Abstract
A simple, inexpensive, environmentally friendly, and efficient route for the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives via the one-pot three-component Biginelli reaction using pentafluorophenylammonium triflate (PFPAT) as a catalyst is described. The organocatalyst is air-stable, cost-effective, easy to handle, and easily removed from the reaction mixtures.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
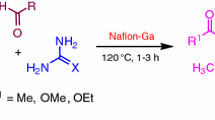
3,4-Dihydropyrimidine-2-(1H)-ones (DHPMs) have received considerable attention in recent times because of their importance in medicinal chemistry. Substituted DHPMs have found applications in diverse therapeutic areas including antiviral, antibacterial, antitumor, and antihypertensive agents, α1a-adrenergic antagonists, and neuropeptide Y (NPY) antagonists [1–5]. Furthermore, these compounds have emerged as the integral backbones of several calcium channel blockers [6]. Some marine alkaloids containing the dihydropyrimidine core unit show interesting biological properties; batzelladine alkaloids are potent HIV gp-120-CD4 inhibitors [7–9]. Moreover, the 3,4-DHPM motif is present in many products isolated from natural materials such as several species of sponges: for example, batzelladines, ptilomycalines, and crambescidines [10, 11] exhibit many biological activities such as anticancer, antifungal, and anti-HIV. In order to improve the yield of DHPMs, a few other multistep approaches using aldehyde [12] or acetoacetate [13] equivalents in modified Biginelli reactions have been developed. Nevertheless, the original Biginelli reaction offers the most simple, cost-effective, and reasonable access to these important compounds. Despite the usefulness of the Biginelli reaction, the efficiency of this method is considerably limited because of the strongly acidic and harsh reaction conditions. Recently, many synthetic methods for preparing these compounds have been developed to improve and modify this reaction by microwave [14–18] and ultrasound irradiation [19–21] and by using Lewis acid as well as Brønsted acid promoters. FeCl3/tetraethyl orthosilicate [22], triflates [23, 24], metal bromide [25, 26], polyoxometalate [27], strontium(II) nitrate [28], cerium(III) chloride [29], Li(OTf) [30], Ln(OTf)3 [31], heteropoly acids [32–36], ion exchange resins, polymer-based solid acid [37, 38], l-proline [39, 40], chiral phosphoric acid [41], TMSCl [42], hexaaquaaluminum(III) tetrafluoroborate [43], and ionic liquids [44, 45] were used to replace the strong protic acid used in the classic Biginelli reaction. Although these methods are quite satisfactory, many of them employ considerable amounts of hazardous organic solvents, which are not environmentally friendly, for carrying out the reactions and/or for extraction and purification (column chromatography). Moreover, several of these reactions are carried out at higher temperatures and using costly reagents. Furthermore, these methods are not suitable in terms of the recent trends in process chemistry, because of the use of metallic catalysts. Therefore, a method using a nonmetallic catalyst is desirable. Pentafluorophenylammonium triflate (PFPAT) has emerged as a highly efficient and effective potential Brønsted acid catalyst imparting high regio- and chemoselectivity in various chemical transformations [46, 47], owing to its low toxicity, air and water compatibility, operational simplicity, and remarkable ability to suppress side reactions in acid-sensitive substrates. In this regard and in connection with our previous work [48, 49], we now describe a one-pot method for the Biginelli reaction using PFPAT as an efficient novel organocatalyst (Scheme 1).
Results and discussion
In order to optimize the reaction conditions, we chose condensation of benzaldehyde, ethyl acetoacetate, and urea catalyzed by PFPAT under different conditions both in the absence and in the presence of PFPAT and results are given in Table 1. It is noteworthy that in the absence of catalyst, the reaction failed to give the desired product, even after a long reaction time (24 h, Table 1, entry 1). Then, the effects of temperature, the amount of catalyst, and the reaction time on the yield of the product were examined. Reaction at room temperature (r.t.) in acetonitrile in the presence of 5 mol% PFPAT afforded the product 4 in 80% yield (Table 1, entry 2). Increasing the amount of catalyst and/or prolonging the reaction time did not improve the yield (Table 1, entry 9). Further studies confirmed that 10 mol% of PFPAT was optimum for this reaction and gave a product yield of 90 % in just 3 h (Table 1, entry 3). The reaction was also examined in solvents such as H2O, THF, CH2Cl2, ethanol, diethyl ether, and toluene. In the presence of these solvents the reaction was sluggish and formation of by-products was observed (Table 1, entries 4–8).
Using these optimized reaction conditions, we explored the scope and efficiency of this approach for the synthesis of a wide variety of substituted 3,4-dihydropyrimidin-2(1H)-ones and results are summarized in Table 2.
A wide range of structurally varied aldehydes reacted smoothly and quickly to give the corresponding DHPMs in high yield and purity as listed in Table 2. In all cases, aromatic aldehydes substituted with either electron-donating or electron-withdrawing groups underwent the reaction smoothly and gave the products in good yields. It could also be concluded that the aldehydes bearing electron-withdrawing groups required shorter time and gave higher yields (Table 2, entries 1–7). This method is even effective with aliphatic aldehydes, which normally produce low yields due to their intrinsic lower reactivity (Table 2, entries 12, 13). The remarkable feature of this improved protocol is the wide stability of a variety of functional groups, such as ethers, alkyl, nitro, and halides under the present reaction conditions. Furthermore, the conditions are mild enough to perform these reactions with acid-sensitive aldehydes such as furfuraldehyde and cinnamaldehyde, without any decomposition or polymerization, and with enolizable aldehydes such as butyraldehyde. In all cases, the pure product was isolated by simple extraction and recrystallization, without any chromatography or cumbersome workup procedure.
In order to show the merit of the present work in comparison with some reported protocols, we compared the results of the synthesis of 4-(chlorophenyl)-5-(ethoxycarbonyl)-3,4-dihydro-6-methylpyrimidin-2(1H)-one (Table 2, entry 1) in the presence of CuI [50], SbCl3 [51], Cu(NTf2)2 [52], H3PMo12O40 [35], propanephosphonic acid anhydride [53], ZrOCl2 [54], pyrazolidine dihydrochloride [55], Zn-MOF [56], sulfated tungstate [57], imidazol-1-yl-acetic acid [58], MoO3–ZrO2 nanocomposite oxide [59], and PFPAT with respect to the reaction times and temperature (Table 3). PFPAT afforded a yield of product superior to that obtained in the presence of all the other catalysts; moreover, most of the other catalysts required longer reaction times and needed higher temperature.
In addition, the PFPAT catalyst was easily separated from the reaction mixture after workup; washing with NaOH aqueous solution removed CF3SO3H, followed by distillation under reduced pressure (C6F5NH2: b.p. 153 °C).
A plausible mechanism for the formation of DHPMs 4a–4o in the presence of PFPAT is proposed in Scheme 2. The highly hydrophobic pentafluorophenyl moiety effectively repels H2O produced by the dehydration steps [46, 47].
In summary, an efficient protocol for a one-pot three-component Biginelli reaction catalyzed by PFPAT was developed. In contrast to the existing methods using potentially hazardous catalysts/additives, the present method offers the following competitive advantages: (1) PFPAT is easy to prepare from commercially available pentafluoroaniline and triflic acid, (2) ease of product isolation/purification by non-aqueous workup, (3) no side reaction, (4) low cost and simplicity in process and handling, and (5) DHPMs are produced by an environmentally benign process.
Experimental
NMR spectra were determined on an FT-NMR Bruker AV-400 spectrometer in CDCl3 or DMSO-d 6 and are expressed in δ values relative to tetramethylsilane; coupling constants (J) are measured in hertz. Melting points were determined on an Electrothermal 9100 apparatus. Infrared spectra were recorded on a Rayleigh WQF-510 Fourier transform instrument. Commercially available reagents were used throughout without further purification.
Typical experimental procedure
A mixture of aldehyde (2 mmol), β-dicarbonyl compound (2 mmol), urea (3 mmol), and 0.04 g PFPAT was stirred at r.t. in a vial. After completion of the reaction as indicated by TLC, the organic phase was washed with 1 cm3 1 M NaOH aqueous solution. The separated organic phase was evaporated under reduced pressure to give a crude product, which was purified by recrystallization from hot ethanol to afford pure products. Products were characterized by comparison of their physical and spectral data with those of authentic samples.
References
Kappe CO (2000) Acc Chem Res 33:879
Kappe CO (1993) Tetrahedron 49:6937
Atwal KS, Roonyak GC, O’Reilly BC, Schwartz J (1989) J Org Chem 54:5898
Kappe CO, Fabian WMF, Semones MA (1997) Tetrahedron 53:2803
Zorkun IS, Sarac S, Celebi S, Erol K (2006) Bioorg Med Chem Lett 14:8582
Kappe CO (2000) Eur J Med Chem 35:1043
Snider BB, Chen J, Patil AD, Freyer A (1996) Tetrahedron Lett 37:6977
Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, De Brosse C, Mai S, Truneh A, Carte B, Faulkner DJ (1995) J Org Chem 60:1182
Aron ZD, Overman LE (2004) Chem Commun 3:253
Heys L, Moore CG, Murphy PJ (2000) Chem Soc Rev 29:57
Bewley CA, Ray S, Cohen F, Collins SK, Overmann LE (2004) J Nat Prod 67:1319
Abdel-Fattah AAA (2003) Synthesis 15:2358
Singh K, Singh J, Deb PK, Singh H (1999) Tetrahedron 55:12873
Manhas MS, Ganguly SN, Mukherjee S, Jain AK, Bose AK (2006) Tetrahedron Lett 47:2423
Shaabani A, Bazgir A (2004) Tetrahedron Lett 45:2575
Gross GA, Wurziger H, Schobert A (2006) J Comb Chem 8:153
Kidwai M, Saxena S, Mohan R, Venkataramanan R (2002) J Chem Soc Perkin Trans 1:1845
Aghayan MM, Bolourtchian M, Hosseini M (2004) Synth Commun 34:3335
Stefani HA, Oliveira CB, Almeida RB, Pereira CMP, Braga RC, Cella R, Borges VC, Savegnago L, Nogueira CW (2006) Eur J Med Chem 41:513
Zhang XL, Li YP, Liu CJ, Wang JD (2006) J Mol Catal A Chem 253:207
Li JT, Han JF, Yang JH, Li TS (2003) Ultrason Sonochem 10:119
Cepanec I, Litvic M, Bartolincic A, Lovric M (2005) Tetrahedron 61:4275
Lu J, Bai Y (2002) Synthesis 47:466
Adapa SR, Anan MM, Varala R (2003) Synlett 67
Hojatollah S, Guo Q (2004) Synth Commun 34:171
Martins MAP, Teixeira MVM, Cunico W, Scapin E, Mayer R, Pereira CMP, Zanatta N, Bonacorso HG, Peppe C, Yuan YF (2004) Tetrahedron Lett 45:8991
Fazaeli R, Tangestaninejad S, Aliyan H, Moghadam M (2006) Appl Catal A: Gen 309:44
Liu C, Wang J, Li Y (2006) J Mol Catal A Chem 258:367
Bose DS, Fatima L, Mereyala HB (2003) J Org Chem 68:587
Lusch MJ, Tallarico JA (2004) Org Lett 6:3237
Huang YJ, Yang FY, Zhu CJ (2005) J Am Chem Soc 127:16386
Rafiee E, Jafari H (2006) Bioorg Med Chem Lett 16:2463
Amini MM, Shaabani A, Bazgir A (2006) Catal Commun 7:843
Maradur PS, Gokavi GS (2007) Catal Commun 8:279
Heravi MM, Bakhtiari K, Bamoharram FF (2006) Catal Commun 7:373
Rafiee E, Shahbazi F (2006) J Mol Catal A Chem 250:57
Joseph JK, Jain SL, Sain B (2006) J Mol Catal A Chem 247:99
Palaniappan S, John A (2005) J Mol Catal A Chem 233:9
Gohain M, Prajapati D, Sandhu JS (2004) Synlett 235
Mabry J, Ganem B (2006) Tetrahedron Lett 47:55
Chen XH, Xu XY, Liu H, Cun LF, Gong LZ (2006) J Am Chem Soc 128:14802
Zhu YL, Huang SL, Wan JP, Yan L, Pan YJ, Wu A (2006) Org Lett 8:2599
Litvić M, Večenaj I, Ladiŝić ZM, Lovrić M, Vinković V, Litvic MF (2010) Tetrahedron 66:3463
Peng JJ, Deng YQ (2001) Tetrahedron Lett 42:5917
Zheng RW, Wang XX, Xu H, Du JX (2006) Synth Commun 36:1503
Funatomi T, Wakasugi K, Misaki T, Tanabe Y (2006) Green Chem 8:1022 (commercially available; TCI-P1626)
Iida A, Osada J, Nagase R, Misaki T, Tanabe Y (2007) Org Lett 9:1859 (commercially available; TCI-P1626)
Montazeri N, Khaksar S, Nazari A, Alavi SS, Vahdat SM, Tajbakhsh M (2011) J Fluorine Chem 132:450
Khaksar S, Ostad S (2011) J Fluorine Chem 132:937
Kalita HR, Phukan P (2007) Catal Commun 8:179
Cepanec I, Litvić M, Filipan-Litvić M, Grüngold I (2007) Tetrahedron 63:11822
Suzuki I, Suzumura Y, Takeda K (2006) Tetrahedron Lett 47:7861
Zumpe FL, Flüβ M, Schmitz K, Lender A (2007) Tetrahedron Lett 48:1421
Rodrίguez-Domίnguez JC, Bernardi D, Kirsch G (2007) Tetrahedron Lett 48:5777
Suzuki I, Iwata Y, Takeda K (2008) Tetrahedron Lett 49:3238
Li P, Regati S, Butcher RJ, Arman HD, Chen Z, Xiang S, Chen B, Zhao C (2011) Tetrahedron Lett 52:6220
Salim SD, Akamanchi KG (2011) Catal Commun 12:1153
Kargar M, Hekmatshoar R, Mostashari A, Hashemi Z (2011) Catal Commun 15:123
Samantaray S, Mishra BG (2011) J Mol Catal A Chem 339:92
Acknowledgments
Financial support of this work from the Islamic Azad University, Ayatollah Amoli Branch is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the Memory of Shahid Dr. Majid Shahriari.
Rights and permissions
About this article
Cite this article
Khaksar, S., Vahdat, S.M. & Moghaddamnejad, R.N. Pentafluorophenylammonium triflate: an efficient, practical, and cost-effective organocatalyst for the Biginelli reaction. Monatsh Chem 143, 1671–1674 (2012). https://doi.org/10.1007/s00706-012-0752-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-012-0752-2