium cyclopium westling. J Chem Soc, Perkin Trans 1:1751–1761. doi:10.1039/P19790001751
Mugishima T, Tsuda M, Kasai Y, Ishiyama H, Fukushi E, Kawabata J, Watanabe M, Akao K, Kobayashi J (2005) Absolute stereochemistry of citrinadins A and B from marine-derived fungus. J Org Chem 70:9430–9435. doi:10.1021/jo051499o
Pettersson M, Knueppel D, Martin SF (2007) Concise, stereoselective approach to the spirooxindole ring system of citrinadin A. Org Lett 9:4623–4626. doi:10.1021/ol702132v
Bian Z, Marvin CC, Martin SF (2013) Enantioselective total synthesis of (-)-citrinadin A and revision of its stereochemical structure. J Am Chem Soc 135:10886–10889. doi:10.1021/ja405547f
Guerrero CA, Sorensen EJ (2011) Concise, stereocontrolled synthesis of the citrinadin B core architecture. Org Lett 13:5164–5167. doi:10.1021/ol2020362
Kong K, Enquist JA Jr, McCallum ME, Smith GM, Matsumaru T, Menhaji-Klotz E, Wood JL (2013) An enantioselective total synthesis and stereochemical revision of (+)-citrinadin B. J Am Chem Soc 135:10890–10893. doi:10.1021/ja405548b
Li X, Li YM, Peng FZ, Wu ST, Li ZQ, Sun ZW, Zhang HB, Shao ZH (2011) Highly enantioselective one-pot synthesis of spirocyclopentaneoxindoles containing the oxime group by organocatalyzed Michael addition/ISOC/fragmentation sequence. Org Lett 13:6160–6163. doi:10.1021/ol2024955
Albertshofer K, Tan B, Barbas CF (2012) Assembly of spirooxindole derivatives containing four consecutive stereocenters via organocatalytic Michael-Henry cascade reactions. Org Lett 14:1834–1837. doi:10.1021/ol300441z
Albertshofer K, Anderson KE, Barbas CF (2012) Assembly of spirooxindole derivatives via organocatalytic iminium-enamine cascade reactions. Org Lett 14:5968–5971. doi:10.1021/ol302876c
Jiang K, Tiwari B, Chi YR (2012) Access to spirocyclic oxindoles via N-heterocyclic carbene-catalyzed reactions of enals and oxindole-derived \({\alpha },{\beta }\)-unsaturated imines. Org Lett 14:2382–2385. doi:10.1021/ol3008028
Sun W, Zhu G, Wu C, Hong L, Wang R (2012) An organocatalytic cascade strategy for the enantioselective construction of spirocyclopentane bioxindoles containing three contiguous stereocenters and two spiro quaternary centers. Chem Eur J 18:6737–6741. doi:10.1002/chem.201200478
Sun W, Zhu G, Wu C, Hong L, Wang R (2012) “Organo-metal” synergistic catalysis: the 1+1\(>\)2 effect for the construction of spirocyclopentene oxindoles. Chem Eur J 18:13959–13963. doi:10.1002/chem.201201976
Tian X, Melchiorre P (2013) Control of remote stereochemistry in the synthesis of spirocyclic oxindoles: vinylogous organocascade catalysis. Angew Chem Int Ed 52:1–5. doi:10.1002/anie.201301017
Ding L-Z, Zhong T-S, Wu T, Wang Y-M (2014) Highly enantioselective construction of spirocyclopentaneoxindoles containing four consecutive stereocenters through an organocatalytic iminium–enamine cascade reaction. Eur J Org Chem 24:5139–5143. doi:10.1002/ejoc.201402687
Trost BM, Cramer N, Silverman SM (2007) Enantioselective construction of spirocyclic oxindolic cyclopentanes by palladium-catalyzed trimethylenemethane-[3+2]-cycloaddition. J Am Chem Soc 129:12396–12397. doi:10.1021/ja075335w
Voituriez A, Pinto N, Neel M, Retailleau P, Marinetti A (2010) An organocatalytic [3+2] cyclisation strategy for the highly enantioselective synthesis of spirooxindoles. Chem Eur J 16:12541–12544. doi:10.1002/chem.201001791
Tan B, Candeias NR, Barbas CF (2011) Core-structure-motivated design of a phosphine-catalyzed [3+2] cycloaddition reaction: enantioselective syntheses of spirocyclopenteneoxindoles. J Am Chem Soc 133:4672–4675. doi:10.1021/ja110147w
Peng J, Huang X, Jiang L, Cui H-L, Chen Y-C (2011) Tertiary amine-catalyzed chemoselective and asymmetric [3+2] annulation of Morita-Baylis-Hillman carbonates of isatins with propargyl sulfones. Org Lett 13:4584–4587. doi:10.1021/ol201776h
Wang Y, Liu L, Zhang T, Zhong N-J, Wang D, Chen Y-J (2012) Diastereo- and enantioselective [3+2] cycloaddition reaction of Morita-Baylis-Hillman carbonates of isatins with n-phenylmaleimide catalyzed by Me-Duphos. J Org Chem 77:4143–4147. doi:10.1021/jo3002535
Jiang T, Kuhen KL, Wolff K, Yin H, Bieza K, Caldwell J, Bursulaya B, Wu TYH, He Y (2006) Design, synthesis and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part I. Bioorg Med Chem Lett 16:2105–2108. doi:10.1016/j.bmcl.2006.01.073
Jiang T, Kuhen KL, Wolff K, Yin H, Bieza K, Caldwell J, Bursulaya B, Wu TYH, Tuntland T, Zhang K, Karanewsky D, He Y (2006) Design, synthesis and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part II. Bioorg Med Chem Lett 16:2109–2112. doi:10.1016/j.bmcl.2006.01.066
Dou X, Lu Y (2012) Diastereodivergent synthesis of 3-spirocyclopropyl-2-oxindoles through direct enantioselective cyclopropanation of oxindoles. Chem Eur J 18:8315–8319. doi:10.1002/chem.201200655
Shaabanzadeh M, Khabari F (2010) One-pot synthesis of new spiro[cyclopropane-1,3\(^{\prime }\)-[3H]indol]-2\(^{\prime }\)(1\(^{\prime }\)H)-ones from 3-phenacylideneoxindoles. J Heterocycl Chem 47:949–953. doi:10.1002/jhet.394
Kumari G, Nutan Modi M, Gupta SK, Singh RK (2011) Rhodium(II) acetate-catalyzed stereoselective synthesis, SAR and anti-HIV activity of novel oxindoles bearing cyclopropane ring. Eur J Med Chem 46:1181–1188. doi:10.1016/j.ejmech.2011.01.037
Muthusamy S, Azhagan D, Gnanaprakasam B, Suresh E (2010) Diastereoselective synthesis of strained spiro-cyclopropanooxindoles from cyclic diazoamides. Tetrahedron Lett 51:5662–5665. doi:10.1016/j.tetlet.2010.07.159
Muthusamy S, Ramkumar R (2014) Solvent- and transition metal-free synthesis of spiro[cyclopropane-1,3- oxindoles] from cyclic diazoamides. Tetrahedron Lett 55:6389–6393. doi:10.1016/j.tetlet.2014.09.086
Oseka M, Noole A, Zari S, Öeren M, Järving I, Lopp M, Kanger T (2014) Asymmetric diastereoselective synthesis of spirocyclopropane derivatives of oxindole. Eur J Org Chem 17:3599–3606. doi:10.1002/ejoc.201402061
Pedras MSC, Okanga FI, Zaharia IL, Khan AQ (2000) Phytoalexins from crucifers: synthesis, biosynthesis, and Biotransformation. Phytochem 53:161–176. doi:10.1016/S0031-9422(99)00494-X
Kutschy P, Salayová A, Curillová Z, Kozár T, Mezencev R, Mojzis J, Pilátová M, Balentová E, Pazdera P, Sabol M, Zburová M (2009) 2-(Substituted phenyl)amino analogs of 1-methoxyspirobrassinol methyl ether: synthesis and anticancer activity. Bioorg Med Chem 17:3698–3712. doi:10.1016/j.bmc.2009.03.064
Kutschy P, Suchý M, Monde K, Harada N, Marušková R, Čurillová Z, Dzurilla M, Miklošová M, Mezencev R, Mojžiš J (2002) Spirocyclization strategy toward indole phytoalexins. The first synthesis of \((\pm )\)-1-methoxyspirobrassinin, \((\pm )\)-1-methoxyspirobrassinol, and \((\pm )\)-1-methoxyspirobrassinol methyl ether. Tetrahedron Lett 43:9489–9492. doi:10.1016/S0040-4039(02)02452-8
Zhang Y, Li ZJ, Xu HS, Zhang Y, Wang W (2011) Organocatalytic asymmetric Henry reaction of isatins: highly enantioselective synthesis of 3-hydroxy-2-oxindoles. RSC Adv 1:389–392. doi:10.1039/C1RA00477H
Chen W-B, Wu Z-J, Hu J, Cun J-F, Zhang X-M, Yuan W-C (2011) Organocatalytic direct asymmetric aldol reactions of 3-isothiocyanato oxindoles to ketones: stereocontrolled synthesis of spirooxindoles bearing highly congested contiguous tetrasubstituted stereocenters. Org Lett 13:2472–2475. doi:10.1021/ol200724q
Han Y-Y, Chen W-B, Han W-Y, Wu Z-J, Zhang X-M, Yuan W-C (2012) Highly efficient and stereoselective construction of dispiro-[oxazolidine-2-thione]bisoxindoles and dispiro[imidazolidine-2-thione]bisoxindoles. Org Lett 14:490–493. doi:10.1021/ol203081x
Jiang X, Cao Y, Wang Y, Liu L, Shen F, Wang R (2010) A unique approach to the concise synthesis of highly optically active spirooxazolines and the discovery of a more potent oxindole-type phytoalexin analogue. J Am Chem Soc 132:15328–15333. doi:10.1021/ja106349m
Badillo JJ, Arevalo GE, Fettinger JC, Franz AK (2011) Titanium-catalyzed stereoselective synthesis of spirooxindole oxazolines. Org Lett 13:418–421. doi:10.1021/ol1027305
Ueda T, Inada M, Okamoto I, Morita N, Tamura O (2008) Synthesis of maremycins a and d1 via cycloaddition of a nitrone with (e)-3-ethylidene-1-methylindolin-2-one. Org Lett 10:2043–2046. doi:10.1021/ol800515w
Singh A, Roth GP (2011) A [3+2] dipolar cycloaddition route to 3-hydroxy-3-alkyl oxindoles: an approach to pyrrolidinoindoline alkaloids. Org Lett 13:2118–2121. doi:10.1021/ol200547m
Bouhfid R, Joly N, Essassi EM, Lequart V, Massoui M, Martin P (2011) Synthesis of new spiro[1,4,2-dioxazole-5,3\(^{\prime }\)-indolin]-2\(^{\prime }\)-one by 1,3-dipolar cycloaddition. Synth Commun 41:2096–2102. doi:10.1080/00397911.2010.497595
Ribeiro CJA, Kumar SP, Moreira R, Santos MMM (2012) Efficient synthesis of spiroisoxazoline oxindoles. Tetrahedron Lett 53:281–284. doi:10.1016/j.tetlet.2011.10.139
Gomez-Monterrey I, Bertamino A, Porta A, Carotenuto A, Musella S, Aquino C, Granata I, Sala M, Brancaccio D, Picone D, Ercole C, Stiuso P, Campiglia P, Grieco P, Ianelli P, Maresca B, Novellino E (2010) Identification of the spiro(oxindole-3,3’-thiazolidine)-based derivatives as potential p53 activity modulators. J Med Chem 53(8319–8329):8319. doi:10.1021/jm100838z
Vintonyak VV, Warburg K, Kruse H, Grimme S, Hubel K, Rauh D, Waldmann H (2010) Identification of thiazolidinones spiro-fused to indolin-2-ones as potent and selective inhibitors of the mycobacterium tuberculosis protein tyrosine phosphatase B. Angew Chem Int Ed 49:5902–5905. doi:10.1002/anie.201002138
Kaminskyy D, Khyluk D, Vasylenko O, Zaprutko L, Lesyk R (2011) A facile synthesis and anticancer activity evaluation of spiro[thiazolidinone-isatin] conjugates. Sci Pharm 79:763–777. doi:10.3797/scipharm.1109-14
Chen H, Shi D (2011) Efficient one-pot synthesis of spiro[indoline-3,40-pyrazolo[3,4-e][1,4]thiazepine]dione via three-component reaction. Tetrahedron 67:5686–5692. doi:10.1016/j.tet.2011.05.069
Cao Y, Shen FF, Zhang FT, Wang R (2012) Catalytic asymmetric michael addition/cyclization of isothiocyanato oxindoles: highly efficient and versatile approach for the synthesis of 3,2\(^{\prime }\)-pyrrolidinyl mono- and bi-spirooxindole frameworks. Chem Eur J 19:1184–1188. doi:10.1002/chem.201204114
Wu H, Zhang LL, Tian ZQ, Huang YD, Wang YM (2012) Highly efficient enantioselective construct hion of bispirooxindoles containing three stereocenters through an organocatalytic cascade michael-cyclization reaction. Chem Eur J 15:1246–1249. doi:10.1002/chem.201203221
Curillova’ Z, Kutschy P, Budovska M, Nakahashib A, Mondeb K (2007) Stereoselective synthesis of (R)-(+)-1-methoxyspirobrassinin, (2R,3R)-(-)-1-methoxyspirobrassinol methyl ether and their enantiomers or diastereoisomers. Tetrahedron Lett 48:8200–8204. doi:10.1016/j.tetlet.2007.09.080
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pavlovska, T.L., Redkin, R.G., Lipson, V.V. et al. Molecular diversity of spirooxindoles. Synthesis and biological activity. Mol Divers 20, 299–344 (2016). https://doi.org/10.1007/s11030-015-9629-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9629-8