Abstract
SYBR Green coupled to melting curve analysis has been suggested to detect RNA viruses showing high genomic variability. Here, a SYBR Green-based real-time RT-PCR assay was developed for simultaneous detection and differentiation of highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) and classical type 2 PRRSV (C-PRRSV). The different strains were identified by their distinctive melting temperatures: 82.98 ± 0.25 °C and 85.95 ± 0.24 °C for HP-PRRSVs or 82.74 ± 0.26 °C for C-PRRSVs. Specificity was tested using nine other viral and bacterial pathogens of swine. The detection limit was 1 TCID50 for HP- or C-PRRSV. Furthermore, the detection results for samples from an animal trial with HP- or C-PRRSV infections showed that the SYBR Green-based real-time RT-PCR was more sensitive than the conventional RT-PCR. Additionally, an analysis of 319 field samples from North China, Central China and Northeast China showed that HP- and C-PRRSVs co-circulated in pig herds. Thus, the SYBR Green-based real-time RT-PCR, which can be performed within one hour, is a rapid, sensitive and low-cost diagnostic tool for rapid differential detection and routine surveillance of HP- and classical type 2 PRRSVs in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Porcine reproductive and respiratory syndrome (PRRS), which is characterized by late-term reproductive failure and respiratory disease in swine, has spread widely throughout many pig-producing countries [1]. This disease is caused by porcine reproductive and respiratory syndrome virus (PRRSV), which is a positive-stranded RNA virus belonging to the family Arteriviridae within the order Nidovirales [2]. PRRSV isolates are classified into European (type 1) and North American (type 2) genotypes based on phylogenetic analysis [3, 4].
The genome of PRRSV is about 15 kb long and contains nine open reading frames (ORFs). ORF1 encodes nonstructural proteins (NSPs), and ORFs 2-7 encode structural glycoproteins (GP2-5), the matrix (M) protein and the nucleocapsid (N) protein [3, 5]. The variable genes, NSP2 and GP5, are usually chosen for analysis of evolution and divergence [6–8]. Because the NSP2 gene is prone to natural point mutations, insertions and deletions, it contributes significantly to genetic variation of PRRSV [7]. Highly pathogenic PRRSV (HP-PRRSV) strains cause high mortality rates and have spread to most areas of China and other Asian countries [9–11]. Genome analysis of these isolates revealed that a discontinuous deletion of 30 amino acids (aa) in the NSP2 region, and this deletion is not present in classical type 2 PRRSVs [10–12]. Although there were several HP-PRRSV isolates with novel deletions within NSP2, the unique discontinuous deletion remains a genetic marker in most isolates [8, 13–15].
PRRS can be diagnosed by virus isolation or detection of antibodies, antigens or nucleic acids. However, molecular-based tools are usually more sensitive and rapid than traditional methods for diagnosis of PRRSV infection [16–19]. Since HP-PRRSV is highly prevalent in China, a rapid and low-cost real-time RT-PCR assay that can detect and discriminate HP-PRRSV and classical type 2 PRRSV (C-PRRSV) is important for routine viral diagnosis and monitoring virus prevalence in pigs. In this study, the development and validation of a SYBR Green-based real-time RT-PCR assay followed by melting curve analysis of the amplicon is described. This assay can be used for rapid discrimination between HP- and C-PRRSVs in virus-infected cells and clinical samples.
Materials and methods
Viruses
PRRSV HuN4 and CH-1a strains, swine influenza virus (SIV), classical swine fever virus (CSFV), porcine circovirus type 2 (PCV2), foot-and-mouth disease virus (FMDV), Japanese encephalitis virus (JEV), pseudorabies virus (PRV), and porcine parvovirus (PPV) were kept at the Harbin Veterinary Research Institute of the Chinese Academy of Agriculture Sciences. HuN4 and CH-1a strains are the standard reference strains of HP- and C-PRRSVs, respectively, in China.
Primers
Preliminary evaluation of published HP- and C-PRRSVs that were isolated in China indicated that the NSP2-encoding sequence is a region that is potentially useful for the particular objective of discriminating between HP- and C-PRRSVs [10, 20, 21]. ORF7 genes from the GenBank database were aligned and used for universal detection of type 2 PRRSVs. The sequences of the various primers, primer locations and product sizes are given in Table 1.
Nucleic acid preparation and reverse transcription
DNA was extracted from cell culture supernatants using a QIAamp DNA Kit (QIAGEN), and the RNA was extracted from cell culture supernatants, sera and tissue homogenates using TRIzol Reagent (Invitrogen) according to the manufacturer’s directions. The RT reaction was performed by random priming using M-MLV reverse transcriptase (Promega). A final volume of 20 μl of RT reaction containing 1 μl of 200 U/μl M-MLV RT, 4 μl of 5× reaction buffer, 2.5 mM each dNTP (Takara), 40 U ribonuclease inhibitor (Takara) and 3 μl of nuclease-free water was performed at 42 °C for 1.5 h, followed by addition of random hexamer primers (Takara) and incubation at 70 °C for 10 min.
SYBR Green-based real-time RT-PCR assay
The SYBR Green-based real-time RT-PCR was carried out on a Rotor Gene 3000 (Corbett) using the double-stranded DNA-binding dye method with a SYBR Green PCR kit (Takara). Each 25-μl reaction mixture contained 19 μl SYBR® Premix Ex TaqTM, 1.2 μl (0.1 nM) each of F1 and R1, 0.8 μl (0.1 nM) each of F2 and R2, and 2 μl of cDNA templates. Cycling conditions were as follows: 95 °C for 10 followed by 42 cycles consisting of 95 °C for 5 s and 62 °C for 25 s. The temperature range for the melting curve analysis was from 79 °C to 95 °C, rising by 0.5 °C each step.
Specificity, sensitivity and reproducibility of the SYBR Green-based real-time RT-PCR assay
DNA or cDNA samples from HP-PRRSV (HuN4 strain), C-PRRSV (CH-1a strain), non-PRRSV (PCV, PPV, PRV, CSFV, JEV, FMDV and SIV), and the bacterial pathogens Mycoplasma hyopneumoniae (Mhp) and Streptococcus suis (S.S.) were examined to verify the specificity of the SYBR Green-based real-time RT-PCR assay. The viral RNAs were extracted from sequential 10-fold dilutions of titrated (104 TCID50/ml to 1 TCID50/ml) HuN4 and CH-1a strain PRRSV. The serial dilutions were run to test the sensitivity of the SYBR Green-based real-time RT-PCR assay. An intra-and inter-test reproducibility assay of the Tm-based method was carried out in triplicate by testing samples within the same run and independently in three different runs on different days.
Animal experiment with HP- and classical type 2 PRRSV
Twelve serologically PRRSV-negative pigs (5 weeks old) were randomly divided into three groups and were housed in isolation units to prevent contact among the groups. Group 1 and group 2 each included five pigs, and group 3 included two pigs. The pigs in group 1 and group 2 were inoculated intranasally with the HuN4 and CH-1a strain, respectively, of type 2 PRRSV at 2 × 105 TCID50 of virus per pig. The pigs in group 3 were inoculated intranasally with phosphate-buffered saline (PBS). During the entire study, whole-blood samples were collected from all of the pigs at 3, 5, 7, 10, 14, 21 days post-inoculation (dpi). Total RNA from blood was extracted and analyzed by SYBR Green-based real-time RT-PCR and conventional RT-PCR. The conventional RT-PCR assays for HP-PRRSV and C-PRRSV were described previously [21, 22].
Practical application to clinical samples
A total of 319 clinical samples (whole-blood samples) were collected from Northeast China, Central China and Northern China in 2006-2010. These samples were obtained from field investigations. Total RNA from the whole-blood samples was extracted and detected by SYBR Green-based real-time RT-PCR. To confirm the detection result of the SYBR Green-based real-time RT-PCR, the positive PCR products were purified using a PCR purification mini kit (Watson Biotechnologies, Inc) and were sequenced.
Fifteen of the HP-PRRSV-positive samples were randomly chosen to reamplify the partial NSP2 gene for sequence analysis. The primers and the procedure for RT-PCR were described previously [21]. For sequencing, the RT-PCR products were purified using a Gel Extraction Kit (Watson Biotechnologies) and ligated separately into a pMD-18T vector according to the manufacturer’s instructions (Takara). The targeted fragments were sequenced. Sequence data were assembled and analyzed using the software Lasergen (DNAstar). Multiple-sequence alignments were done using Clustal W. The plylogenetic tree was constructed by using the neighbor-joining method with MEGA4. The robustness of the neighbor-joining tree was estimated by bootstrap analysis with 1,000 replicates.
Interpretation of results and data analysis
Specimens were considered HP-PRRSV or C-PRRSV positive when (i) the curve in the amplification plot showed an exponential increase, (ii) the Ct value was below 40, and (iii) specific melting peaks with two Tm values positive for HP-PRRSV or with one Tm value positive for C-PRRSV were obtained (Fig. 1).
Fluorescence melting curve analysis of SYBR Green-based real-time RT-PCR. Melting peaks of PRRSV cDNA template (HP- or classical type 2 PRRSV) were observed, and no specific curve of a non-template control (NTC) was observed. The HuN4 and CH-1a strains are reference strains of HP-PRRSV and classical type 2 PRRSV (C-PRRSV), respectively. Samples were detected in separate wells during the same PCR run. Fluorescence data were converted into melting peaks by plotting the negative derivative of fluorescence with respect to temperature vs. temperature (-d(F)/dt vs. °C)
The Tm values of the melting curve of SYBR Green-based real-time RT-PCR were generated using each instrument’s incorporated software. The means and coefficients of variation (CoVs) of the measured Tm values were calculated using Microsoft Excel.
Results
SYBR Green-based real-time RT-PCR assay and melting curve analysis
To develop the SYBR Green-based real-time RT-PCR for discrimination between specific amplification products of HP- and C-PRRSVs, two sets of primers were used in a single reaction mix. For the analysis, HuN4 and CH-1a were used as the reference strains for HP- and C-PRRSVs. As shown in Fig. 1, two melting peaks were produced by the amplification of HP-PRRSV (Tm1 = 82.98 ± 0.25 °C, Tm2 = 85.95 ± 0.24 °C), while one melting peak was observed for C-PRRSV (Tm = 82.74 ± 0.26 °C), indicating that the SYBR Green real-time RT-PCR was able to discriminate between HP-PRRSV and C-PRRSV.
Specificity of SYBR Green-based real-time RT-PCR assay
To verify the specificity of the SYBR Green-based real-time RT-PCR assay, the HuN4 and CH-1a strains of type 2 PRRSV, non-PRRSV (PCV, PPV, PRV, CSFV, JEV, FMDV and SIV) and bacterial pathogens Mhp and S.S. were tested. The result is shown in Fig. 2. The HuN4 and CH-1a strains were detected based on the melting curve analysis, while no specific melting peak was observed for non-PRRSV and bacterial pathogens.
Sensitivity of the SYBR Green-based real-time RT-PCR assay
To determine the sensitivity of the SYBR Green-based real-time RT-PCR, viral RNA, which was extracted from sequential tenfold dilutions of titrated (104 TCID50/ml to 1 TCID50/ml) HuN4 and CH-1a strain PRRSVs, was tested in triplicate. The detection limit was 1 TCID50 per reaction of HuN4 (HP-PRRSV) or CH-1a (C-PRRSV) in the SYBR Green-based real-time RT-PCR (Fig. 3).
Intra- and inter-assay variability of SYBR Green-based real-time RT-PCR assay
To assess the reproducibility of the SYBR Green-based real-time RT-PCR, three replicates for HuN4 strain PRRSV (102 TCID50), CH-1a strain PRRSV (102 TCID50) and two clinical positive samples that had been evaluated previously by conventional RT-PCR were examined. The result is shown in Table 2. The mean intra-assay CoVs were below 0.1 % for cells infected with HuN4 or CH-1a strain PRRSV and below 0.2 % for the clinically positive samples; the mean inter-assay CoVs were below 0.35 % for virus-infected cells and below 0.5 % for clinically positive samples.
Detection of virus RNA in samples from pigs experimentally infected with PRRSV
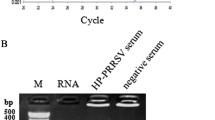
Whole-blood samples from pigs infected with HP-PRRSV (HuN4 strain), C-PRRSV (CH-1a strain) and negative control pigs were collected at 3, 5, 7, 10, 14 and 21 dpi and were detected separately using the SYBR Green-based real-time RT-PCR and conventional RT-PCR. As shown in Table 3, all challenged samples were positive for HP- or C-PRRSV by the SYBR Green-based real-time RT-PCR, whereas eight of the samples were negative by the conventional RT-PCR. The coincidence between two diagnostic methods was 86.67 %, suggesting that the SYBR Green-based real-time RT-PCR assay is more sensitive than the conventional RT-PCR.
Detection of virus RNA in clinical samples collected from three areas of China
To evaluate the performance of the SYBR Green-based real-time RT-PCR in clinical samples, we analyzed 319 whole-blood samples collected during field investigations from North China, Central China and Northeast China from 2006 to 2010. As shown in Table 4, the detection results were 72 positive samples for HP-PRRSV and 106 positive samples for C-PRRSV. Additionally, the positive samples were confirmed by sequencing. These results indicated that the SYBR Green-based real-time RT-PCR could be applied for detection of virus in clinical samples and for epidemiological investigation.
The reamplified PCR products of randomly chosen HP-PRRSV-positive samples were purified and sequenced. The sequences obtained were compared with HuN4 and CH-1a PRRSVs sequences, which are the respective references for HP- and classic type 2 PRRSVs in China. Phylogenetic trees were constructed based on a partial sequence of the NSP2 gene (Fig. 4). The phylogenetic analysis showed that all of the HP-PRRSV samples from 2010 and some of the samples from 2009 were clustered in a different branch from the samples collected in 2006-2008. However, they still had the genetic marker for HP-PRRSV, a unique 30-amino-acid deletion (data not shown).
Phylogenetic tree based on nucleotide sequences of the partial NSP2 genes from random samples that were positive for HP-PRRSV in the three areas of China. Phylogenetic analysis was conducted using MEGA4. The reliability of the tree was assessed by bootstrap analysis of 1,000 replications. NC, CC and NEC are the abbreviations of North China, Central China and Northeast China, respectively. The year in which the samples were collected follows the geographic area
Discussion
PRRSV has brought great losses to Chinese pig husbandry, particularly since the emergence of HP-PRRSV in 2006. Currently, HP-PRRSV, which causes persistent recurrences and sporadic outbreaks, still threatens the Chinese pig industry. Therefore, the rapid and reliable detection of HP-PRRSV and classical type 2 PRRSV is essential for herd management and the prevention of disease spread. In this study, a SYBR Green-based real-time RT-PCR was developed and validated. The assay allows the rapid detection all PRRSV strains circulating in swine herds in China and specific discrimination between HP- and C-PRRSV by melting curve analysis.
The SYBR Green real-time PCR system provides a reliable method for detection and discrimination among different target genes [23, 24]. Variations of 1 °C in the Tm values of the amplicons should be enough to discriminate the viruses using melting curve analysis [25]. Furthermore, the melt ramp times have been shown to affect the acquisition of melt peaks in the multiplex real-time PCR assay [25]. In this study, the melting peaks were 82.98 ± 0.25 °C and 85.95 ± 0.24 °C for HP-PRRSVs and 82.74 ± 0.26 °C for C-PRRSVs using a melt ramp time of 0.5 °C/s, which facilitated the discrimination between the different amplicons of the HP- and C-PRRSVs. When different co-amplifications are performed in the same tube, an appropriate selection of primers plays an important role in the SYBR Green-based real-time PCR system [26]. A primer-competition effect could occur, and the specificity and sensitivity of multiplex PCR might be decreased [27]. Here, the detection limit of SYBR Green-based real-time RT-PCR is 1 TCID50 per reaction (Fig. 3), indicating that this diagnostic method is highly sensitive. Furthermore, this assay was specific for detection of type 2 PRRSV. When other viral and bacterial pathogens of swine were tested, no specific amplicons were obtained (Fig. 2), demonstrating that this assay reliably distinguished type 2 PRRSV from other swine pathogens. The detection results obtained using clinical samples from the animal experiment and field samples (Tables 3 and 4) indicated that this assay could be used to test field samples. In the whole-blood samples obtained from the animal experiment, the viral genome was first detectable using the SYBR Green-based real-time RT-PCR at 3 dpi, and the positive detection rate was much higher than that of the conventional RT-PCR (Table 3), demonstrating that the assay used in this study was more sensitive than the conventional RT-PCR and could be suitable for the early diagnosis of type 2 PRRSV infection. Seventy-two of 319 clinical samples were positive for HP-PRRSV, and 106 clinical samples were positive for C-PRRSV using the SYBR Green-based real-time RT-PCR, suggesting that the HP- and C-PRRSV strains coexist in swine herds in China and that the predominant strains of PRRSV in infected pigs belong to the type 2 genotype. In the phylogenetic tree obtained by neighbor-joining analysis, the partial NSP2 sequence data revealed no correlations between the date or place of collection and the distribution of HP-PRRSV in three areas of China (Fig. 4). Despite the presence of some naturally occurring mutations, the SYBR Green-based real-time RT-PCR assay was effective. More importantly, the assay can be performed within 1 h, and the interpretation of result is so easy that it is feasible for testing of field samples.
Multiplex real-time PCR assays have been developed for diagnostic and research purposes. They have been used to discriminate among different genotypes of viral and bacterial pathogens [28–32]. All of these multiplex real-time PCR assays, which are characterized by high sensitivity, specificity and reliability, are suitable for diagnosis of multiple infectious diseases. To date, real-time-PCR-based methods have been established for detection and differentiation of PRRSV. A multiplex-like TaqMan RT-PCR assay has been developed for detecting and differentiating type 1 and type 2 PRRSV [33]. Since the emergence of HP-PRRSV, a duplex real-time PCR assay using minor groove binder (MGB) probes has been developed for differential detection of the two type 2 PRRSV isolates [34]. Nevertheless, the rapid rate of PRRSV gene mutation remains a huge challenge for these fluorescent-probe-based real-time PCR assays. Even if a single mutation occurs in the fluorescent probe, it may result in detection failure. Therefore, SYBR Green-based real-time RT-PCR assay should be more reliable for rapidly evolving RNA viruses. A SYBR Green-based real-time RT-PCR assay, which could detect and discriminate between type 1 and type 2 PRRSV has been reported [17]. We have also utilized the advantage of SYBR Green fluorescence dye to develop a real-time RT-PCR with specificity, sensitivity and reproducibility. However, our assay has disadvantages. First, this assay is a qualitative method and cannot be used to calculate the viral load. Furthermore, it cannot differentiate HP-PRRSV in samples from a mixed infection with HP- and C-PRRSVs, although, to our knowledge, there have been no cases of HP- and C-PRRSV co-infection in Chinese herds. Additionally, the SYBR Green-based real-time RT-PCR cannot detect type 1 PRRSV, although there has been only one report of the emergence of type 1 PRRSV in 2011 in China [35]. Recently, a multiplex real-time RT-PCR based on specific probes has been developed for type 1, type 2 and HP-PRRSVs [36, 37]. Although the novel method can detect most PRRSV strains, it is prone to false-negative results, a disadvantage of methods based on fluorescent probes [36]. In the future, we will develop a SYBR Green-based real-time RT-PCR assay that has the characteristics of low cost and a low false-negative rate for detection of type 1, type 2 and HP-PRRSV based on this study.
In summary, a SYBR Green-based real-time RT-PCR assay coupled with melting curve analysis was developed for detection and differentiation of HP- and C-PRRSV. This method is a rapid, sensitive and low-cost diagnostic tool for routine surveillance of viral infections in pigs. Furthermore, the analytical and diagnostic performance observed using field samples demonstrates the usefulness of this tool for laboratory diagnosis and epidemiological investigations.
References
Rossow KD (1998) Porcine reproductive and respiratory syndrome. Vet Pathol 35:1–20
Cavanagh D (1997) Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol 142:629–633
Murtaugh MP, Elam MR, Kakach LT (1995) Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch Virol 140:1451–1460
Nelson EA, Christopher-Hennings J, Drew T, Wensvoort G, Collins JE, Benfield DA (1993) Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J Clin Microbiol 31:3184–3189
Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ (1993) Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology 192:62–72
Zhou L, Zhang J, Zeng J, Yin S, Li Y, Zheng L, Guo X, Ge X, Yang H (2009) The 30-amino-acid deletion in the Nsp2 of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China is not related to its virulence. J Virol 83:5156–5167
Zhou L, Chen S, Zhang J, Zeng J, Guo X, Ge X, Zhang D, Yang H (2009) Molecular variation analysis of porcine reproductive and respiratory syndrome virus in China. Virus Res 145:97–105
Zhou Z, Ni J, Cao Z, Han X, Xia Y, Zi Z, Ning K, Liu Q, Cai L, Qiu P, Deng X, Hu D, Zhang Q, Fan Y, Wu J, Wang L, Zhang M, Yu X, Zhai X, Tian K (2011) The epidemic status and genetic diversity of 14 highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) isolates from China in 2009. Vet Microbiol 150:257–269
Ni J, Yang S, Bounlom D, Yu X, Zhou Z, Song J, Khamphouth V, Vatthana T, Tian K (2012) Emergence and pathogenicity of highly pathogenic Porcine reproductive and respiratory syndrome virus in Vientiane, Lao People’s Democratic Republic. J Vet Diagn Invest 24:349–354
Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, Hu Y, Chen X, Hu D, Tian X, Liu D, Zhang S, Deng X, Ding Y, Yang L, Zhang Y, Xiao H, Qiao M, Wang B, Hou L, Wang X, Yang X, Kang L, Sun M, Jin P, Wang S, Kitamura Y, Yan J, Gao GF (2007) Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE 2:e526
Tong GZ, Zhou YJ, Hao XF, Tian ZJ, An TQ, Qiu HJ (2007) Highly pathogenic porcine reproductive and respiratory syndrome, China. Emerg Infect Dis 13:1434–1436
Li Y, Wang X, Bo K, Wang X, Tang B, Yang B, Jiang W, Jiang P (2007) Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the Mid-Eastern region of China. Vet J 174:577–584
Li B, Fang L, Liu S, Zhao F, Jiang Y, He K, Chen H, Xiao S (2010) The genomic diversity of Chinese porcine reproductive and respiratory syndrome virus isolates from 1996 to 2009. Vet Microbiol 146:226–237
Li B, Fang L, Guo X, Gao J, Song T, Bi J, He K, Chen H, Xiao S (2011) Epidemiology and evolutionary characteristics of the porcine reproductive and respiratory syndrome virus in China between 2006 and 2010. J Clin Microbiol 49:3175–3183
Yu X, Chen N, Wang L, Wu J, Zhou Z, Ni J, Li X, Zhai X, Shi J, Tian K (2012) New genomic characteristics of highly pathogenic porcine reproductive and respiratory syndrome viruses do not lead to significant changes in pathogenicity. Vet Microbiol 158:291–299
Van Woensel P, Van Der Wouw J, Visser N (1994) Detection of porcine reproductive respiratory syndrome virus by the polymerase chain reaction. J Virol Methods 47:273–278
Martinez E, Riera P, Sitja M, Fang Y, Oliveira S, Maldonado J (2008) Simultaneous detection and genotyping of porcine reproductive and respiratory syndrome virus (PRRSV) by real-time RT-PCR and amplicon melting curve analysis using SYBR Green. Res Vet Sci 85:184–193
Balka G, Hornyak A, Balint A, Benyeda Z, Rusvai M (2009) Development of a one-step real-time quantitative PCR assay based on primer-probe energy transfer for the detection of porcine reproductive and respiratory syndrome virus. J Virol Methods 158:41–45
Li Q, Q-f Zhou, C-y Xue, J-y Ma, D-z Zhu, Y-c Cao (2009) Rapid detection of porcine reproductive and respiratory syndrome virus by reverse transcription loop-mediated isothermal amplification assay. J Virol Methods 155:55–60
Xiao XL, Wu H, Yu YG, Cheng BZ, Yang XQ, Chen G, Liu DM, Li XF (2008) Rapid detection of a highly virulent Chinese-type isolate of Porcine Reproductive and Respiratory Syndrome virus by real-time reverse transcriptase PCR. J Virol Methods 149:49–55
Zhou YJ, Hao XF, Tian ZJ, Tong GZ, Yoo D, An TQ, Zhou T, Li GX, Qiu HJ, Wei TC, Yuan XF (2008) Highly virulent porcine reproductive and respiratory syndrome virus emerged in China. Transbound Emerg Dis 55:152–164
Li XS, Fu F, Lang YK, Li HZ, Wang W, Chen X, Tian HB, Zhou YJ, Tong GZ, Li X (2011) Development and preliminary application of an immunochromatographic strip for rapid detection of infection with porcine reproductive and respiratory syndrome virus in swine. J Virol Methods 176:46–52
Tam S, Clavijo A, Engelhard EK, Thurmond MC (2009) Fluorescence-based multiplex real-time RT-PCR arrays for the detection and serotype determination of foot-and-mouth disease virus. J Virol Methods 161:183–191
Varga A, James D (2005) Detection and differentiation of Plum pox virus using real-time multiplex PCR with SYBR Green and melting curve analysis: a rapid method for strain typing. J Virol Methods 123:213–220
Papp AC, Pinsonneault JK, Cooke G, Sadee W (2003) Single nucleotide polymorphism genotyping using allele-specific PCR and fluorescence melting curves. Biotechniques 34:1068–1072
Pérez LJ, Perera CL, Frías MT, Núñez JI, Ganges L, de Arce HD (2012) A multiple SYBR Green I-based real-time PCR system for the simultaneous detection of porcine circovirus type 2, porcine parvovirus, pseudorabies virus and Torque teno sus virus 1 and 2 in pigs. J Virol Methods 179:233–241
Belak S (2007) Molecular diagnosis of viral diseases, present trends and future aspects: a view from the OIE collaborating centre for the application of polymerase chain reaction methods for diagnosis of viral diseases in veterinary medicine. Vaccine 25:5444–5452
Gagnon CA, del Castillo JR, Music N, Fontaine G, Harel J, Tremblay D (2008) Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of Porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J Vet Diagn Invest 20:545–558
Templeton KE, Scheltinga SA, Beersma MFC, Kroes ACM, Claas ECJ (2004) Rapid and sensitive method using multiplex real-time pcr for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol 42:1564–1569
Lehmann LE, Hunfeld K-P, Emrich T, Haberhausen G, Wissing H, Hoeft A, Stüber F (2008) A multiplex real-time PCR assay for rapid detection and differentiation of 25 bacterial and fungal pathogens from whole blood samples. Med Microbiol Immunol 197:313–324
Brittain-Long R, Nord S, Olofsson S, Westin J, Anderson L-M, Lindh M (2008) Multiplex real-time PCR for detection of respiratory tract infections. J Clin Virol 41:53–56
Huang YW, Dryman BA, Harrall KK, Vaughn EM, Roof MB, Meng XJ (2010) Development of SYBR green-based real-time PCR and duplex nested PCR assays for quantitation and differential detection of species- or type-specific porcine Torque teno viruses. J Virol Methods 170:140–146
Egli C, Thür B, Liu L, Hofmann MA (2001) Quantitative TaqMan® RT-PCR for the detection and differentiation of European and North American strains of porcine reproductive and respiratory syndrome virus. J Virol Methods 98:63–75
Chen NH, Chen XZ, Hu DM, Yu XL, Wang LL, Han W, Wu JJ, Cao Z, Wang CB, Zhang Q, Wang BY, Tian KG (2009) Rapid differential detection of classical and highly pathogenic North American Porcine Reproductive and Respiratory Syndrome virus in China by a duplex real-time RT-PCR. J Virol Methods 161:192–198
Chen N, Cao Z, Yu X, Deng X, Zhao T, Wang L, Liu Q, Li X, Tian K (2011) Emergence of novel European genotype porcine reproductive and respiratory syndrome virus in mainland China. J Gen Virol 92:880–892
Wernike K, Bonilauri P, Dauber M, Errington J, Leblanc N, Revilla-Fernández S, Hjulsager C, Isaksson M, Stadejek T, Beer M, Hoffmann B (2012) Porcine reproductive and respiratory syndrome virus: interlaboratory ring trial to evaluate real-time reverse transcription polymerase chain reaction detection methods. J Vet Diagn Invest 24:855–866
Wernike K, Hoffmann B, Dauber M, Lange E, Schirrmeier H, Beer M (2012) Detection and typing of highly pathogenic porcine reproductive and respiratory syndrome virus by multiplex real-time RT-PCR. PLoS ONE 7:e38251
Acknowledgement
This study was supported by grants from Major International Scientific Cooperation Projects in the National Animal Zoonoses Prevention and Control of Key Technologies (2010DFB33920).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chai, Z., Ma, W., Fu, F. et al. A SYBR Green-based real-time RT-PCR assay for simple and rapid detection and differentiation of highly pathogenic and classical type 2 porcine reproductive and respiratory syndrome virus circulating in China. Arch Virol 158, 407–415 (2013). https://doi.org/10.1007/s00705-012-1504-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-012-1504-7