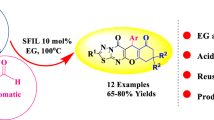
The investigation presents a straightforward synthesis of fourteen novel 2-amino-4-aryl-3-(4-fluorophenyl)-4,6,7,8-tetrahydroquinolin-5(1H)-one derivatives via a catalyst-free one-pot four-component cyclocondensation reaction of dimedone, various substituted benzaldehydes, 4-fluorophenylacetonitrile, and ammonium acetate in water under the influence of ultrasound. In comparison with the literature methods, our approach is more effective and offers several advantages, such as safe handling, excellent yields, shorter reaction time, and a simple workup procedure. All the synthesized derivatives were obtained in 87–97% yields and were characterized by IR, 1H, 13C NMR, and ESI mass spectra and elemental analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
One-pot multicomponent reactions (MCRs) have been considered an important strategy in synthesizing and designing extremely complex scaffolds possessing significant biological activity. Today, it has become a methodology of choice in various fields of research like organic, medicinal, and combinatorial chemistry.1,2,3,4,5,6, – 7 MCRs are successfully employed in the construction of the target molecules in a single operation, obtaining atom economy8 and reducing the reaction times.9
Recently, the application of ultrasound (US) has witnessed a phenomenal growth and has changed the landscape of synthetic and medicinal chemistry due to its operational simplicity, enhanced reaction rates, higher yields, and, importantly, compliance to the principles of green chemistry as advantages over traditional methods.10,11, – 12 Sonication in conjunction with catalyst-free13,14,15, – 16 synthesis has proven to be highly challenging and reliable strategy in varied chemical transformations.17
Quinolones are the structural motifs of keen interest in the field of medicinal chemistry as they have helped in establishing vital breakthroughs in antibacterial drug discovery They are extensively used in the treatment of the respiratory tract and urinary tract diseases, septicemia, nose/ear/throat infections, meningitis, endocarditis, liver and bile infections and, in addition, also possess attributes such as high combining potency with minimal side effects.18 The considerable potential of these biologically active quinolone moieties have continuously fuelled the interest of synthetic chemists to develop new or improved protocols for their synthesis and enhance their useful properties by amalgamation of diverse pharmacophoric groups in one molecular framework.
Several related approaches have been documented in the literature for the synthesis of 5-quinolinones, which generally involve the cyclocondensation of aldehydes, dimedone, an active methylene compound, and ammonium acetate.19,20,21,22, – 23 Microwave irradiation22 and solventless grinding19 have been used as activation conditions, as well as various catalysts, such as nano-ZrO2,24 Fe3O4–TiO2,25 nano-MgO,26 and nano-Fe3O4.27 Recently, we have reported a synthetic strategy for obtaining 2-amino-4-aryl-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carbo-nitriles from arylaldehydes, dimedone, malononitrile, and ammonium acetate using K2CO3 as a base in water with ultrasound treatment.28 The literature survey also reveals that phenylacetonitrile has not been used as the active methylene component in the synthesis of 5-quinolinones. In addition, the reported protocols suffer from one or several drawbacks, such as prolonged reaction time, harsh conditions, complicated preparation of the catalyst, unsatisfactory yield, and lack of generality.
As a part of our growing interest in ultrasound chemistry and with the aim of synthesizing novel 2-amino-4-aryl-3-fluorophenyl-4,6,7,8-tetrahydroquinolin-5(1H)-ones, we decided to carry out a systematic study on the preparation of the target heterocyclic compounds under significantly milder and environmentally benign conditions. Herein, we present a catalyst-free strategy for the synthesis of fourteen novel 2-amino-4-aryl-3-fluorophenyl-4,6,7,8-tetrahydroquinolin-5(1H)-one derivatives 4a–n via a one-pot fourcomponent cyclocondensation reaction of dimedone (1), various substituted benzaldehydes 2a–n, 4-fluoro-phenylacetonitrile (3), and ammonium acetate in water under ultrasonic irradiation (Scheme 1).

Scheme 1
To examine the generality and feasibility of the sonicated MCR, the reaction conditions, including catalyst, reaction medium, and energy efficiency, were optimized to inspect their influence on the rates and yield of the reaction. The interaction of dimedone (1), benzaldehyde (2a), 4-fluorophenylacetonitrile (3), and ammonium acetate in water was chosen as the model reaction.
To investigate the effect of the catalysts (10 mol %), various catalysts were screened, and the reaction was also carried out without any catalyst (Table 1). It was found that the desired product was obtained to a maximum of only about 45% with InCl3 under sonication (entry 1). Unsatisfactory yields were also obtained when other catalysts were used (entries 2–9). Fortunately, reaction without a catalyst afforded compound 4a in 97% yield (entry 10), hence, in the further studies, the reactions were carried out in the absence of catalyst under sonication.
To assess the effect of solvent, various solvents were tried out, along with a solvent-free experiment, in the synthesis of compound 4a, and the results are shown in Table 2. It was found that under the solvent-free conditions, the reaction afforded only 20% yield (entry 1). However, switching to chloroform and toluene under the same conditions failed to deliver the product at all (entries 2 and 3), whereas trace amounts of the product were detected in acetonitrile and n-hexane (entries 4 and 5). Low to moderate yields were registered in dichloromethane, DMF, THF, acetic acid, glycerol, and ethanol (entries 6–11). The best yield was obtained when H2O was employed as the solvent (entry 12). The use of polar protic solvents afforded the highest yields (entries 9–12). Hence, the best solvent for this reaction turned out to be water, and application of ultrasound has a prominent effect in improving the yield while keeping reaction time short. Hence for further studies water was chosen as solvent.
To assess the scope of the presented synthetic protocol, we subjected dimedone (1), various substituted benzaldehydes 2a–n bearing electron-donating or electron-withdrawing substituents, 4-fluorophenylacetonitrile (3), and ammonium acetate to MCR at the optimized conditions. In all the cases, these four components readily underwent cyclization into the corresponding 2-amino-4-aryl-3-(4-fluorophenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1H)-one derivatives 4a–n in excellent yields (87–97%). It was also noted that the electronic effects of the substituents did not have significant impact on the product yield.
The IR spectra of compounds 4a–n confirmed the presence of aromatic C–H, alkyl C–H, ketone C=O, and aromatic C–C bonds due to the appearance of absorption bands at 3150–3180 (compounds 4a–i), 2190–2920 (compounds 4a–l), 1710–1720, and 1585–1620 cm–1, respectively. In the 1H NMR spectra, the two singlets at 1.22–1.56 ppm were assigned to the two geminal methyl groups. The two pairs of doublets observed at 1.83–2.38 ppm correspond to the two CH2 groups and the singlet at 9.40–9.91 ppm – to the NH group of the hexahydroquinoline ring system. The singlet at 6.27–6.82 ppm was assigned to the 2-NH2 group. The disappearance of a singlet at ~10.5 ppm of CHO group clearly confirmed the cyclization of the Knoevenagel intermediate. The integrals for the signals of aromatic protons corresponded to those of the quinoline ring. The 13C NMR spectra showed the characteristic signals that were expected for the proposed structure. The 1H–13C HSQC spectrum of compound 4i showed the exact correlation between the carbon atoms linked to hydrogen atoms. The ESI mass spectra showed protonated molecular ion signals corresponding to the calculated molecular mass. The obtained elemental analysis values are in good agreement with theoretical data.
The synthesis of novel 2-amino-4-aryl-3-(4-fluorophenyl)-4,6,7,8-tetrahydroquinolin-5(1H)-ones 4a–n delineates the role of ultrasound in enhancing the rate of the reaction. The driving force for the increased efficacy of the construction of product motifs by ultrasound is the upsurge in the temperature and pressure owed to the formation of hot spots and the surge in the reactant interaction surface area through a process entitled as acoustic cavitation. Upon irradiation with ultrasound, the formation, growth, and implosive breakdown of bubbles take place, that ultimately creates an extreme chemical and physical environments in the solid–liquid systems, leading to short-lived localized hot spots with elevated pressure and temperature where the reaction rate is accelerated many times.29 Moreover, when cavitation occurs it leads to cavity collapse30 , 31 and results in the formation of liquid jets near the surface of the reaction vessel. Thus, the reaction rate is enhanced also by mechanochemical effects, along with the thermal ones. The use of water provides for compression-rarefaction cycles with a large pressure amplitude32 thereby facilitating the formation of cavitation bubbles, which allows the conversion of the reactants to happen swiftly.
It is reasonable to assume that, the first step of the reaction may involve the reaction of dimedone (1) with ammonia (generated from ammonium acetate) under sonication to give aminal A and, upon elimination of water, β-enaminone B that may attack the activated aldehyde 2 to form adduct C. The latter may lose another molecule of water to give adduct D. The Knoevanagel adduct D may react with anion E (formed from the electron-poor nitrile 3) to give the intermediate F which undergoes intramolecular cyclization to the final product 4 (Scheme 2).

Scheme 2
In other solvents and in the presence of catalysts, a range of secondary reactions are possible involving the starting materials and intermediates, which may explain the low yields under these conditions. It appears from our results that the mechanical effect initiated by the ultrasound along with water as a solvent play a major role in the formation of the target products by facilitating and accelerating the desired reaction.
We have developed an elegant, efficient, easy, and direct procedure for the synthesis of fourteen novel 2-amino-4-aryl-3-(4-fluorophenyl)-4,6,7,8-tetrahydroquinolin-5(1H)-ones under ultrasonic irradiation using dimedone, substituted benzaldehydes, 4-fluorophenylacetonitrile, and ammonium acetate in water as a solvent. The effect of water as a solvent and the ultrasound technique is in the preparation of the products in excellent yield under the aspect of environmentally benign processes. This methodology has numerous and significant advantages, such as those including atom economy, the use of a green solvent, mild catalyst-free conditions, short reaction time, as well as an easier workup procedure when compared with the conventional methods.
Experimental
FT-IR (ATR) spectra were recorded on a Cary 630 spectrophotometer equipped with an Agilent diffuse reflectance sampling interface. 1H and 13C NMR spectra were recorded on a Bruker Avance III FT-NMR spectrometer (400 and 100 MHz, respectively) in DMSO-d 6. ESI mass spectra were recorded on a Bruker Daltonics HCT Ultra ETD with ESI source. Elemental analysis was carried out using a vario MICRO cube CHN-analyzer. Melting points were determined on a RAAGA apparatus. Reagents and solvents of commercial grade were used without further purification. Liquid aldehydes were distilled before use. Sonication was performed using a SIDILU sonic bath operating at a constant frequency of 35 kHz and an output power of 80 W at 26°C (maintained by circulating water). The reactions were performed in open vessels without any external mechanical stirring. The progress of the reactions and the purity of products were assessed by TLC on Merck60 F254 analytical silica gel plates.
Synthesis of 2-amino-4-aryl-3-(4-fluorophenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1 H )-ones 4a–n (General method). Dimedone (1) (1.00 mmol, 0.140 g), an appropriate benzaldehyde 2a–n (1 mmol), 4-fluorophenylacetonitrile (3) (1.00 mmol, 0.135 g), ammonium acetate (1.00 mmol, 0.077 g) in H2O (3 ml) were placed into a conical flask and sonicated in a sonic bath for 20 min. In order to follow the reaction, an aliquot of the reaction mixture was taken in a small test tube and extracted with a few drops of diethyl ether, and then TLC was performed using hexane–AcOEt, 7:3, as the eluent. After the completion of the reaction, the reaction mixture was quenched with crushed ice, the precipitated solid was filtered off, washed with water, and recrystallized from ethanol.
2-Amino-3-(4-fluorophenyl)-7,7-dimethyl-4-phenyl-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4a). Yield 97%, white solid, mp 153–154°C. IR spectrum, ν, cm–1: 3150, 2900, 1710, 1600, 1350, 980, 620. 1H NMR spectrum, δ, ppm (J, Hz): 1.30 (3H, s, CH3); 1.45 (3H, s, CH3); 1.90 (1H, d, J = 7.6) and 2.09 (1H, d, J = 7.6, CH2); 2.29 (1H, d, J = 8.4) and 2.33 (1H, d, J = 7.6, CH2); 4.37 (1H, s, CH); 6.69 (2H, s, NH2); 6.90 (2H, d, J = 8.4, H Ar); 7.00 (1H, t, J = 8.8, H Ar); 7.20–7.22 (2H, m, H Ar); 7.40 (2H, t, J = 8.8, H Ar); 6.65 (2H, d, J = 9.0, H Ar); 9.61 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.2; 35.4; 36.9; 44.5; 57.1; 90.0; 107.6; 114.1 (d, 2 JCF = 21.0); 122.5; 127.5; 129.3 (d, 3 JCF = 8.0); 130.4; 132.5; 134.2; 140.4; 148.4; 160.3 (d, 1 JCF = 242.0); 196.6. Mass spectrum, m/z: 363 [M+H]+. Found, %: C 76.36; H 6.52; N 7.67. C23H23FN2O. Calculated, %: C 76.22; H 6.40; N 7.73.
2-Amino-3,4-bis(4-fluorophenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4b). Yield 90%, white solid, mp 146–147°C. IR spectrum, ν, cm–1: 3170, 2910, 1710, 1620, 1330, 960, 600. 1H NMR spectrum, δ, ppm (J, Hz): 1.39 (3H, s, CH3); 1.50 (3H, s, CH3); 1.83 (1H, d, J = 7.6) and 2.06 (1H, d, J = 7.6, CH2); 2.28 (1H, d, J = 7.6) and 2.32 (1H, d, J = 7.6, CH2); 4.40 (1H, s, CH); 6.69 (2H, s, NH2); 7.07 (2H, d, J = 8.6, H Ar); 7.27–7.31 (2H, m, H Ar); 7.54 (2H, d, J = 9.6, H Ar); 7.74–7.77 (2H, m, H Ar); 9.61 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.7; 35.6; 36.8; 44.7; 53.4; 89.9; 107.4; 112.8 (d, 2 JCF = 22.0); 113.1 (d, 2 JCF = 23.0); 127.4 (d, 3 JCF = 8.0); 129.4 (d, 3 JCF = 8.0); 135.6; 137.6; 140.2; 148.6; 159.5 (d, 1 JCF = 244.0); 160.5 (d, 1 JCF = 243.0); 195.8. Mass spectrum, m/z: 381 [M+H]+. Found, %: C 72.69; H 5.73; N 7.40. C23H22F2N2O. Calculated, %: C 72.61; H 5.83; N 7.36.
2-Amino-4-(2-chlorophenyl)-3-(4-fluorophenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4c). Yield 92%, white solid, mp 166–167°C. IR spectrum, ν, cm–1: 3160, 2900, 1710, 1625, 1340, 965, 610. 1H NMR spectrum, δ, ppm (J, Hz): 1.36 (3H, s, CH3); 1.59 (3H, s, CH3); 1.90 (1H, d, J = 8.0) and 2.08 (1H, d, J = 8.0, CH2); 2.21 (1H, d, J = 7.6) and 2.25 (1H, d, J = 8.0, CH2); 4.52 (1H, s, CH); 6.70 (2H, s, NH2); 6.87 (2H, d, J = 8.6, H Ar); 7.15–7.20 (1H, m, H Ar); 7.35–7.39 (1H, m, H Ar); 7.73 (1H, d, J = 8.8, H Ar); 7.89 (1H, d, J = 7.2, H Ar); 8.07–8.12 (2H, m, H Ar); 9.40 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.2; 35.6; 36.4; 44.7; 53.7; 89.9; 107.7; 113.4 (d, 2 JCF = 21.0); 123.5; 125.6; 127.4; 129.3 (d, 3 JCF = 8.0); 131.6; 133.6; 135.8; 137.8; 145.2; 151.7; 160.5 (d, 1 JCF = 242.0); 196.6. Mass spectrum, m/z: 397 [M+1]+. Found, %: C 69.70; H 5.63; N 7.11. C23H22ClFN2O. Calculated, %: C 69.60; H 5.59; N 7.06.
2-Amino-4-(4-chlorophenyl)-3-(4-fluorophenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4d). Yield 90%, white solid, mp 186–187°C. IR spectrum, ν, cm–1: 3175, 2920, 1710, 1615, 1330, 975, 600. 1H NMR spectrum, δ, ppm (J, Hz): 1.37 (3H, s, CH3); 1.54 (3H, s, CH3); 1.96 (1H, d, J = 8.0) and 2.18 (1H, d, J = 8.0, CH2); 2.33 (1H, d, J = 8.0) and 2.37 (1H, d, J = 8.0, CH2); 4.64 (1H, s, CH); 6.74 (2H, s, NH2); 6.97 (2H, d, J = 7.6, H Ar); 7.28 (2H, d, J = 6.8, H Ar); 7.34 (2H, d, J = 8.8, H Ar); 7.55 (2H, d, J = 7.6, H Ar); 9.87 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.3; 35.8; 36.5; 44.8; 53.5; 90.0; 107.9; 113.3 (d, 2 JCF = 22.0); 125.9; 127.5; 129.5 (d, 3 JCF = 8.0); 135.1; 137.4; 140.5; 148.1; 153.6; 160.3 (d, 1 JCF = 242.0); 195.5. Mass spectrum, m/z: 397 [M+H]+. Found, %: C 69.68; H 5.62; N 7.18. C23H22ClFN2O. Calculated, %: C 69.60; H 5.59; N 7.06.
2-Amino-4-(3-bromophenyl)-3-(4-fluorophenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4e). Yield 90%, white solid, mp 177–178°C. IR spectrum, ν, cm–1: 3165, 2905, 1710, 1615, 1330, 965, 620. 1H NMR spectrum, δ, ppm (J, Hz): 1.29 (3H, s, CH3); 1.51 (3H, s, CH3); 1.87 (1H, d, J = 8.0) and 2.09 (1H, d, J = 8.0, CH2); 2.30 (1H, d, J = 8.0) and 2.34 (1H, d, J = 8.0, CH2); 4.59 (1H, s, CH); 6.69 (2H, s, NH2); 6.93 (2H, d, J = 7.2, H Ar); 7.16–7.23 (2H, m, H Ar); 7.41–7.44 (1H, m, H Ar); 7.55–7.59 (1H, m, H Ar); 7.72–7.76 (2H, m, H Ar); 9.91 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.8; 35.5; 36.6; 44.8; 53.4; 90.2; 107.1; 113.2 (d, 2 JCF = 21.0); 124.0; 125.7; 127.7 (2C); 129.6 (d, 3 JCF = 8.0); 135.3; 137.5; 140.8; 148.6; 154.0; 160.7 (d, 1 JCF = 242.0); 196.7. Mass spectrum, m/z: 441 [M+H]+. Found, %: C 62.62; H 5.09; N 6.33. C23H22BrFN2O. Calculated, %: C 62.59; H 5.02; N 6.35.
2-Amino-4-(4-bromophenyl)-3-(4-fluorophenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4f). Yield 87% yield, white solid, mp 162–163°C. IR spectrum, ν, cm–1: 3160, 2905, 1715, 1625, 1335, 970, 615. 1H NMR spectrum, δ, ppm (J, Hz): 1.34 (3H, s, CH3); 1.45 (3H, s, CH3); 1.89 (1H, d, J = 7.2) and 2.15 (1H, d, J = 7.2, CH2); 2.29 (1H, d, J = 7.2) and 2.33 (1H, d, J = 7.2, CH2); 4.45 (1H, s, CH); 6.69 (2H, s, NH2); 6.90 (2H, d, J = 7.2, H Ar); 7.11–7.13 (2H, m, H Ar); 7.29 (2H, m, H Ar); 7.50 (2H, d, J = 7.6, H Ar); 9.45 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.5; 35.5; 36.6; 44.6; 53.5; 90.2; 111.5; 113.4 (d, 2 JCF = 21.0); 119.6; 122.2; 126.0; 127.6; 129.5 (d, 3 JCF = 8.0); 135.3; 137.5; 143.0; 151.3; 160.8 (d, 1 JCF = 242.0); 197.2. Mass spectrum, m/z: 441 [M+H]+. Found, %: C 62.59; H 5.04; N 6.38. C23H22BrFN2O. Calculated, %: C 62.59; H 5.02; N 6.35.
2-Amino-3-(4-fluorophenyl)-7,7-dimethyl-4-(4-methylphenyl)-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4g). Yield 90%, white solid, mp 158–159°C. IR spectrum, ν, cm–1: 3150, 2910, 1710, 1615, 1320, 975, 615. 1H NMR spectrum, δ, ppm (J, Hz): 1.25 (3H, s, CH3); 1.41 (3H, s, CH3); 1.85 (1H, d, J = 7.6) and 2.01 (1H, d, J = 7.6, CH2); 2.18 (1H, d, J = 7.2) and 2.22 (1H, d, J = 7.2, CH2); 2.32 (3H, s, CH3); 4.64 (1H, s, CH); 6.75 (2H, s, NH2); 6.97 (2H, d, J = 8.0, H Ar); 7.17–7.20 (2H, m, H Ar); 7.28–7.31 (2H, m, H Ar); 7.99 (2H, d, J = 8.8, H Ar); 9.67 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 24.4; 26.4; 35.4; 36.4; 44.7; 53.7; 90.0; 101.5; 114.6 (d, 2 JCF = 21.0); 118.8; 125.4; 127.8 (2C); 129.3 (d, 3 JCF = 8.0); 137.4; 142.6; 150.7; 161.0 (d, 1 JCF = 242.0); 196.6. Mass spectrum, m/z: 377 [M+H]+. Found, %: C 76.40; H 6.53; N 7.41. C24H25FN2O. Calculated, %: 76.57; H 6.69; N 7.44.
2-Amino-4-(3,4-dimethoxyphenyl)-3-(4-fluorophenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4h). Yield 95%, white solid, mp 170–171°C. IR spectrum, ν, cm–1: 3170, 2920, 1710, 1625, 1330, 1110, 955, 625. 1H NMR spectrum, δ, ppm (J, Hz): 1.35 (3H, s, CH3); 1.56 (3H, s, CH3); 1.85 (1H, d, J = 7.2) and 2.03 (1H, d, J = 7.2, CH2); 2.26 (1H, d, J = 7.2) and 2.30 (1H, d, J = 7.2, CH2); 3.57 (3H, s, OCH3); 3.70 (3H, s, OCH3); 4.74 (1H, s, CH); 6.54 (2H, s, NH2); 6.74–6.76 (2H, m, H Ar); 6.95–6.98 (1H, m, H Ar); 7.10–7.13 (1H, m, H Ar); 7.32 (1H, s, H Ar); 7.54–7.58 (2H, m, H Ar); 9.81 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.3; 35.6; 36.5; 44.7; 53.6; 60.1; 90.0; 113.1 (d, 2 JCF = 21.0); 119.4; 121.4; 122.4; 125.1; 129.2 (d, 3 JCF = 8.0); 135.0; 137.6; 138.5; 142.6; 150.7; 151.8; 160.3 (d, 1 JCF = 242.0); 196.6. Mass spectrum, m/z: 423 [M+H]+. Found, %: C 71.10; H 6.32; N 6.69. C25H27FN2O3. Calculated, %: C 71.07; H 6.44; N 6.63.
2-Amino-3-(4-fluorophenyl)-7,7-dimethyl-4-(3,4,5-trimethoxyphenyl)-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4i). Yield 95%, buff solid, mp 182–183°C. IR spectrum, ν, cm–1: 3180, 2910, 1715, 1615, 1335, 1100, 950, 635. 1H NMR spectrum, δ, ppm (J, Hz): 1.27 (3H, s, CH3); 1.42 (3H, s, CH3); 1.99 (1H, d, J = 8.0) and 2.15 (1H, d, J = 8.0, CH2); 2.30 (1H, d, J = 8.0) and 2.34 (1H, d, J = 8.0, CH2); 3.58 (6H, s, OCH3); 3.67 (3H, s, OCH3); 4.65 (1H, s, CH); 6.27 (2H, s, NH2); 6.97 (2H, d, J = 8.4, H Ar); 7.25 (2H, s, H Ar); 7.52 (2H, d, J = 8.4, H Ar); 9.78 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.1; 35.6; 36.4; 44.5; 50.2; 56.6; 57.4; 90.0; 111.2; 113.3 (d, 2 JCF = 22.0); 119.1; 129.9 (d, 3 JCF = 8.0); 135.0; 137.4; 138.7; 140.3; 143.5; 150.6; 160.3 (d, 1 JCF = 243.0); 197.4. Mass spectrum, m/z: 453 [M+H]+. Found, %: C 69.07; H 6.95; N 6.22. C26H29FN2O4. Calculated, %: C 69.01; H 6.46; N 6.19.
2-Amino-3-(4-fluorophenyl)-4-(2-hydroxyphenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4j). Yield 90%, pale-yellow solid, mp 169–170°C. IR spectrum, ν, cm–1: 3450, 3010, 2915, 1710, 1590, 1300, 945, 630. 1H NMR spectrum, δ, ppm (J, Hz): 1.30 (3H, s, CH3); 1.41 (3H, s, CH3); 1.94 (1H, d, J = 8.4) and 2.15 (1H, d, J = 8.4, CH2); 2.31 (1H, d, J = 8.0) and 2.35 (1H, d, J = 7.6, CH2); 4.61 (1H, s, CH); 5.59 (1H, s, OH); 6.76 (2H, s, NH2); 6.88–6.92 (2H, m, H Ar); 7.10 (1H, d, J = 8.4, H Ar); 7.28−7.35 (2H, m, H Ar); 7.52 (1H, d, J = 8.4, H Ar); 7.77 (2H, d, J = 8.8, H Ar); 9.68 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.5; 35.3; 36.1; 44.7; 53.5; 90.0; 111.2; 113.3 (d, 2 JCF = 21.0); 116.5; 121.7; 125.6; 127.1; 129.4 (d, 3 JCF = 8.0); 133.4; 135.3; 137.7; 150.6; 155.1; 160.4 (d, 1 JCF = 242.0); 198.1. Mass spectrum, m/z: 379 [M+H]+. Found, %: C 73.07; H 6.19; N 7.46. C23H23FN2O2. Calculated, %: C 73.00; H 6.13; N 7.40.
2-Amino-3-(4-fluorophenyl)-4-(3-hydroxyphenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4k). Yield 90%, white solid, mp 153–154°C. IR spectrum, ν, cm–1: 3020, 2900, 1710, 1585, 1320, 965, 620. 1H NMR spectrum, δ, ppm (J, Hz): 1.22 (3H, s, CH3); 1.43 (3H, s, CH3); 1.95 (1H, d, J = 7.6) and 2.19 (1H, d, J = 7.6, CH2); 2.33 (1H, d, J = 7.6, CH2); 2.36 (1H, d, J = 7.6, CH2); 4.49 (1H, s, CH); 5.54 (1H, s, OH); 6.82 (2H, s, NH2); 6.99 (2H, d, J = 7.2, H Ar); 7.20 (2H, d, J = 8.0, H Ar); 7.39 (1H, d, J = 7.6, H Ar); 7.52 (1H, d, J = 8.8, H Ar); 7.72 (2H, d, J = 7.6, H Ar); 9.85 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.4; 35.6; 36.6; 44.5; 53.2; 90.1; 108.4; 111.2; 113.7 (d, 2 JCF = 21.0); 116.4; 125.9; 127.3; 129.5 (d, 3 JCF = 8.0); 133.3; 136.0; 137.8; 150.6; 155.8; 160.6 (d, 1 JCF = 242.0); 197.6. Mass spectrum, m/z: 379 [M+H]+. Found, %: C 73.12; H 6.20; N 7.42. C23H23FN2O2. Calculated, %: C 73.00; H 6.13; N 7.40.
2-Amino-3-(4-fluorophenyl)-4-(4-hydroxyphenyl)-7,7-dimethyl-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4l). Yield 90%, white solid, mp 171–172°C. IR spectrum, ν, cm–1: 3480, 3010, 2905, 1715, 1610, 1310, 955, 615. 1H NMR spectrum, δ, ppm (J, Hz): 1.21 (3H, s, CH3); 1.46 (3H, s, CH3); 2.00 (1H, d, J = 8.0) and 2.18 (1H, d, J = 8.0, CH2); 2.38 (1H, d, J = 8.0) and 2.42 (1H, d, J = 8.0, CH2); 4.39 (1H, s, CH); 5.58 (1H, s, OH); 6.82 (2H, s, NH2); 6.84–6.88 (2H, m, H Ar); 7.06–7.09 (2H, m, H Ar); 7.30–7.33 (2H, m, H Ar); 7.52–7.55 (2H, m, H Ar); 9.71 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.3; 35.5; 36.5; 44.5; 53.5; 90.0; 107.1; 113.1 (d, 2 JCF = 21.0); 127.2; 129.3 (d, 3 JCF = 8.0); 135.4; 137.9; 140.4; 148.1; 158.3; 161.1 (d, 1 JCF = 242.0); 196.5. Mass spectrum, m/z: 379 [M+H]+. Found, %: C 73.04; H 6.18; N 7.49. C23H23FN2O2. Calculated, %: C 73.00; H 6.13; N 7.40.
7-Amino-6-(4-fluorophenyl)-2,2-dimethyl-5-(3-nitrophenyl)-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4m). Yield 95%, yellow solid, mp 162–163°C. IR spectrum, ν, cm–1: 3020, 1720, 1615, 1515, 1350, 1320, 975, 615. 1H NMR spectrum, δ, ppm (J, Hz): 1.37 (3H, s, CH3); 1.58 (3H, s, CH3); 1.92 (1H, d, J = 8.0) and 2.10 (1H, d, J = 8.0, CH2); 2.33 (1H, d, J = 7.6) and 2.36 (1H, d, J = 8.0, CH2); 4.70 (1H, s, CH); 6.78 (2H, s, NH2); 7.05–7.07 (2H, m, H Ar); 7.30 (1H, d, J = 7.6, H Ar); 7.48 (1H, d, J = 8.4, H Ar); 7.60–7.73 (2H, m, H Ar); 8.09 (2H, d, J = 8.4, H Ar); 9.42 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.4; 35.6; 36.5; 44.7; 53.8; 90.0; 107.5; 113.2 (d, 2 JCF = 22.0); 123.8; 125.4; 127.9 (2C); 129.6 (d, 3 JCF = 8.0); 135.6; 137.3; 141.5; 147.9; 155.7; 160.9 (d, 1 JCF = 242.0); 197.8. Mass spectrum, m/z: 408 [M+H]+. Found, %: C 67.84; H 5.46; N 10.30. C23H22FN3O3. Calculated, %: C 67.80; H 5.44; N 10.31.
2-Amino-3-(4-fluorophenyl)-7,7-dimethyl-4-(4-nitrophenyl)-4,6,7,8-tetrahydroquinolin-5(1 H )-one (4n). Yield 90%, pale-yellow solid, mp 175–176°C. IR spectrum, ν, cm–1: 3010, 1710, 1610, 1510, 1350, 1320, 975, 610. 1H NMR spectrum, δ, ppm (J, Hz): 1.27 (3H, s, CH3); 1.41 (3H, s, CH3); 1.89 (1H, d, J = 8.4) and 2.11 (d, J = 8.4, CH2); 2.29 (1H, d, J = 7.6) and 2.32 (1H, d, J = 7.6, CH2); 4.49 (1H, s, CH); 6.58 (2H, s, NH2); 6.83 (2H, d, J = 8.8, H Ar); 7.15 (2H, d, J = 8.8, H Ar); 7.79 (2H, d, J = 9.6, H Ar); 8.02 (2H, d, J = 8.8, H Ar); 9.54 (1H, br. s, NH). 13C NMR spectrum, δ, ppm (J, Hz): 26.6; 35.8; 36.4; 44.6; 53.4; 90.3; 107.1; 112.7 (d, 2 JCF = 21.0); 126.2; 127.6; 129.8 (d, 3 JCF = 8.0); 135.3; 137.7; 140.5; 148.6; 153.3; 161.1 (d, 1 JCF = 242.0); 196.7. Mass spectrum, m/z: 408 [M+H]+. Found, %: C 67.86; H 5.55; N 10.38. C23H22FN3O3. Calculated, %: C 67.80; H 5.44; N 10.31.
The Supplementary information file containing 1H and 13C NMR spectra and ESI mass spectra of compounds 4a–n and 1H–13C HSQC spectrum of compound 4i, is available from the journal website at http://springerlink.bibliotecabuap.elogim.com/journal/10593.
References
Zhu, J.; Bienaymé, H.: Multicomponent Reactions; Wiley-VCH: Weinheim, 2005.
Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899.
Dömling, A. Chem. Rev. 2006, 106, 17.
Dömling, A.; Ugi, I. Angew. Chem. Int. 2000, 39, 3168.
Kappe, C. O. QSAR Comb. Sci. 2003, 22, 630.
Ahmed, N.; van Lier, J. E. Tetrahedron Lett. 2007, 48, 5407.
Ugi, I.; Werner, B.; Dömling, A. Molecules 2003, 8, 53.
Trost, B. M. Acc. Chem. Res. 2002, 35, 695.
Shaterian, H. R.; Yarahmadi, H. Tetrahedron Lett. 2008, 49, 1297.
Pasha, M. A.; Swamy, N. R.; Jayashankara, V. P. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2005, 44B, 823.
Pasha, M. A.; Jayashankara, V. P. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2007, 46B, 1328.
Datta, B.; Pasha, M. A. Ultrason. Sonochem. 2011, 18, 624.
Wang, S. X.; Li, Z. Y.; Zhang, J.-C.; Li, J. T. Ultrason. Sonochem. 2008, 15, 677.
Shekouhy, M.; Hasaninejad, A. Ultrason. Sonochem. 2012, 19, 307.
Safari, J.; Banitaba, S. H.; Khalili, S. D. Ultrason. Sonochem. 2012, 19, 1061.
Banitaba, S. H.; Safari, J.; Khalili, S. D. Ultrason. Sonochem. 2013, 20, 401.
Rama, K.; Pasha, M. A. Ultrason. Sonochem. 2005, 12, 437.
Pintilie, L.; Negut, C.; Oniscu, C.; Caproiu, M. T.; Nechifor, M.; Iancu, L.; Ghiciuc, C.; Ursu, R. Rom. Biotech. Lett. 2009, 14, 4756.
Kumar, S.; Sharma, P.; Kapoor, K. K.; Hundal, M. S. Tetrahedron 2008, 64, 536.
Lichitsky, B. V.; Dudinov, A. A.; Krayushkin, M. M. ARKIVOC 2001, (ix), 73.
Elnagdi, M. H.; Aal, A.; Maksoud, F. A.; Yassin, Y. M. J. Prakt. Chem. 1989, 331, 971.
Tu, S.; Zhang, J.; Zhu, X.; Zhang, Y.; Wang, Q.; Xu, J.; Jiang, B.; Jia, R.; Zhang, J.; Shi, F. J. Heterocycl. Chem. 2006, 43, 985.
Said, S. A.; Moustafa, A. H. J. Chem. Res. 2010, 34, 528.
Amoozadeh, A.; Rahmani, S.; Bitaraf, M.; Abadi, F. B.; Tabrizian, E. New J. Chem. 2016, 40, 770.
Tabrizian, E.; Amoozadeh, A. Catal. Sci. Technol. 2016, 6, 6267.
Abaszadeh, M.; Seifi, M.; Asadipour, A. Synth. React. Inorg., Met.-Org., Nano-Met. Chem. 2016, 46, 512.
Amirheidari, B.; Seifi, M.; Abaszadeh, M. Res. Chem. Intermed. 2016, 42, 3413.
Siddekha, A.; Azzam, S. H. S.; Pasha, M. A. Synth. Commun. 2014, 44, 424.
Safari, J.; Zarnegar, Z. Ultrason. Sonochem. 2013, 20, 740.
Gogate, P. R.; Mujumdar, S.; Pandit, A. B. Adv. Environ. Res. 2003, 7, 283.
Mason, T. J. Ultrason. Sonochem. 2003, 10, 175.
Carnell, M. T.; Gentry, T. P.; Emmony, D. C. Ultrasonics 1998, 36, 689.
The authors gratefully acknowledge the financial assistance by the VGST, Department of Information Technology, Biotechnology and Science & Technology, Government of Karnataka for the CESEM Award Grant No. 24 (2010-2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(11), 964–969
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 7840 kb)
Rights and permissions
About this article
Cite this article
Govindaraju, S., Tabassum, S., Khan, RuR. et al. Catalyst-free green synthesis of novel 2-amino-4-aryl-3-(4-fluorophenyl)-4,6,7,8-tetrahydroquinolin-5(1H)-ones via a one-pot four-component reaction under ultrasonic condition. Chem Heterocycl Comp 52, 964–969 (2016). https://doi.org/10.1007/s10593-017-1994-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-1994-z