Abstract
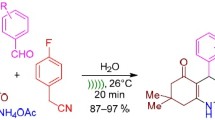
A facile and efficient one-pot, four-component synthesis of polyhydroquinoline derivatives via the Hantzsch reaction using Nafion-H® as heterogeneous catalyst in PEG 400–water solvent system is described herein. The present methodology offers several advantages such as excellent yields, simple procedure, shorter reaction times, and milder conditions with remarkable recyclability.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, multicomponent reactions (MCRs) have gained wide applicability in the field of synthetic organic chemistry. MCRs are one-pot processes that combine three or more substrates simultaneously [1–5]. Such processes are of great interest in diversity-oriented synthesis, especially to generate compound libraries for screening purposes.
In recent years, much attention has been directed towards synthesis of 1,4-dihydropyridyl (1,4-DHP) compounds (Fig. 1) due to a wide range of biological activities associated with this heterocyclic nucleus [6–11]. 1,4-Dihydropyridyl compounds are well known as calcium channel blockers. Cardiovascular agents such as nifedipine [12], nicardipine [13], amlodipine [14], and other related derivatives are 1,4-dihydropyridyl compounds, which are effective for treatment of hypertension [15]. They have been reported to act as vasodilators [16] and bronchodilators [17], and to possess antiatherosclerotic [18], antitumor [19], geroprotective [20], hepatoprotective [21], and antidiabetic [22] activities. Furthermore, current literature has revealed that 1,4-DHPs exhibit several medicinal applications [23–26]. For these reasons, polyhydroquinolines (PHQs) not only have attracted the attention of chemists to synthesize these compounds but also represent an interesting research challenge.
In view of the importance of polyhydroquinoline derivatives, numerous methods have been developed for their synthesis [27–30]. Experimentally, preparation of 1,4-DHPs was first reported in 1882 by Hantzsch, involving one-pot, three-component coupling of an aldehyde with ethyl acetoacetate and ammonia in acetic acid or in refluxing alcohol [31, 32]. However, these methods suffered from drawbacks such as long reaction time, excess of organic solvent, low yields, and harsh refluxing conditions. Therefore, there is scope for further improvement towards milder reaction conditions and higher yields, and progress in this area is remarkable, including the recent use of microwaves [33], TMSCl [34], ionic liquid [35], polymer [36, 37], molecular I2 [38], CAN [39], Yb(OTf)3 [40], HClO4-SiO2 [41], heteropolyacid [42], and MCM-41 [43]. Each of the above methods for the Hantzsch reaction has its own merits, while some of the methods are plagued by limitations of poor yield, longer reaction time, tedious workup, and effluent pollution. Moreover, the main disadvantage of almost all existing methods is that the catalysts are destroyed in the workup procedure and cannot be recovered or reused. Moreover, there are a relatively limited number of reports on synthesis of polyhydroquinolines compared with synthesis of four-substituted 1,4-DHPs. Therefore, the search continues for a better catalyst and an ecofriendly approach for generating polyhydroquinolines in terms of operational simplicity, reusability, economic viability, and greater selectivity.
Over the years, Nafion-H®, a superacidic perfluorinated resin sulfonic acid, has enjoyed immense popularity as a solid acid catalyst for a wide variety of synthetic transformations [44]. The estimated Hammett (H 0) value for Nafion-H® is comparable to that of 96–100% H2SO4 (H 0 = −12.0) [45]. The high catalytic activity, its selectivity, recyclability, its superior chemical and thermal stability, ease of separation from the reaction mixture, and ecofriendly nature as compared with other available heterogeneous acid catalysts [46–48] place it in a unique position as an attractive and promising heterogeneous acid catalyst for organic synthesis. In recent years, replacement of hazardous substances with environmentally benign solvents [49, 50] has been one of the key areas of green chemistry [51]. While use of water as a solvent is probably the most desirable approach, this is often not possible due to the hydrophobic nature of the reactants and sensitivity of many catalysts to aqueous conditions. Recently, PEG and its solutions have been introduced as interesting green solvent systems [52–54]. These have replaced many other “neoteric solvents” such as ionic liquids, supercritical carbon dioxide, and micellar systems, whose toxicological properties, short- and long-term hazardous nature, and biodegradability have not been established completely. Low cost, reduced flammability and toxicity, recyclability, completely nonhalogenated composition, easy degradability, and miscibility with a wide variety of organic solvents are some of the prominent features that render PEG a benign alternative solvent in organic synthesis. Its use as a reaction medium in organic reactions is relatively recent [55–57]. As a part of our ongoing research program to devise cheap synthetic methodologies using PEG 400 and exploration of catalytic potential of Nafion-H® [58, 59], herein we report a simple and efficient protocol for one-pot synthesis of polyhydroquinolines using Nafion-H® as catalyst coupled with aqueous PEG 400 medium (Scheme 2).
Results and discussion
First, a model reaction was carried out using 1 equivalent each of benzaldehyde (1a), ethyl acetoacetate (2), dimedone (3a), and ammonium acetate (4). These were stirred at ambient temperature in ethanol. After 5 h, only 54% of the expected product 5a was obtained. To improve the yield and optimize the reaction conditions, the same reaction was carried out in presence of Nafion-H® as catalyst. Surprisingly, a significant improvement was observed and the yield dramatically increased to 84% after stirring the mixture for only 2 h. Thus, the catalytic efficiency of Nafion-H® was definitely identified.
To increase the product yield further, we used polyethylene glycol as solvent system for the same representative reaction scheme. The yield was better and increased to 92% after stirring the mixture for only 1.5 h. Prompted by these promising outcomes, we further investigated the best reaction conditions by using different ratios of the PEG 400–water solvent system. A decrease in the amount of PEG 400 from 100% to 60% increased the product yield slightly from 92% to 96%. However, a further decrease in the ratio of PEG 400 reduced the product yield, which is attributed to loss of solubility of the reactants. Also, the model reaction was examined in various solvents commonly used in organic synthetic procedures (Table 1). Polar solvents such as ethanol and acetonitrile were much better than nonpolar solvents such as toluene in terms of better yields and shorter reaction times, due to the enhanced solubility of reactants and the ability of the solvent to swell Nafion-H®, which resulted in its high catalytic activity. Nafion-H®, being nonporous, relies on solvation of the ionic groups by an appropriate polar solvent to form solvent channels and clusters. Low yields are observed in case of nonpolar solvent due to failure of the substrate to be able to access the catalyst [60].
The temperature of the reaction was kept at 50 °C unlike in the reactions which used HClO4–SiO2 [41], heteropolyacid [42], and MCM-41 [43] as catalyst. These reactions occurred at refluxing temperatures. It was also observed that, on increasing the temperature, the reaction time decreased, but we continued to use this time to maintain the ambient conditions of the reaction.
The scope and generality of this four-component, one-pot synthesis of polyhydroquinoline derivatives through the Hantzsch reaction was illustrated with different aldehydes, and the results are summarized in Table 2. It is worthwhile to mention that the electronically and structurally diverse aldehydes do not show any obvious effect on this conversion, because the desired products were obtained in good to excellent yields in relatively short reaction times (Scheme 2). However, aliphatic substrates did not undergo any reaction under similar conditions.
Next, we investigated the reusability and recyclability of Nafion-H® (Fig. 2) for the model reaction including 1 equiv. each of benzaldehyde (1a), ethyl acetoacetate (2), dimedone (3a), and ammonium acetate (4) giving product 5a under the optimized conditions (Scheme 1). The catalyst was removed by simple filtration after completion of the reaction [64] and was washed with acetone and dried overnight at 105 °C. The catalyst was reused as such for subsequent reactions (four runs) with fresh substrates under the same conditions. The catalyst showed excellent recyclability in this reaction, as the reaction times and yield remained almost the same without loss of catalytic activity.
X-ray crystallography
Compound 5l was recrystallized from its solution in ethanol. The packing pattern of the compound is shown in Fig. 3. The details of crystal data, intensity data collection, and refinement are given in the Supplementary Material. The DIFABS absorption correction was not applied due to the small size of the crystals (0.2 × 0.4 × 0.2 mm3).
In conclusion, this paper describes a simple and efficient method for synthesis of polyhydroquinoline derivatives via improved Hantzsch condensation using Nafion-H® as reusable catalyst in PEG 400–water solvent system. The mildness of the conversion, experimental simplicity, compatibility with various functional groups, inexpensive reagents, high yields, short reaction times, and easy workup procedure make this protocol very attractive to synthesize a variety of these derivatives.
Experimental
All chemicals were purchased from Sigma-Aldrich and were used without further purification. All reactions and purity of polyhydroquinolines were monitored by thin-layer chromatography (TLC) using aluminum plates coated with silica gel F254 (Merck) using 30% ethyl acetate and 70% petroleum ether as eluent. The spots were detected either under ultraviolet (UV) light or by placing in an iodine chamber. Melting points were determined using a Thomas Hoover melting point apparatus. Infrared (IR) spectra were recorded on a PerkinElmer FTIR-1710 spectrophotometer using Nujol film. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a JEOL JNM-ECX 400P FT NMR system using tetramethylsilane (TMS) as internal standard. Chemical shift values are recorded on the δ scale, and coupling constant values are in Hertz (Hz). Elemental analysis was performed on a Heraeus CHN rapid analyzer. The temperature of the reaction mixture was measured through a noncontact infrared mini gun thermometer (AZ minigun type, model 8868).
General procedure for synthesis of polyhydroquinolines
A 50-cm3 round-bottomed flask was charged with 1 equiv. each of aldehydes 1a–1k (1 mmol), ethyl acetoacetate (2), dimedone (3a) or 1,3-cyclohexanedione (3b), ammonium acetate (4), and Nafion-H® (one bead, i.e., 30 mg) along with 5 cm3 PEG 400–water (60:40) solvent system. The mixture was then stirred at 50 °C until the reaction was complete. The completion of the reaction was monitored through TLC. After the reaction was complete, the reaction mixture was allowed to cool to room temperature, the catalyst Nafion-H® was removed by simple filtration, and the product was extracted with ethyl acetate (3 × 5 cm3). The combined organic layer was dried over anhydrous sodium sulfate and filtered. The solvent was evaporated under vacuo to give the crude product. After extraction with ethyl acetate, the remaining solution of PEG 400 and water was concentrated to recover pure PEG 400, which was then reused for the subsequent reactions [65]. The crude products obtained were subjected to purification via recrystallization or through column chromatography on silica gel (100–200 mesh size) using 25% ethyl acetate in petroleum ether as eluent to yield polyhydroquinolines 5a–5l. The structures of all products were established on the basis of spectral analysis (IR, 1H NMR, and 13C NMR), elemental analysis, and melting point determination. X-ray analysis was carried out for compound 5l (Figs. 3, 4).
Regeneration of catalyst
After filtration, the catalyst was washed successively with acetone and deionized water and dried overnight at 105 °C. The obtained catalyst had the same catalytic activity as the fresh catalyst.
Ethyl 1,4,5,6,7,8-hexahydro-4-(3-hydroxyphenyl)-2,7,7-trimethyl-5-oxoquinoline-3-carboxylate (5f, C21H25NO4)
IR (Nujol): \( \overline{\nu } \) = 3,245, 2,959, 1,600, 1,453, 1,379, 1,267, 1,149, 707 cm−1; 1H NMR (DMSO-d 6 , 400 MHz): δ = 0.90 (s, 3H, CH3), 1.02 (s, 3H, CH3), 1.16 (t, 3H, J = 7.04 Hz, CH3), 2.13–2.29 (m, 4H, 2 × CH2), 2.32 (s, 3H, CH3), 4.02 (q, 2H, J = 7.4 Hz, CH2), 4.96 (s, 1H, CH–Ar), 5.85 (br s, 1H, NH), 6.75–6.99 (m, 4H, Ar–H), 7.81 (s, 1H, OH) ppm; 13C NMR (DMSO-d 6 , 100 MHz): δ = 13.4, 18.4, 19.8, 27.3, 28.7, 32.3, 35.6, 50.9, 58.8, 100.4, 105.1, 112.0, 112.9, 114.9, 119.3, 128.2, 144.3, 148.5, 149.3, 167.4, 194.9 ppm.
Ethyl 1,4,5,6,7,8-hexahydro-4-(2-hydroxy-3-methoxyphenyl)-2-methyl-5-oxoquinoline-3-carboxylate (5j, C20H23NO5)
IR (Nujol): \( \overline{\nu } \) = 3,342, 2,946, 1,733, 1,693, 1,607, 1,523, 1,390, 1,078 cm−1; 1H NMR (CDCl3, 400 MHz): δ = 1.21 (t, 3H, J = 7.32 Hz, CH3), 1.88–2.27 (m, 6H, 3 × CH2), 2.47 (s, 3H, CH3), 3.85 (s, 3H, OCH3), 4.10 (q, 2H, J = 7.3 Hz, CH2), 5.17 (s, 1H, CH–Ar), 6.14 (br s, 1H, NH), 6.69–6.79 (m, 3H, Ar–H), 9.26 (s, 1H, OH) ppm; 13C NMR (CDCl3, 100 MHz): δ = 14.0, 19.5, 21.2, 25.4, 27.7, 29.7, 30.3, 36.2, 43.6, 55.8, 60.3, 81.2, 110.2, 120.8, 140.3, 148.7, 158.7, 169.9, 194.9 ppm.
Ethyl 1,4,5,6,7,8-hexahydro-4-(1-naphthyl)-2-methyl-5-oxoquinoline-3-carboxylate (5l, C23H23NO3)
IR (Nujol): \( \overline{\nu } \) = 3,292, 3,082, 2,952, 1,696, 1,609, 1,487, 1,379, 1,222 cm−1; 1H NMR (CDCl3, 400 MHz): δ = 0.87 (t, 3H, J = 7.32 Hz, CH3), 1.86–2.27 (m, 6H, 3 × CH2), 2.40 (s, 3H, CH3), 3.88 (q, 2H, J = 7.08 Hz, CH2), 5.84 (s, 1H, CH–Ar), 6.21 (br s, 1H, NH), 7.33–7.73 (m, 7H, naphthyl) ppm; 13C NMR (CDCl3, 100 MHz): δ = 14.1, 19.1, 21.0, 27.1, 31.8, 37.1, 59.8, 107.7, 114.5, 125.3, 125.4, 125.6, 126.9, 127.9, 143.2, 146.5, 150.5, 167.8, 196.4 ppm.
X-ray crystallography
X-ray diffraction data were collected using an Enraf–Nonius CAD4 diffractometer. The structure of compound 5l was determined by a direct method using the program SHELXS 97 and difference Fourier calculation. The coordinates of nonhydrogen atoms were refined anisotropically using the program SHELXL 97 [66]. The positions of hydrogen atoms were determined from difference Fourier maps and were included in the final cycles of refinement using isotropic temperature factors of the nonhydrogen atoms to which they were attached. The final R-factor for observed 7,518 reflections [I ≥ 2σ(I)] was 0.1824. The atomic scattering factors used in these calculations were those of Cromer and Mann [67] for nonhydrogen atoms and of Stewart et al. [68] for hydrogen atoms.
Crystallographic data for the structure 5l have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 796332.
References
Guillena G, Ramon DJ, Yus M (2007) Tetrahedron Asymmetry 18:693
Domling A (2006) Chem Rev 106:17
Simon C, Constantieux T, Rodrigeuz J (2004) Eur J Org Chem 4957
Domling A, Ugi I (2000) Angew Chem Int Ed 39:3168
Ramon DJ, Yus M (2005) Angew Chem Int Ed 44:1602
Mauzerall D, Westheimer FH (1955) J Am Chem Soc 77:2261
Baraldi PG, Budriesi R, Cacciari B, Chiarini A, Garuti L, Giovanninetti G, Leoni A, Roberti M (1992) Collect Czech Chem Commun 57:169
Di Stilo A, Visentin S, Cena C, Gasco AM, Ermondi G, Gasco A (1998) J Med Chem 41:5393
Kawase M, Shah A, Gaveriya H, Motohashi N, Sakagami H, Varga A, Molnar J (2002) Bioorg Med Chem 10:1051
Suarez M, Verdecia Y, Illescas B, Martinez-Alvarez R, Alvarez A, Ochoa E, Seoane C, Kayali N, Martin N (2003) Tetrahedron 59:9179
Shan R, Velazquez C, Knaus EE (2004) J Med Chem 47:254
Bossert F, Meyer H, Wehinger E (1981) Angew Chem Int Ed 20:762
Takenaka T, Usuda S, Nomura T, Maeno H, Sado T (1976) Arzneim Forsch 26:2172
Julius S (1988) J Cardiovasc Pharmacol 12:S27
Liang J-C, Yeh J-L, Wang C-S, Liou S-F, Tsai C-H, Chen I-J (2002) Bioorg Med Chem 10:719
Boschi D, Caron G, Visentin S, Di Stilo A, Rolando B, Fruttero R, Gasco A (2001) Pharm Res 18:987
Godfraind T, Miller R, Wibo M (1986) Pharmacol Rev 38:321
Fleckenstein-Gruen G, Thimm F, Czirfuzs A, Matyas S, Frey M (1994) J Cardiovasc Pharmacol 24:75
Manpadi M, Uglinskii PY, Rastogi SK, Cotter KM, Wong Y-SC, Anderson LA, Ortega AJ, Van Slambrouck S, Steelant WFA, Rogelj S, Tongwa P, Antipin MY, Magedov IV, Kornienko A (2007) Org Biomol Chem 5:3865
Emanuel NM, Obukhova LK, Dubur GJ, Tirzit GD, Uldrikis JR (1985) Dokl Akad Nauk SSSR 284:1271
Bird GLA, Prach AT, McMahon AD, Forrest JAH, Mills PR, Danesh BJ (1998) J Hepatol 28:194
Ogawa AK, Willoughby CA, Bergeron R, Ellsworth KP, Geissler WM, Myers RW, Yao J, Harris G, Chapman KT (2003) Bioorg Med Chem Lett 13:3405
Klusa V (1995) Drugs Future 20:135
Bretzol RG, Bollen CC, Maester E, Federlin KF (1993) Am J Kidney Dis 21:53
Bretzel RG, Bollen CC, Maeser E, Federlin KF (1992) Drugs Future 17:465
Boer R, Gekeler V (1995) Drugs Future 20:499
Eisner U, Kuthan J (1972) Chem Rev 72:1
Stout DM, Meyers AI (1982) Chem Rev 82:223
Kutney JP (1977) Heterocycles 7:593
Widdowson DA (1978) In: Van Tamelen EE (ed) Bioorganic chemistry, Series 4. Academic, New York, p 239
Hantzsch A (1882) Justus Liebigs Ann Chem 215:1
Loev B, Snader KM (1965) J Org Chem 30:1914
Tu S-J, Zhou J-F, Deng X, Cai P-J, Wan H, Feng J-C (2001) Chin J Org Chem 21:313
Sabitha G, Reddy GSKK, Reddy CS, Yadav JS (2003) Tetrahedron Lett 44:4129
Ji S-J, Jiang Z-Q, Lu J, Loh T-P (2004) Synlett 5:831
Breitenbucher JG, Figliozzi G (2000) Tetrahedron Lett 41:4311
Dondoni A, Massi A, Minghini E, Bertolasi V (2004) Tetrahedron 60:2311
Ko S, Sastry MNV, Lin C, Yao C-F (2005) Tetrahedron Lett 46:5771
Ko S, Yao C-F (2006) Tetrahedron 62:7293
Wang L-M, Sheng J, Zhang L, Han J-W, Fan Z-Y, Tian H, Qian C-T (2005) Tetrahedron 61:1539
Maheswara M, Siddaiah V, Damu GLV, Rao CV (2006) Arkivoc 2:201
Heravi MM, Bakhtiari K, Javadi NM, Bamoharram FF, Saeedi M, Oskooie HA (2007) J Mol Catal A Chem 264:50
Nagarapu L, Kumari MD, Kumari NV, Kantevari S (2007) Catal Commun 8:1871
Olah GA, Iyer PS, Prakash GKS (1986) Synthesis 513
Olah GA, Prakash GKS, Sommer J (1985) Superacids. Wiley Interscience, New York
Arata K (1990) Adv Catal 37:165
Yeo SC, Eisenberg A (1977) J Appl Polym Sci 21:875
Yeager HL, Eisenberg A (1982) In: Eisenberg A, Yeager HL (eds), Perflourinated ionomer membranes. ACS Symposium series, vol. 180, American Chemical Society, Washington, DC, p 1
Polshettiwar V, Varma RS (2008) Acc Chem Res 41:629
Polshettiwar V, Varma RS (2008) Chem Soc Rev 37:1546
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, Oxford
Chen J, Spear SK, Huddleston JG, Rogers RD (2005) Green Chem 7:64
Zhang Z-H, Yin L, Wang Y-M, Liu J-Y, Li Y (2004) Green Chem 6:563
Kumar R, Chaudhary P, Nimesh S, Chandra R (2006) Green Chem 8:356
Winter A, van den Berg AMJ, Hoogenboom R, Kickelbick G, Schubert US (2006) Synthesis 2873
Han W, Liu C, Jin Z-L (2007) Org Lett 9:4005
Smith CB, Raston CL, Sobolev AN (2005) Green Chem 7:650
Kidwai M, Bhatnagar D, Chauhan R (2011) J Heterocycl Chem. doi:10.1002/jhet.1037
Kidwai M, Chauhan R, Bhatnagar D (2011) J Sulfur Chem 32:37
Seen AJ (2001) J Mol Catal A Chem 177:105
Kumar S, Sharma P, Kapoor KK, Hundal MS (2008) Tetrahedron 64:536
Mobinikhaledi A, Foroughifar N, Fard MAB, Moghanian H, Ebrahimi S, Kalhor M (2009) Synth Commun 39:1166
Bandgar BP, More PE, Kamble VT, Totre JV (2008) Arkivoc 15:1
Lingaiah BV, Ezikiel G, Yakaiah T, Reddy GV, Rao PS (2006) Synlett 15:2507
Zhu D, Chen J, Xiao H, Liu M, Ding J, Wu H (2009) Synth Commun 39:2895
Sheldrick GM (1997) SHELXL 97, A program for the determination of crystal structure. Anorganisch-Chemisches Institut der Universität Göttingen, Germany
Cromer DT, Mann JB (1968) Acta Cryst A24:321
Stewart RF, Davidson ER, Simpson WT (1965) J Chem Phys 42:3175
Acknowledgments
Ritika Chauhan is grateful to UGC (University Grants Commission) for providing junior research fellowship. We express our thanks to the Director of University Science and Instrumentation Centre, University of Delhi, Delhi, for providing the spectral data and also acknowledge the Head of the Biophysics Department, All India Institute of Medical Sciences, New Delhi, for carrying out the X-ray studies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kidwai, M., Chauhan, R., Bhatnagar, D. et al. Nafion-H®-catalyzed synthesis of polyhydroquinolines via the Hantzsch multicomponent reaction. Monatsh Chem 143, 1675–1680 (2012). https://doi.org/10.1007/s00706-012-0742-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-012-0742-4