Abstract
A new type of solid-base catalyst for aldol condensation reaction was prepared by modifying commercial CaO with benzyl bromide in a simple way. It was found that modified CaO can effectively catalyze the aldol condensation of acetophenone and benzaldehyde to produce chalcone with a high conversion and good selectivity. The catalyst gave a higher yield (90.5%) of chalcone than commercial CaO. The high catalytic activity and stability of this catalyst was related to the organic modifier with a hydrophilic functional group that improved the diffusion of grease to the catalyst surface and prevented its hydration. The influence of several reaction parameters, such as temperature, catalyst loading and the moisture absorption rate of modified CaO, was investigated. From the results, the basic centers of modified CaO are stable and hardly poisoned by CO2 unlike commercial CaO. The catalyst was completely recyclable without significant loss in activity up to five reaction cycles. Moreover, this catalyst showed a promising future in providing an environmentally clean process for the industrial sector.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the fine chemical industry, the aldol condensation of acetone is an important reaction involving carbon–carbon bonds formation for synthesis of a variety of organic compounds [1–3]. This kind of reaction can take place catalytically in the presence of a strong base or acid in liquid phase [4–6]. However, high operating costs and serious environmental issues associated with product separation, purification, corrosion, and waste generation have attracted great efforts toward the development of processes mediated by heterogeneous catalysts. Solid-base catalysts constitute a class of heterogeneous catalysts that are capable of catalyzing the C–C bond-forming reactions. Different families of solid bases have been found active in this reaction, including alkali-exchanged zeolites [1], ion-exchange resins [2, 3], alumina-supported hydroxide [7, 8], Mg–Al hydrotalcites [9, 10], and alkaline-earth metal oxides [11, 12]. In particular, alkaline-earth metal oxides have been regarded as the most potential catalysts for industrial application for their strong basicity and low cost, especially for CaO. However, this solid base has not widely reached the industrial scene because of technical aspects concerning the stability of these catalysts upon exposure to moist air. The basic sites of alkaline-earth metal oxides or hydrotalcite-like samples surface can be easily poisoned by air due to the adsorption of CO2 and H2O on the surface [13–15]. Furthermore, the single contact between reagents and catalysts causes the catalyst to be less active than a homogeneous strong base like sodium hydroxide. Many efforts have been taken toward the activity enhancement of such solid base catalysts by increasing their basicity. Diez et al. [16] reported that magnesium oxide doped with 0.5 wt% lithium formed an active catalyst for aldol condensation. The presence of Li+ correlates with an increase in the density of strongly basic sites. It should be stressed that all operations during preparation, processing, storage, and use of the catalyst were undertaken extremely carefully in an inert gas atmosphere to avoid contact with air.
In this work, a high-efficiency CaO was developed by a simple method using an organic reagent as modifier over commercial CaO. After modifying, the three-phase reaction system will be weakened and the stability of the modified catalyst in moist air is greatly improved. The modification conditions and various reaction conditions as well as characterization of the samples have also been investigated.
Experimental
Modification of CaO
The surface modification of CaO was carried out by stirring 1 g commercial CaO particles (160–200 mesh) in a certain amount of benzyl bromide (0.01–0.5%) in 2.5 mL hexane at room temperature. After 24 h, the mixture was filtrated and washed with hexane to remove unreacted modifier, and then the modified CaO was obtained after a vacuum drying process.
Humidity test
Commercial CaO and modified CaO particles were kept in a container containing saturated humidity at room temperature for several days to accelerate the moisture absorption. The tested samples were weighed at regular intervals, and the moisture absorption degree (%) was evaluated by the following equation.
In the equation, the ∆m was signed as increased weight, and the m 0 as the initial weight of the sample.
Characterizations
X-ray diffraction (XRD) patterns were collected on a D/Max-3C X-ray powder diffractometer (Rigalcu, Japan) using a Cu-K α source fitted with an Inel CPS 120 hemispherical detector. Data were collected in the 2θ range of 10–80° with an angular step of 0.01° and a counting time of 10.4 s per step. The Fourier transform-infrared (FT-IR) spectra were recorded by using Nicolet Nexus 670 spectrophotometers. Scanning electron microscope (SEM) photographs were taken by Quanta 200 SEM equipped with an energy dispersive spectrometer (Philips-FEI, the Netherlands).
Catalytic activity measurement
Amounts of 5.0 mmol acetophenone and 7.5 mmol purified benzaldehyde were added to 20 mL MeOH, and then 20% catalyst was added under stirring. After reacting for 6 h at room temperature, the liquid was flitted from the mixture and analyzed by gas chromatography (Agilent 6890) using a FID detector and HP-5 column. The yield and selectivity were calculated by area normalization method on a carbon basis and the carbon balances within 100 ± 5%.
Results and discussion
The loading of modifier, benzyl bromide, on CaO was investigated for moisture absorption and catalytic activity for aldol condensation. Too much modifier will lead to the catalytic activity decreasing for the occupation of active sites of CaO, while less modifier is insufficient for the formation of the hydrophobic layer over the catalyst surface. On the other hand, too much benzyl bromide will lead to a large amount of CaBr2 forming over the CaO surface, which is highly water-absorptive and decreases the resistance to moisture of the modified CaO. Humidity tests were carried out for various coated products with modifier amounts varying from 0.01 to 0.5%; the unmodified sample was also included as a comparison. The tested samples were kept in a container at saturated humidity condition at room temperature to accelerate the moisture absorption. The weight of each CaO sample modified with various amounts of benzyl bromides at different times are shown in Fig. 1. It was noted that the moisture absorption continues rising for all samples at different time periods. For the commercial CaO particles, the moisture absorption degree reached nearly 100% within 50 h, while for the modified CaO, only a slight weight increase can be found over the same period. It is obvious that the surface modification can improve the moisture resistance of commercial CaO effectively.
Figure 2 shows the IR spectra of commercial CaO and modified CaO. The bands at 1,621 and 3,460 cm−1 are associated with the adsorbed water. The spectra display bands at 867 and 1,477 cm−1 correspond to vibration modes of mono and bidentate carbonates. This is also evidenced from the characteristic absorption of C=O between 2,000 and 1,500 cm−1, which indicates the presence of calcium carbonate in the two samples. The vibration of aromatic carbon double-bond bands causes an increase of intensity at 1,590 cm−1. The sp 3 hybridized C–H stretching vibration at 2,800–3,000 cm−1 and bending at 1,440 cm−1 [17] of modified CaO indicates the successfully chemical bonding of the modifier and CaO [18].
The TG/DSC thermogram profiles for the modified CaO sample is illustrated in Fig. 3. As shown in Fig. 3, no obvious peak corresponding to the elimination of the surface loosely held water, at a temperature below 473 K, was observed in the thermal analysis profiles, suggesting that the surface physisorbed water was removed during the course of drying. In addition, two steps in the TG curve between the temperature range from 400 to 700 °C were due to the loss of H2O and CO2 remaining in the form of CaO. Furthermore, the DSC curve of the modified CaO presents two broad peaks at 450 and 700 °C corresponding to the decomposition of Ca(OH)2 and CaCO3 due to the hydration and carbonation of CaO [19], which moves to higher temperature ranges compared to commercial CaO reported by Ngamcharussrivichai et al. [20]. The results suggest that the thermal stability of CaO is enhanced after modifying. By comparing with the commercial samples, the percentage of water loss of 2.414% in modified CaO is the same as in commercial CaO samples. Since the percentage of water weight loss is directly correlated to the amounts of Ca(OH)2, these results suggest that Ca(OH)2 formed during the modifying procedure but there is relatively little storage. Furthermore, the step and peak in the TG/DSC thermogram profiles corresponding to decomposition of modifier do not appear, suggesting the amount of modifier over the CaO surface is too small.
The optimized reaction condition of the amount of catalyst was investigated over commercial CaO after reacting for 6 h at room temperature with acetophenone/benzaldehyde molar ratio of 1:1.5 (Table 1). With a low CaO amount (<10%), relatively low conversion of acetophenone (60.2%) was observed. On increasing the catalyst amount to 20%, conversion of acetophenone increased up to 75.7% with 99.1% selectivity of chalcone (1,3-diphenyl-2-propen-1-one). Too much catalyst produces a large number of basic sites, which lead to the selectivity of chalcone decrease with the formation of 1,3,5-triphenylpentan-1,5-dione by Michael addition [20]. The Michael reaction is usually considered to require a strong basicity. The effect of reaction temperature on the conversion of acetophenone was investigated with a 20% amount of catalyst, and the results are shown in Table 2. Conversion of acetophenone increases as the reaction temperature rises, and reaches the highest value of 80.8% at the reaction temperature of 60 °C, while the selectivity to chalcone increased slightly along with the conversion. Further increases of the temperature cause a slight decrease of the yield and selectivity. To assess the influence of the molar ratio on the conversion of acetophenone, the acetophenone/benzaldehyde ratio was varied from 1:1.1 to 1:2.0 at 60 °C (Table 3). The conversion of acetophenone was 72.1% with an acetophenone/benzaldehyde ratio of 1:1.1. The conversion of acetophenone enhances as the acetophenone/benzaldehyde ratio increases, and the ratio of 1:1.5 results in a conversion as high as 80.8%. In comparison, Lopez et al. [21] obtained the conversion of 80% over KF/Al2O3 at an extremely high reaction temperature of 473 K with an acetophenone/benzaldehyde ratio of 1:1.
In our work, the influence of the substitutes of aldehydes on the conversion and the selectivity was also tested using modified CaO as catalyst (Table 4). The p-nitrobenzaldehyde, o-nitrobenzaldehyde and 2-furaldehyde (with strong electron-withdrawing groups, entries 2, 3, and 10) involved reactions giving close to 100% conversion of acetophenone but poor selectivity to chalcone, while aldehydes with relatively weak electron-withdrawing groups, such as p-chlorobenzaldehyde, o-chlorobenzaldehyde, and naphthaldehyde, lead to high yields of chalcone (conversion of acetophenone × selectivity to chalcone, entries 4, 5, and 9). On the other hand, the reactions of p-methoxybenzaldehyde, p-methybenzaldehyde, and piperonyl aldehyde (with electron-donor groups, entries 6, 7, and 8) give moderate conversion of acetophenone. In general, electron-withdrawing groups of benzaldehyde can activate the aldehyde group leading to high conversion. However, with the basicity of the catalyst as high as CaO, the selectivity to chalcone decreases due to more side reactions. So, the relative weak electron-withdrawing groups lead to the highest yields.
The catalytic data over commercial CaO and modified CaO with different amounts of benzyl bromide at the reaction temperature of 60 °C with an acetophenone/benzaldehyde molar ratio of 1:1.5 are displayed in Table 5. From the results, it can be seen that the conversion of acetophenone over modified CaO is much higher than that of commercial CaO, and the catalytic activity increases with the amount of modifier. The highest yield of chalcone (90.5%) was obtained over modified CaO with 0.2% benzyl bromide. The main reason for the high activity for modified CaO can be attributed to the role of the benzyl groups, which form a hydrophobic layer over the CaO surface to improve the absorption of grease to the catalyst surface and so increase the efficiency of basic sites over the CaO surface.
As the reaction completed, the reaction mixture was filtered by vacuum and the catalysts were washed with 5 mL MeOH and used directly in subsequent aldol reactions without adding any new catalyst, in order to reuse the catalyst and study the catalyst’s stability. In each reuse, the same amounts of substrate were used. The results showed that the catalyst essentially retained its catalytic activity without any decrease of selectivity and yield for five cycles (Table 6).
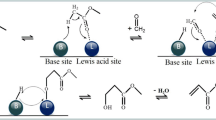
The possible reaction mechanism of aldol condensation between acetophenone (A) and benzaldehyde (B) over CaO is illustrated in Scheme 1. In this case, the solid base catalysis captures a proton and promotes the rearrangement reaction of acetophenone to form C, which is the active media of the aldol reaction. After a classical nucleophile attacking of C to benzaldehyde (B), intermediate D is formed. Then, the Ca2+ picks off an O− from D to form a carbocation E, and the deprotonation of E finally results in the formation of product F.
Conclusion
This work developed an efficient catalyst for the aldol condensation of acetophenone and benzaldehyde by modifying commercial CaO with benzyl bromide. The results showed that the conversion of acetophenone over modified CaO was greatly enhanced, to 90.5% compared with 80.8% over commercial CaO, under the same reaction condition. Part of the explanation for such a finding is the role of the benzyl group which improves the adsorption of reactant to the catalyst surface and resists the adsorption of water to ther catalyst surface. Our strategy to design a highly efficient heterogeneous catalyst with good stability offers the possibility of industrial application and environmentally benign carbon–carbon bond-forming reactions.
References
J.C.A.A. Roelofs, D.J. Lensveld, A.J. van Dillen, K.P. de Jong, J. Catal. 203, 184–191 (2001)
I. Cota, R. Chimentao, J. Sueiras, F. Medina, Catal. Comm. 9, 2090–2094 (2008)
L. Zhong, Q. Gao, J.B. Gao, C. Li, J. Catal. 250, 360–364 (2007)
Y. Ono, J. Catal. 216, 406 (2003)
H. Hattori, Appl. Catal. A 222, 247 (2001)
K. Tanabe, W.F. Hölderich, Appl. Catal. A 181, 399 (1999)
Y.W. Xie, K.K. Sharma, A. Anan, G. Wang, A.V. Biradar, T. Asefa, J. Catal. 265, 131–140 (2009)
G.G. Podrebarac, F.T.T. Ng, G.L. Rempel, Chem. Eng. Sci. 52, 2991–3002 (1997)
V. Serra-Holm, T. Salmi, J. Multamäki, J. Reinik, P. Mäki-Arvela, R. Sjöholm, L.P. Lindfors, Appl. Catal. A 198, 207–221 (2000)
V. Raju, R. Radhakrishnan, S. Jaenicke, G.K. Chuah, Catal. Today 164, 139–142 (2011)
J.M. Clacens, D. Genuit, L. Delmotte, A. Garcia-Ruiz, G. Bergeret, R. Montiel, J. Lopez, F. Figueras, J. Catal. 221, 483–490 (2004)
H.H. Liu, W.J. Xu, X.H. Liu, Y. Guo, Y.L. Guo, G.Z. Lu, Y.Q. Wang, Kinet. Catal. 51, 75–80 (2010)
D. Tichit, D. Lutic, B. Coq, R. Durand, R. Teissier, J. Catal. 219, 167–175 (2003)
W.J. Ji, Y. Chen, H.H. Kung, Appl. Catal. A 161, 93–104 (1997)
M.J. Climent, A. Corma, S. Iborra, A. Veltry, Catal. Lett. 79, 157–162 (2002)
V.K. Díez, J.I. Di Cosimo, C.R. Apesteguía, J. Catal 240, 235–244 (2006)
K. Ebitani, K. Motokura, K. Mori, T. Mizugaki, K. Kaneda, J. Org. Chem. 71, 5440–5447 (2006)
S. Abelló, D. Bijaya-Shankar, J. Pérez-Ramírez, Appl. Catal. A 342, 119–125 (2008)
L.C. Meher, M.G. Kulkarni, A.K. Dalai, S.N. Naik, Eur. J. Lipid Sci. Technol. 108, 389–397 (2006)
C. Ngamcharussrivichai, W. Wiwatnimit, S. Wangnoi, J. Mol. Catal. A 272, 24–33 (2007)
J. Lopez, J. Sanchez Valente, J.M. Clacens, F. Figueras, J. Catal. 208, 30–37 (2002)
Acknowledgments
This work was financially supported by grants from Natural Science Research Plan Projects of Shaanxi Science and Technology Department (2011JQ2014), Scientific Research Program Funded by Shaanxi Provincial Education Department (No. 11JK0591) and the Open Founds of the Shanghai Key Laboratory of Green Chemistry and Chemical Process.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, Y., Chen, G. & Lu, Y. Efficient aldol condensation by using modified CaO as solid-base catalysts. Res Chem Intermed 38, 937–946 (2012). https://doi.org/10.1007/s11164-011-0430-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-011-0430-8