Abstract
Mayaro fever, caused by Mayaro virus (MAYV) is a sub-lethal disease with symptoms that are easily confused with those of dengue fever, except for polyarthralgia, which may culminate in physical incapacitation. Recently, outbreaks of MAYV have been documented in metropolitan areas, and to date, there is no therapy or vaccine available. Moreover, there is no information regarding the three-dimensional structure of the viral proteins of MAYV, which is important in the search for antivirals. In this work, we constructed a three-dimensional model of protein C of MAYV by homology modelling, and this was employed in a manner similar to that of receptors in virtual screening studies to evaluate 590 molecules as prospective antiviral agents. In vitro bioassays were utilized to confirm the potential antiviral activity of the flavonoid epicatechin isolated from Salacia crassifolia (Celastraceae). The virtual screening showed that six flavonoids were promising ligands for protein C. The bioassays showed potent antiviral action of epicatechin, which protected the cells from almost all of the effects of viral infection. An effective concentration (EC50) of 0.247 μmol/mL was observed with a selectivity index (SI) of 7. The cytotoxicity assay showed that epicatechin has low toxicity, with a 50% cytotoxic concentration (CC50) greater than 1.723 µmol/mL. Epicatechin was found to be twice as potent as the reference antiviral ribavirin. Furthermore, a replication kinetics assay showed a strong inhibitory effect of epicatechin on MAYV growth, with a reduction of at least four logs in virus production. Our results indicate that epicatechin is a promising candidate for further testing as an antiviral agent against Mayaro virus and other alphaviruses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mayaro virus (MAYV), which in Brazil was previously considered to be restricted to Amazon riverside regions, has been increasingly studied after being isolated in recent years in urban centres of the states of Mato Grosso [1], Goiás, Tocantins [2] and São Paulo [3]. In forest areas, mosquitoes of the genus Haemagogus are the principal vectors for MAYV. However, the confirmation of their potential to be transmitted by Aedes aegypti reveals a latent public health threat [4]. Mayaro fever is a debilitating and widely neglected disease that is beginning to threaten urban areas [5]. In the endemic region of Amazônia, many cases of Mayaro fever go undiagnosed. It is estimated that approximately 1% of all cases diagnosed as dengue in the northern regions of South America are caused by MAYV [6]. This fact, together with insufficient epidemiological surveillance and the lack of precise diagnostic methods, is among the reasons for the low level of recognition of this disease.
MAYV is closely related to chikungunya virus (CHIKV) and produces a highly debilitating clinical condition, characterized by severe arthralgia that can last for weeks or months [7]. According Halsey et al., 54% of the patients infected by MAYV develop persistent arthralgia affecting the major joints [8]. Little is known regarding the process of induction of arthralgia in infected individuals. There is evidence that arthropathy caused by arthritogenic viruses is due to inflammatory responses induced by the virus or the viral products inside the joint tissues [9].
Currently, there is no vaccine or therapy available for MAYV infection. Symptoms are treated using simple analgesics and/or non-steroidal anti-inflammatory drugs, which provide relief to the patients [10]. Santiago et al. studied the long-term immune response in patients infected by MAYV and showed that all of the patients produced antibody against MAYV [11]. Additionally, the intensity of the response did not differ between patients who developed severe arthralgia and those who recovered completely. This finding revealed that a neutralizing antibody response was not sufficient to protect the individuals from developing arthralgia, especially because the illness correlated with the capacity of the antibodies to reach and neutralize the virus possibly located in various joints. These authors therefore suggest that the development of vaccines seems to be an ineffective option for protection against chronic arthralgia and that antiviral substances still represent a better option for the treatment of infections by arthritogenic alphaviruses. In this context, the identification of compounds with antiviral potential as well as anti-MAYV targets is of great importance.
There is still a lack of data available in the literature on the three-dimensional structures of MAYV proteins. Most of the information available is related to other alphaviruses. The genome of MAYV consists of a single, positive-sense RNA strand containing two open reading frames (ORF) that are separated by an intergenic region. The first ORF of the virus genome can be read directly and codes for four non-structural proteins, nsP1, nsP2, nsP3 and nsP4, which are involved in the transcription and replication of the viral RNA [12]. The second ORF is translated as a result of programmed ribosomal frameshifting events from a subgenomic 26S RNA. After undergoing cleavage by enzymes present in the host and by autoproteolytic activity of the C-terminal domain, the structural proteins E1, E2, E3, 6k and C or TF, E2, E3, 6k and C [12,13,14,15] are produced in the host cell. The C protein is a component of the nucleocapsid, responsible for the packaging of the viral RNA [16] and is involved in viral particle formation through its interaction with the cytoplasmic domain of glycoprotein E2 in the cell membrane [17]. The structure of the C protein has been determined for several alphaviruses and has been found to contain a conserved hydrophobic pocket that is involved in protein-protein interactions and represents a promising target for the development of antiviral agents [18,19,20].
Natural products have a large variety in terms of their structure and physicochemical and biological properties, which makes them attractive in drug development studies. Compounds obtained from members of the genus Salacia are utilized in different countries in folk medicine, primarily against diabetes and as anti-inflammatory drugs. Compounds of the pentacyclic triterpene (TTPC) and flavonoid classes are primarily found in Salacia crassifolia leaf extracts [21].
In view of the important role of protein C in the replication cycle of alphaviruses, in this work, we attempted to model the three-dimensional structure of the MAYV protein C and use it as target for virtual screening studies for different compounds. Modelling results were confirmed by bioassays in order to search for novel drugs against MAYV.
Materials and methods
Source of the primary sequence and building the model
The primary sequence of MAYV protein C was obtained from the National Center for Biotechnology Information (NCBI) database [22] in the FASTA format. The MAYV protein C model was built using the Swiss-Model portal, based on a target-template alignment, using the structure of the Aura virus C protein complexed to a dioxane molecule (PDB accession no. 4AGJ_A) [23] as a model. The search of suitable templates was performed using the Basic Local Alignment Search Tool (BLASTp).
Evaluation of the quality of the model
The global quality of the model was evaluated based on the root-mean-square deviation (RMSD) value, obtained by overlapping the peptide chain of the conserved regions of the generated model using the Swiss-PdbViewer programme [24]. Validation of the stereochemical quality of the model was performed using a Ramachandran plot, which was generated using RAMPAGE [25], and by the QMEAN Z-score [26], which relates the scores obtained for parameters such as local geometry, distance-dependent interaction potential, and solvation potential of the constructed model, with scores being obtained for reference structures determined experimentally by X-ray crystallography.
Inverse virtual screening
Inverse virtual screening was performed using the Octopus 1.0 framework [27]. The ligands were obtained from the ZINC database [28] and the related literature. The predicted charges of the compounds at physiological pH were calculated, and possible tautomers were evaluated using the Marvin Sketch programme [29]. Since the protein C model had already been built and validated, re-docking of the crystallographic ligand present in the template (dioxane) was performed in order to validate the methodology. To explore the binding site of the dioxane and the flexibility of the ligand, a box was built for the modelled protein C of MAYV using the AutoDock Tools programme [30]. This box was defined as a cube with dioxane-centric geometry with dimensions of 14 x 14 x 14 Å, spacing of 1 Å and coordinates X = -23.833, Y = 11.281 and Z = -9.142. Next, the other ligands were docked against the MAYV protein C model utilizing the AutoDockVina 1.0.2 programme [31].
Phytochemical study
The leaves of Salacia crassifolia (Mart. ex Schult.) G. Don, Celastraceae, were collected in Montes Claros, Minas Gerais, Brazil, and the species was identified by Dr. Maria Olívia Mercadante-Simões of Universidade Estadual de Montes Claros, Brazil. A voucher specimen (No. HBCB 144624) was preserved in the herbarium of Instituto de Ciências Biológicas, UFMG. The plant materials were collected as authorized by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (Process: 010119/2014-0).
The purification processes were performed by column chromatography (CC) using silica gel 60 (0.063-0.200 mm) as the stationary phase. Pure organic solvents or organic solvents in mixtures of increasing polarity were used as mobile phases. Silica gel 60 (Merck) was used to prepare plates (0.25 mm) for analytical thin-layer chromatography (TLC).
The ethyl acetate extract (10 g) from S. crassifolia was subjected to silica gel CC eluted with hexane (Hex), chloroform (CHCl3), ethyl acetate (EtOAc), and pure methanol (MeOH) or in mixtures of increasing polarity. The fraction (439.1 mg) obtained with EtOAc/MeOH (4:6) after the removal of the solvent was subjected to silica gel CC, eluted with Hex, CHCl3, EtOAc and MeOH, pure or in mixtures of increasing polarity. An AcOEt/MeOH (25:75) fraction was isolated as a solid. The weight of the sample was 139.0 mg, and the melting point was 154.2-158.8°C.
1H (400 MHz) and 13C (100 MHz) NMR experiments were carried out on a Bruker Avance DRX-400 instrument operating at 300 K. The chemical shift assignments (δ) were expressed in parts per million (ppm), and coupling constants (J) were expressed in Hertz (Hz). Tetramethylsilane was used as an internal standard (δ H = δ C= 0). Melting points were determined on an MQAPF-302 apparatus (Microquímica Equipamentos Ltda) [32].
Cells and viruses
Vero cells (derived from African green monkey kidney, ATCC CCL-81) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Cultilab, BRA) supplemented with 5% fetal bovine serum (FBS) (Cultilab, BRA) and 100 U of penicillin, 100 μg of streptomycin, and 2.5 μg of amphotericin B (Sigma-Aldrich, USA) per mL. Cells were maintained at 37°C in 5% CO2.
Mayaro virus strain BeAr 20290, kindly provided by Dr. Maurício Lacerda Nogueira (Faculdade de Medicina de São José do Rio Preto / Famerp), was propagated in Vero cells and titrated using a plaque assay [33]. The viral stock was kept at -80°C until the time of use.
Cytotoxicity assay
Cytotoxicity was evaluated using the colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method [34]. Vero cells grown in 96-well microplates (5 × 104 cells/well) were treated with different concentrations of compounds (0 to 1.723 μmol/mL) diluted in DMEM (containing 2.5% FBS) and incubated at 37°C. After 48 hours, the medium of the wells was replaced by 25 μL of MTT solution (2 mg/mL in 1x PBS), and the plates were incubated for 2 hours at 37°C in 5% CO2 to allow the tetrazolium salt to be metabolized by the viable cells, after which 100 μL of DMSO was added. The optical density of the solution in the wells of the microplate was determined by spectrophotometry at 492 nm. Using regression analysis, absorbance values to determine of the concentration of the compound that reduced cell viability by 50% (CC50) compared to the viability of the untreated cells.
Global antiviral activity assay
For the global antiviral activity, 90% confluent Vero cells (5 × 104 cells/well) in 96-well microplates and the viral inoculum were pre-treated for 30 minutes at 37°C with different dilutions of the compounds, ranging from 0.013 to 0.574 μmol/mL. This pre-treatment was performed to include all of the stages of the viral cycle at which the compound could act, i.e., in adsorption, penetration, viral replication or virucidal effect. Next, the pre-treated cells were infected with the pretreated viral inoculum at a multiplicity of infection (moi) of 0.1 or 5. To evaluate other conditions, the treatment was done at different times in the viral life cycle. To analyze the virucidal effect, the drug was mixed with the viral inoculum 1 hour before infection. After 48 hours of incubation at 37°C in 5% CO2, cell viability was also determined using the MTT colorimetric method [34]. The optical density of the solution was determined by spectrophotometry at 492 nm. Using regression analysis, absorbance values were used to calculate the 50% effective concentration (EC50), the concentration that resulted in protection of 50% of the cells from viral infection. Ribavirin (Sigma-Aldrich, USA) and DMSO (Sigma-Aldrich, USA) were used as a positive and a negative control, respectively. The selectivity index (SI) was expressed as the CC50/EC50 ratio, and the relative potency (RP) was expressed as the ratio of the EC50 of ribavirin (reference antiviral) to that of the tested compound. When appropriate, the virus concentration (in plaque-forming units [PFU] per mL), was determined using the same culture supernatant.
Replication kinetics
To examine the replication kinetics of Mayaro virus in the absence or presence of epicatechin, 90% confluent Vero cells (5 × 104 cells/well) in 96-well microplates and the viral inoculum (moi, 5) were pre-treated for 30 minutes at 37°C with epicatechin at a concentration of 0.344 µmol/mL. The pre-treated cells were then infected with the pre-treated viral inoculum for 1 hour at 37°C. After viral adsorption, the inoculum was removed and the cells were washed with 1x PBS, after which DMEM medium containing epicatechin at a concentration of 0.344 µmol/mL was added. The supernatants from the wells were collected at 1, 3, 6, 12, 24 and 36 hours postinfection (hpi), and the virus yield was determined by plaque assay [33].
Statistical analysis
Regression analysis was performed and the mean ± standard deviation of triplicate samples was determined using the statistical software GraphPad Prism® v. 7 (GraphPad Software, Inc. La Jolla, California, USA). A one-way ANOVA test was used to compare treatments. Dunnett’s test was used to determine differences between experimental groups and a control group (Statistica software 7.0, StatSoft, Inc., 2004). All tests were considered statistically significant when the P-value was less than 0.05.
Results
Homology modelling
A template search using an X-ray crystal structure of Aura virus protein C complexed to a dioxane molecule (PDB 4AGJ_A com) at 1.98 Å resolution, a similarity of 63% with the protein C of the MAYV was determined with a satisfactory score of 201 and an e-value of 3e−64 with limit values of 30% and 50e−7, respectively [35]. The values obtained corresponded to a root-mean-square deviation (RMSD) of 0.44 Å for the carbon alpha atoms (αC) (Fig. 1a), which was lower than that of 2 Å and therefore, considered of good quality [36]. The stereochemical quality was confirmed by a Ramachandran plot in which 93.2% of the amino acid residues were in favourable regions, 4.7% were in allowed regions, and only 2.0% were in disallowed regions (Fig. 2). The quality of the model was confirmed by a QMEAN Z-score that was close to zero, as would be expected for crystallographic structures with high resolution [26] (Fig. 3). The model was therefore regarded as appropriate for use in the subsequent docking study.
Construction of a three-dimensional model of MAYV protein C by homology modelling. (a) Superimposition of the crystal structure of Aura virus protein C (red) used as a template and the model of the MAYV protein C (yellow) (b) Three-dimensional structure of the model of MAYV protein C (ribbon representation) complexed with dioxane (represented by tubes)
Inverse virtual screening
Re-docking was performed to validate the virtual screening method. A comparison of the crystallographic and the docked dioxane conformations yielded an RMSD value of 1.92 Å (Fig. 4a). Values around 2 Å are considered satisfactory for re-docking, [37]. The binding of the dioxane to MAYV protein C exhibited a binding energy value of -2.8 kcal/mol. Then, 590 molecules were docked to the protein C site, and their respective binding energy was compared to the value (-4.9 kcal/mol) obtained for the docking of the 1,3-bis{(R)-[1,4]dioxan-2-il}propane (bisdioxane) molecule to MAYV protein C. According to Kim et al. [38], this substance exhibited potential for interaction with a similar binding site on the Sindbis virus protein C and induced antiviral effects in bioassays performed by the same authors. Of the 590 molecules evaluated, various molecules were predicted to have higher affinity for the binding site of the MAYV protein C than that of bisdioxane (Online Resource 1). Among the substances with potential antiviral activity, flavonoids stood out as a promising compound class. Six flavonoids compounds (1 to 6) were therefore docked to the binding site of the MAYV protein C with the same conformation (Fig. 4b). All of the flavonoids evaluated bound to the binding site with favourable free energy values (Online Resource 2). Of these, epicatechin (-6.8 kcal/mol) was considered the most promising. Thus, to confirm the antiviral activity in this compound, we performed bioassays using epicatechin isolated from S. crassifolia (Celestraceae).
Cytotoxicity, antiviral activity and replication kinetics
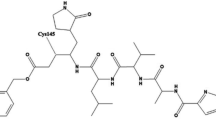
Epicatechin was used in biological assays, as it was one of the six flavonoids with favourable binding energy to MAYV protein C (-6.8 kcal/mol). Its purity was confirmed by hydrogen and carbon (1H and 13C NMR) nuclear magnetic resonance spectroscopy (Online Resource 3).
In the cytotoxicity assay, treatment of cells with epicatechin, even at the highest concentration used, did not reduce the cell viability to 50% (CC50). However, potential cytotoxicity of epicatechin was detected at 1.704 ± 0.101 μmol/mL, with only 10% reduction in cell viability.
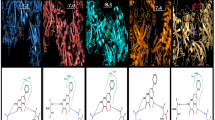
To evaluate global antiviral activity by the MTT technique, 0.430 μmol of epicatechin per mL (Fig. 5d) was able to protect approximately 100% of the MAYV-infected cells when compared to infected and untreated cells (p < 0.001) (Fig. 5b). The nucleoside analogue ribavirin (Fig. 5e), which was used as a classical positive control, was found to have an EC50 above 0.486 ± 0.008 μmol/mL and was therefore less potent than epicatechin. Because reducing the concentration also decreased the protection of cells from viral infection, the antiviral effect of epicatechin appears to be dose dependent (Fig. 6a). The EC50 value was 0.247 ± 0.026 μmol/mL (Table 1). A dose-dependent antiviral effect of epicatechin also was observed at an moi of 5, with an EC50 value of 0.413 ± 0.004 μmol/mL (Fig. 6b).
Antiviral activity of epicatechin in Vero cells infected with MAYV. Vero cells were treated with epicatechin or ribavirin and infected with MAYV (moi, 0.1) and photographed after 48 hours of infection. (a) Uninfected and untreated cells, (b) infected and untreated cells, (c) infected cells treated with DMSO, (d) infected cells treated with 0.430 μmol of epicatechin per mL, (e) infected cells treated with 1.024 μmol of Ribavirin per mL (magnification, 100x)
Anti-MAYV activity of epicatechin. The anti-MAYV activity of epicatechin was evaluated by treating MAYV-infected cells with 0–0.574 μmol of this compound per mL for 48 hours. The antiviral effect of epicatechin was dose dependent in (a) cells infected with MAYV at an moi of 0.1 and (b) cells infected with MAYV at a moi 5. MAYV-infected cells at an moi of 0.1 were also treated with ribavirin (reference substance) and DMSO. The data presented are the mean ± SD
The selectivity index (SI) and relative potency (RP) are used as selection criteria for promising drugs. The SI value for epicatechin was 7, making it twice as potent as ribavirin.
Analysis of replication kinetics showed a strong inhibitory effect of epicatechin on MAYV growth, with reduction of least four logs when compared with the MAYV-infected cells without drug treatment (Fig. 7). In the earliest phase (0 to 6 hours), MAYV was not detected. After 12 hours, virus was detected without significant variation in the yield (less than one log).
Growth kinetics of MAYV in Vero cells. Cells and virus were treated with 0.344 µmol of epicatechin per mL before and during infection (moi, 5). Infected and untreated cells were used as virus controls. Viral progeny in the supernatant was quantified at various time points postinfection by plaque assay
Discussion
The three-dimensional structure of MAYV protein C was built from the coordinates of the Aura virus protein C complexed to a dioxane molecule (accession no. PDB 4AGJ_A), to which it had 63% sequence identity. According to Takeda-Shitaka et al., models built with more than 50% sequence identity are sufficiently accurate and suitable for application in the development of new drugs [39]. The evaluated three-dimensional structure confirmed the quality of the model, confirming its possible application in molecular docking studies proposed for antiviral development.
Aggarwal et al. [23] determined the X-ray crystal structure of Aura virus protein C, which was utilized as a template in this work, and found that a dioxane solvent molecule, which was used in the crystallization process, occupied the protein C hydrophobic pocket in the a region that is believed interact the viral glycoprotein E2. According to these authors, the dioxane was inserted in the same position that would be occupied by the pyrrolidine ring of the proline 405 residue (Pro 405), a constituent of the loop region of the helix-turn-helix motif of glycoprotein E2, which is conserved in alphaviruses [23]. This suggests that the dioxane or a similar molecule may compete with binding of Pro 405 in this hydrophobic pocket and disrupt the protein C-E2 interaction, preventing the spread of the infection. The dioxane molecule is smaller than the binding site (Fig. 1b), and therefore, the few contacts that it makes culminate in a binding energy value of only -2.8 kcal/mol. In elucidating the three-dimensional structure of Sindbis virus protein C, Lee et al. also found that dioxane bound to a hydrophobic core [40]. However, in that study, the hydrophobic core was occupied by two dioxane molecules. Based on those results, Kim et al. [38] synthesized molecules containing two covalently bound dioxane molecules that were predicted to interact more strongly than the dioxane molecule alone. In vitro biological tests performed by these authors with this molecule, bisdioxane, demonstrated an antiviral effect of the compound against Sindbis virus [38]. The docking of bisdioxane to the modelled protein of MAYV resulted in a binding energy of -4.9 kcal/mol, and this value was used as a parameter for the selection of promising antiviral molecules against MAYV, since that compound showed activity against a virus that also belongs to the genus Alphavirus. Of the 590 molecules docked to protein C, six flavonoids bound to the binding site with favourable energy, and all showed higher affinity than bisdioxane.
The potential of flavonoids as antivirals has been described in the literature for some viruses. For example, studies have shown antiviral activity of these compounds against dengue virus [41,42,43,44,45], chikungunya virus [46], hepatitis C virus [47] and influenza virus [48]. Flavonoids have been reported to negatively impact the formation of the viral RNA polymerase complex [49] and the hemifusion between virus particles and the cell membrane. This reduces the integrity of the viral membrane, resulting in the loss of the possible internalization of virus particles [50] in the final stages of the viral replication cycle [51]. Additionally, flavonoids are potent inhibitors of entry, acting on the viral particle even before it is adsorbed to cellular receptors [52]. However, it remains unclear whether the antiviral activity of flavonoids is based on their interactions with one or more targets.
In the present study, molecules with promising antiviral properties against Mayaro virus were identified by virtual screening. The activity of epicatechin flavonoid (EC50, 0.247 μmol/mL) isolated from Salacia crassifolia, a plant species of the family Celastraceae, was confirmed by in vitro biological assays. This result was consistent with those reported by Takeshita et al., who demonstrated antiviral activity of epicatechin against hepatitis C virus (HCV), with an EC50 of 0.317 μmol/mL. Like MAYV, HCV is an enveloped virus with a single-stranded positive-sense RNA genome [53]. In cytotoxicity assays, epicatechin was found to be non-cytotoxic to Vero cells (CC50 > 1.723 μmol/mL). The SI value of 7 demonstrates that epicatechin is able to inhibit viral replication at a concentration seven times lower than the cytotoxic dose [54]. Epicatechin showed strong inhibition of MAYV growth at 36 hours postinfection (reduction of ~4 logs). After 12 hpi, MAYV was detected in small amounts without significant variations in its kinetics. The reason why the effect of epicatechin is decreasing after 12 hours is not clear. Since a high moi was used, it could be speculated that some virus evaded the virucidal action of epicatechin and initiated a subtle infection. Moreover, few viruses could escape, due to the presence of epicatechin in the extracellular environment, culminating a single cycle of infection and a low level of virus production.
In the search for mechanisms of action against MAYV, preliminary results in our laboratory indicate that a blockade of infection by epicatechin is easily observed when the compound is added in advance to the viral inoculum (Table 2). When cells and virus inoculum (global antiviral effect) or only viral inoculum (virucidal effect) were pre-treated with epicatechin before infection, a powerful effect was observed (p < 0.001). When only the cells were treated (1 hour) and rinsed prior to viral adsorption (adsorption effect), no inhibitory effects were observed (p > 0.05). Under the effective antiviral conditions, a six-log reduction in virus production was observed. When added after infection, epicatechin on average reduced virus production by two logs in the first 12 hours (data not shown). This is thought to be due to the action of epicatechin in the second cycle of infection, when mature virions are released into the extracellular environment, which is common to alphaviruses. Considering the low moi, we also do not rule out an intracellular action of epicatechin in the late stages of the viral replication. This action would be able to block important stages of the cycle and formation of new viral progeny. Thus, our data together indicate that epicatechin acts by binding to components of the viral particle, but not to cellular components. Details of the mechanism of action, such as the stage of the viral cycle during and/or after viral penetration, possible synergistic interactions with other antivirals, and development of an in vivo assay are part of an upcoming study by our group. This study was able to identify promising antiviral molecules by virtual screening, and the antiviral activity of epicatechin was confirmed by bioassays.
References
Vieira CJ, Silva DJ, Barreto ES, Siqueira CE, Colombo TE et al (2015) Detection of Mayaro virus infections during a dengue outbreak in Mato Grosso, Brazil. Acta Trop 147:12–16
Vasconcelos PFC, Da Travassos Rosa APA, Pinheiro FP, Shope RE, da Travassos Rosa JFS et al (1998) Arboviruses pathogenic for man in Brazil. In: da Travassos Rosa APA, Vasconcelos PFC, Da Travassos Rosa JFS (eds) An overview of arbovirology in Brazil and neighbouring countries. Instituto Evandro Chagas, Belém, pp 72–99
Coimbra TLM, Santos CLS, Suzuki A, Petrella SMC, Bisordi I et al (2007) Mayaro virus: imported cases of human infection in São Paulo state, Brazil. Rev Inst Med Trop São Paulo 49:221–224
Long KC, Ziegler SA, Thangamani S, Hausser NL, Kochel TJ et al (2011) Experimental transmission of Mayaro virus by Aedes aegypti. Am J Trop Med Hyg 85:750–757
Mourão MP, Bastos Mde S, de Figueiredo RP, Gimaque JB, Galusso Edos S et al (2012) Mayaro fever in the city of Manaus, Brazil, 2007–2008. Vector Borne Zoonotic Dis 12:42–46
Forshey BM, Guevara C, Laguna-Torres VA, Cespedes M, Vargas J et al (2010) Arboviral etiologies of acute febrile illnesses in western South America, 2000–2007. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0000787
Pinheiro FP, Freitas RB, Travassos da Rosa JF, Gabbay YB, Mello WA et al (2010) An outbreak of Mayaro virus disease in Belterra, Brazil. I. Clinical and virological findings. Am J Trop Med Hyg 30:674–681
Halsey ES, Siles C, Guevara C, Vilcarromero S, Jhonston EJ et al (2013) Mayaro virus infection, Amazon Basin region, Peru, 2010–2013. Emerg Infect Dis 19:1839–1842
Suhrbier A, Mahalingam S (2009) The immunobiology of viral arthritides. Pharmacol Ther 124:301–308
Figueiredo MLG, Figueiredo LTM (2014) Emerging alphaviruses in the Americas: Chikungunya and Mayaro. Rev Soc Bras Med Trop 47:677–683
Santiago FW, Halsey ES, Siles C, Vilcarromero S, Guevara C et al (2015) Long-term arthralgia after Mayaro virus infection correlates with sustained pro-inflammatory cytokine response. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0004104
Lavergne A, de Thoisy B, Lacoste V, Pascalis H, Pouliquen JF et al (2006) Mayaro virus: complete nucleotide sequence and phylogenetic relationships with other alphaviruses. Virus Res 117:283–290
Strauss JH, Strauss EG (1994) The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 58:491–562
Firth AE, Chung BYW, Fleeton MN, Atkins JF (2008) Discovery of frameshifting in alphavirus 6K resolves a 20-year enigma. Virol J. https://doi.org/10.1186/1743-422x-5-108
Snyder JE, Kulcsar KA, Schultz KLW, Riley CP, Neary JT et al (2013) Functional characterization of the alphavirus TF protein. J Virol 87:8511–8523
Gaspar LP, Terezan AF, Pinheiro AS, Foquel D, Rebello MA et al (2001) The metastable state of nucleocapsids of enveloped viruses as probed by high hydrostatic pressure. J Biol Chem 276:7415–7421
Napoleão-Pego P, Gomes LP, Provance DW, De Simone SG (2014) Mayaro virus disease. J Hum Virol Retrovirol. https://doi.org/10.15406/jhvrv.2014.01.00018
Cheng RH, Kuhn RJ, Olson NH, Rossmann MG, Choi HK et al (1995) Nucleocapsid and glycoprotein organization in an enveloped virus. Cell 80:621–630
Choi HK, Tong L, Minor W, Dumas P, Boege U et al (1991) Structure of Sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature 354:37–43
Choi HK, Lu G, Lee S, Wengler G, Rossmann MG (1997) Structure of Semliki Forest virus core protein. Proteins Struct Funct Genet 27:345–359
Rodrigues VG, Duarte LP, Silva RR, Silva GDF, Mercadante-Simões MO et al (2015) Salacia crassifolia (Celastraceae): chemical constituents and antimicrobial activity. Quim Nova 38:237–242
National Center for Biotechnology Information (NCBI) (2015) http://www.ncbi.nlm.nih.gov. Accessed 21 Jul 2015
Aggarwal M, Tapas S, Preeti Siwach A, Kumar P et al (2012) Crystal structure of aura virus capsid protease and its complex with dioxane: new insights into capsid-glycoprotein molecular contacts. PLoS One. https://doi.org/10.1371/journal.pone.0051288
Guex N, Peitsch MC (1997) SWISS-MODEL and the SwissPdb Viewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Lovell SC, Davis IW, Arendall WB 3rd, de Bakker PI, Word JM et al (2003) Structure validation by Calpha geometry: phi, psi and Cbeta deviation. Proteins 50:437–450
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27:343–350
Maia EHB, Campos VA, Dos Reis Santos B, Costa MS, Lima IG, Greco SJ, Ribeiro RIMA, Munayer FM, Da Silva AM, Taranto AG (2017) Octopus: a platform for the virtual high-throughput screening of a pool of compounds against a set of molecular targets. J Mol Model 23:23–26
Zinc12 (2015) http://zinc.docking.org. Accessed 17 Nov 2015
Chemaxon (2015) MarvinSketch, an advanced chemical editor for drawing chemical structures, queries and reactions. https://www.chemaxon.com/products/marvin/marvinsketch/. Accessed 19 Nov 2015
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK et al (2009) Autodock4 and AutoDockTools4: automated docking with selective receptor flexiblity. J Comput Chem 16:2785–2791
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Rodrigues VG (2015) Estudo fitoquímico, biológico e de atividades antioxidante e inibitória da acetilcolinesterase de Salacia crassifolia e Maytenus imbricata. Universidade Federal de Minas Gerais, Tese
Dulbecco R (1952) Production of plaques in monolayer tissue cultures by single particles of an animal virus. Proc Natl Acad Sci USA 38:747–752
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Pearson WR (2013) An introduction to sequence similarity (“Homology”) searching. Curr Protoc Bioinform. https://doi.org/10.1002/0471250953.bi0301s42
Vyas VK, Ukawala RD, Ghate M, Chintha C (2012) Homology modeling a fast tool for drug discovery: current perspectives. Indian J Pharm Sci 74:1–17
Brozell SR, Mukherjee S, Balius TE, Roe DR, Case DA et al (2012) Evaluation of DOCK 6 as a pose generation and database enrichment tool. J Comput Aided Mol Des 26:749–773
Kim YH, Patkar C, Warrier R, Kuhn R, Cushman M (2005) Design, synthesis, and evaluation of dioxane-based antiviral agents targeted against the Sindbis virus capsid protein. Bioorg Med Chem Lett 15:3207–3211
Takeda-Shitaka M, Takaya D, Chiba C, Tanaka H, Umeyama H (2004) Protein structure prediction in structure based drug design. Curr Med Chem 11:551–558
Lee S, Kuhn RJ, Rossmann MG (1998) Probing the potential glycoprotein binding site of Sindbis virus capsid protein with dioxane and model building. Proteins 33:311–317
Sánchez I, Gómez Garibay F, Taboada J, Ruiz BH (2000) Antiviral effect of flavonoids on the dengue virus. Phytother Res 14:89–92
Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR (2011) Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol J. https://doi.org/10.1186/1743-422x-8-560
Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR (2011) In vitro antiviral activity of fisetin, rutin and naringenin against Dengue virus type-2. J Med Plant Res 5:5534–5539
Allard PM, Dau ET, Eydoux C, Guillemot JC, Dumontet V et al (2011) Alkylated flavanones from the bark of Cryptocarya chartacea as dengue virus NS5 polymerase inhibitors. J Nat Prod 11:2446–2453
Frabasile S, Koishi AC, Kuczera D, Silveira GF, Verri WA Jr et al (2017) The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci Rep. https://doi.org/10.1038/srep41864
Lin SC, Chen MC, Li S, Lin CC, Wang TT (2017) Antiviral activity of nobiletin against chikungunya virus in vitro. Antivir Ther. https://doi.org/10.3851/imp3167
Calland N, Albecka A, Belouzard S, Wychowski C, Duverlie G et al (2012) (−)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 55:720–729
Grienke U, Richter M, Walther E, Hoffmann A, Kirchmair J et al (2016) Discovery of prenylated flavonoids with dual activity against influenza virus and Streptococcus pneumoniae. Sci Rep. https://doi.org/10.1038/srep27156
Kuzuhara T, Iwai Y, Takahashi H, Hatakeyama D, Echigo N (2009) Green tea catechins inhibit the endonuclease activity of influenza A virus RNA polymerase. PLoS Curr. https://doi.org/10.1371/currents.rrn1052
Kim M, Kim SY, Lee HW, Shin JS, Kim P et al (2013) Inhibition of influenza virus internalization by (−)-epigallocatechin-3-gallate. Antiviral Res 100:460–472
Goldwasser J, Cohen PY, Lin W, Kitsberg D, Balaquer P et al (2011) Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J Hepatol 55:963–971
Tsukuda S, Watashi K, Hojima T, Isogawa M, Iwamoto M et al (2017) A new class of Hepatitis B and D Virus entry inhibitors, Proanthocyanidin and its analogs, that directly act on the viral large surface proteins. Hepatology 65:1104–1116
Takeshita M, Ishida Y, Akamatsu E, Ohmori Y, Sudoh M et al (2009) Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. J Biol Chem 284:21165–21176
Bézivin C, Tomasi S, Lohézic-Le Dévéhat F, Boustie J (2003) Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 10:499–503
Acknowledgements
We thank Brazilian Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the granting of Scientific Initiation grants. We thank the Universidade Federal de São João del-Rei for granting Master’s degree grants and the facilities to carry out this work.
Funding
This work was funded by the Brazilian Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) CBBAPQ01028-14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Handling Editor: Patricia Aguilar.
Electronic supplementary material
Below is the link to the electronic supplementary material.
705_2018_3774_MOESM2_ESM.pdf
Supplementary material 2 Binding energy of the flavonoids and dioxanes docked to the protein C of the MAYV. *Molecules used as parameters for the evaluation of the binding energies obtained in Virtual Screening study with the Protein C of the MAYV (PDF 82 kb)
705_2018_3774_MOESM3_ESM.pdf
Supplementary material 3 NMR spectrum of epicatechin from the extract of ethyl acetate from leaves of Salacia crassifolia. (a) 1H NMR (CD3OD, 400 MHz). (b) 13C NMR (CD3OD, 100 MHz) and the chemical structure of epicatechin (PDF 224 kb)
Rights and permissions
About this article
Cite this article
Ferreira, P.G., Ferraz, A.C., Figueiredo, J.E. et al. Detection of the antiviral activity of epicatechin isolated from Salacia crassifolia (Celastraceae) against Mayaro virus based on protein C homology modelling and virtual screening. Arch Virol 163, 1567–1576 (2018). https://doi.org/10.1007/s00705-018-3774-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3774-1