Abstract
Porcine endogenous retroviruses (PERV) are widely distributed in the genomes of pigs. PERV-A and PERV-B are present in all pigs. They infect human cells in vitro and therefore represent a risk for xenotransplantation when pig cells, tissues or organs are used. PERV-C infects only pig cells and is not present in the genomes of all pigs. However, PERV-A/C recombinants infecting human cells and characterized by high replication titers were found in pigs. To select PERV-C-free animals that cannot generate such recombinants, PCR-based assays were developed (Kaulitz et al., J Virol Methods, 175:60, 2011). When screening for PERV-C in German wild boars (Sus scrofa scrofa), applying these methods, a new variant of PERV-C was identified. Whereas in all 125 wild boar only the new variant of PERV-C was found, different variants were present in some landrace pigs, and most importantly, some pigs were totally free of PERV-C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Porcine endogenous retroviruses (PERV) pose a special risk in xenotransplantation using pig cells, tissues or organs to overcome the shortage of human allotransplants [1]. Endogenous retroviruses have been detected in the genomes of all vertebrate species, and their general organization corresponds to that of exogenous retroviruses [2, 3]. Most of the endogenous retroviruses are replication incompetent, while only a minority still produce infectious virus particles that are able to infect cells of the same (ecotropic) or of other species (polytropic, xenotropic) [4, 5].
In pigs, three different classes exist, PERV-A, PERV-B and PERV-C, which all belong to the gammaretroviruses [6–8]. In addition, betaretroviruses are also found in the genomes of pigs [9]. PERV-A and PERV-B infect human cells in vitro as well as cells from other species [7, 10–13]. In contrast, PERV-C is an ecotropic virus infecting only pig cells, and it is not present in the genomes of all pigs [6, 7]. PERVs may be the result of a transspecies transmission, possibly of murine viruses to pigs [14]. PERV-A integrated into the pig genome approximately 7.6 million years ago (MYA), whereas PERV-C integrated in S. barbatus approximately 1.5 MYA [15].
In addition, replication-competent PERV-A/C recombinants have been found in normal pigs [16–19] and in pigs carrying melanomas [20]. The recombinants were found de novo integrated in the genome of somatic cells of these animals, but they are not present in the germ line [16–22]. PERV-A/C isolates are characterized by high-titer replication and specific mutations when compared with the parental PERV-A [18]. After passaging on human cells, the virus titer has been found to increase significantly due to genetic alterations in the LTR [23, 24]. Similar genetic alterations in the LTR have also been detected in PERV-A passaged in human cells [23, 25]. A consensus statement of the International Xenotransplantation Association has suggested breeding pigs for xenotransplantation that do not harbor PERV-C in order to avoid generating such high-titer PERV-A/C recombinants [26]. To detect PERV-C-free pigs, sensitive PCR detection methods for PERV-C have been developed, and 92 % of farm animals and multi-transgenic animals bred for xenotransplantation have been identified as PERV-C positive [27, 28]. Previously a 100 % prevalence of PERV-C in 18 wild boars living near Berlin in the Federal State of Brandenburg, Germany, was reported [27]. In contrast, Mang et al. [29] found no PERV-C in a wild boar using a nested PCR; however they tested only one animal. When analyzing the presence of PERV-C in wild boars using the newly developed methods, some, but not all, of the new PCR assays were able to detect PERV-C. To clarify this discrepancy, sequence analysis was performed, indicating sequence differences in the binding sites of primers and probes that impaired hybridization. The new sequences were identified as variants of PERV-C. Whereas in 125 tested wild boars only one new variant PERV-C was found, in some landrace pigs, two different variants were present, but most importantly, some pigs were totally free of PERV-C, including these new variants.
Materials and methods
Animals and DNA isolation
One hundred twenty-five wild boars were analyzed, 18 of which had been collected at four different locations near Berlin [27], and 107 animals were from four other geographical regions in Germany: Baden-Wuerttemberg (11 animals), Brandenburg (18 animals), Rhineland-Palatinate (50 animals) and Saxony (28 animals). The sampling details and the procedure for DNA preparation from liver tissue of these samples were described in previous studies in which the presence of hepatitis E virus [30] and hokovirus [31] in these animals was investigated. For comparative purposes, DNA from 169 German landrace pigs was analyzed [28].
PCR
In addition to the PCR based on primers published by Takeuchi et al. [7] (designated PCR1), other PCR assays were developed based on different primer pairs (PCR2 to PCR7) (Fig. 1; Table 1). The sensitivity of PCR1 was estimated to be 1.1 × 104 plasmid molecules, using a molecular PERV-C clone in the presence of DNA from the pig cell line PK-15 [28].
Real-time PCR
For the quantification of envC-specific proviral DNA, the primers “envC real forward” and “envC real reverse” and a corresponding probe (Table 1; Fig. 1) were designed. A duplex real-time PCR was performed based on cyclophilin (cyp) as a reference gene using the primers “cyp real-time for” and “cyp real-time rev” and quantifying the PCR with a cyp probe (Table 1). The 25-μl reaction mixture consisted of 2.5 μl 10× PCR buffer (100 mM Tris-HCl, 500 mM KCl, 15 mM MgCl2, 0.01 % [w/v] gelatin), 3 μl MgCl2 (25 mM), 0.5 μl dNTP (20 mM), 0.5 μl (10 μM) of each primer, 0.5 μl of both probes (10 μM), 0.1 μl ROX (Invitrogen, Darmstadt, Germany), 0.3 μl AmpliTaq Gold® polymerase (Roche, Mannheim, Germany) (5 U/μl), nuclease-free water and the template DNA (100 ng). The thermal cycling conditions used were 7 minutes at 95 °C followed by 50 cycles of 95 °C for 20 seconds and 58 °C for 30 seconds in a Stratagene MX4000 machine (Agilent, Waldbronn, Germany). The efficiency of this assay was determined by measuring tenfold serial dilutions of envC-positive genomic DNA from 1.1 × 1010 to 0.2 plasmid copies per reaction. Amplicons generated by real-time PCR were subcloned into the vector pBluescript II KS (Agilent, Waldbronn, Germany) and sequenced, and the plasmid was used in the real-time PCR to measure the copy number. As an internal standard for quantification and for quality control of the template DNA, a part of the housekeeping gene encoding cyclophilin was co-amplified. There was no interference between both real-time PCRs, as confirmed by an analysis of serial dilutions of genomic DNA from one pig, which was PERV-C positive. The PCR showed an efficiency of 93.7 % for cyclophilin and 99.3 % for PERV-C [28]. The detection limit of the real-time PCR was 100 copies, as determined using a plasmid standard, and linear co-amplification was obtained between 1 pg and 100 ng of genomic template DNA.
Sequencing
Amplicons generated by PCR3, PCR5, PCR6 and PCR7, as indicated in Table 1, were sequenced bidirectionally using Sanger’s dideoxy method, using 10 ng of amplicon, 5 pmol of primer, 1 μl of 5× buffer, 2 μl of BigDye (Applied Biosystems), and the corresponding primers.
Phylogenetic tree analysis
The PERV-C sequences from GenBank were analyzed using the software Lasergene®-MegAlign (DNASTAR). A 500-bp sequence of wild boar PERV-C was used to identify homologous regions. The alignment of sequences was done using the ClustalW method. A phylogenetic tree was constructed by neighbor joining. To compare the amino acid sequences of wild boar and landrace PERV-C (AM229312), the env regions were translated and aligned.
Results
Application of PCR-based detection methods developed for PERV-C screening to wild boars
To analyze the prevalence of PERV-C in wild boars, different PCRs and a real-time PCR were used that had been developed to screen for the presence of PERV-C proviruses (Fig. 1A) [28]. When PCR1 was used to detect PERV-C, all 18 animals from the first study and 107 new wild boars from four different regions of Germany were found to be positive. Using PCR3, all 125 wild boars were also found to be positive for PERV-C.
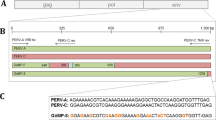
(A) Schematic presentation of the location of the primers and probes for the PCRs and real-time PCRs. The length of the amplicons in bp and the approximate locations of variable regions A (VRA) and B (VRB) and the proline-rich region (PRR) in the receptor-binding site of the surface envelope protein of PERV-C are indicated. (B) Sequence comparison of part of the surface envelope protein of PERV-C (envC), the PERV-C new variant (envC*), PERV-A (envA) and PERV-B (envB). The sequences of the outer primers (PCR3, PCR5, PCR4, PCR6 and PCR7) and inner primers (PCR2) for the nested PCR as well as primers and probes for the real-time PCR are indicated. PCR1-6 were designed using the sequence of PERV-C. A real-time PCR was developed for PERV-C, and a real-time PCR was designed for the new variant (nv). PCR 7 was designed only for the new variant of PERV-C (nv). (B) Amino acid changes in a region of the surface envelope protein of the PERV-C variant found in wild boars compared with the sequence in landrace pigs
However, wild boars were negative when using the PERV-C-specific real-time PCR, whereas German landrace pigs and transgenic pigs that were positive in PCR1 were also positive in this real-time PCR. To analyze the discrepancy, PCR3 and PCR5, both of which produced products that overlapped with the sequences analyzed in the real-time PCR, were performed, and the amplicons were sequenced. When comparing these sequences with published sequences [32] and sequences isolated from landrace pigs and sequenced by us, differences in the sequences including the primer- and probe-binding sites of the real-time PCR were detected (Fig. 1B). These differences explained the negative result obtained in the real-time PCR. The differences in the nucleotide sequence resulted in amino acid exchanges in the proline-rich region (PRR) and the variable region B (VRB) of the surface envelope protein (Fig. 1C). This new variant of PERV-C had not yet been reported.
Design of PCRs and real-time PCR specific for the new variant and distribution of variants of PERV-C
New primers and a new probe were designed based on the sequence of the new variant of PERV-C (Table 1) and applied in PCR1 (new variant) as well as in the real-time PCR (new variant) (Table 2). The efficiency of the real-time PCR specific for the new variant of PERV-C was comparable with that of the real-time PCR for the old variant [28] (Fig. S1). In addition, a probit analysis was performed for this real-time PCR. The real-time PCR was performed 10 times with probes from eight dilutions at the margin of detection, and at 0.0252 ng DNA, all samples were positive, e.g., they had measurable Ct values. Using the real-time PCR specific for the new variant of PERV-C, all 125 wild boars were found positive (Table 2 shows 10 animals). PCR1 [7] and the PCR1 (new variant) did not discriminate between the two variants of PERV-C (Table 2).
Using the real-time PCR (new variant), which is specific for the new variant of PERV-C, only positive reactions were also detected in some landrace pigs (7 animals out of 10 in Table 2). All together, 169 landrace animals were included in these analyses, and 147 (84 %) were positive in the PERV-C-specific real-time PCR. Of these animals, 32 were tested in the real-time PCR specific for the new variant, and 26 of them (81 %) were found positive, suggesting that some animals carry both sequences (Table 2, animals GL 1, 7, 8, 9).
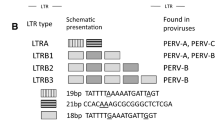
Three main types of German landrace animals were found as a result of this screening (Table 2): (i) pigs that were PERV-C positive in PCR1, PCR1 (new variant) and PCR3 as well as in the real-time PCR and real-time PCR (new variant) (animals GL1, GL7-9), (ii) pigs that were negative in PCR1 but positive in the real-time PCR (new variant) (pigs GL3-6), and finally, (iii) pigs that were negative in all four of these assays (pigs GL2 and 10). In a further analysis of these animals using PCR2 to PCR7, the data obtained previously using PCR1 and real-time PCR were confirmed, but five different groups of animals were found (Table 3). Among the animals GL3-6, belonging to the second group, GL 4 and 5 were positive in PCR7, and GL3 was positive in PCR6.
PCR6 amplifies a region overlapping the sequence between the VRA (variable region A) and PRR (prolin-rich region) (Fig. 1; Table 1) and allows investigating whether mutations in the forward primer may cause negative results in PCR1 and PCR1 (new variant). Sequencing of the amplicons revealed two additional variants of PERV-C in landrace pigs with differences in the binding sites of the primers (Fig. 2). The sequence of these variants had been reported previously (AF402663; DQ996276; [33]).
Differences in the sequences of PERV-C AM229312 [32], of the new variant, a second variant (2.v.) that is almost identical to DQ996276, and AF402663 in the region of primers and probes for PCR1 and the real-time PCR. Substitutions are indicated by small letters
Most importantly, some landrace pigs were negative in all PCRs and real-time PCRs performed (Fig. 3; Table 2, animal GL2), indicating that they were also free of the new variants of PERV-C. The pig kidney cell line PK15 was also PERV-C negative in all assays.
PCR analysis of DNA from one wild boar (WB1), three German landrace pigs (GL1-3), PK15 cells, and a no-template control using primers designed for PCR1 [7] and for the PCR1 specific for the new variant. M, marker. These data indicate the presence of PERV-C new variant in GL1 and WB1, and the absence of any PERV-C in GL 2 and 3. The weak bands seen in addition to the main amplicon were nonspecific, as shown by sequencing
Phylogenetic trees revealed that the new variant of PERV-C found in all wild boars is equally distant to a PERV-C sequence described in bearded pigs (Sus barbatus) and other PERV-C variants (Fig. 4).
Discussion
In this study, a new variant of PERV-C was found in all wild boars that were tested and in some landrace pigs. In addition, the repertoire of methods for the detection of PERV-C was extended by establishing additional PCRs and real-time PCRs. This will allow better screening for PERV-C-free animals.
In previous studies, 92 % of the German landrace and multi-transgenic animals were found to be positive for PERV-C [27, 28]. Here a smaller population was analyzed, which has already been partially selected for the absence of PERV-C, explaining the lower percentage of 84 %.
All wild boars analyzed carried the new variant of PERV-C, which was equally distantly related to a PERV-C in bearded pigs (Sus barbatus) and other PERV-C variants (Fig. 4). It had been calculated that PERV-C was integrated into the genome of S. barbatus approximately 1.5 MYA [15], but the relationship between the sequences is still unclear. It also remains unclear, but was not the aim of this study, whether the new variants of PERV-C are replication competent and how many proviruses can be found in wild boars and other pigs. However, it is likely that even replication-incompetent variants of PERV-C can participate in recombination events, leading to PERV-A/C. Harrison et al. [18] showed that mutations in the proline-rich region (PRR) are relevant for the high-titer replication of PERV-A/C. Therefore, the observation that sequences of the newly described variant of PERV-C have changes in the PRR may also impact infectivity and/or host range.
The identification and characterization of the new variant of PERV-C is of importance for the virus safety of xenotransplantation, and the aim of the study was to improve the detection methods. Although PERV-C does not infect human cells, recombination between PERV-A and PERV-C might result in a high-titer PERV-A/C strain that is able to infect human cells [11, 16–22], and selection of PERV-C-negative animals would prevent such recombinants from arising.
The fact that PCR1 [7] is not sufficient to screen for all PERV-C variants is one of the most important findings of this study. The combined application of PCR1 and the PCR methods based on the sequence of the new variants of PERV-C will prevent false negative results in the detection of PERV-C. In the case of a negative PCR1 result, a real-time PCR and PCR6 should be performed to determine whether the animal contains the new variant of PERV-C or the sequence described by Hector et al. [33]. Most importantly, using the new detection methods, some animals have been identified as being negative for the known PERV-C. These animals may be used as source for the generation of genetically modified animals designed for xenotransplantation. Since it is not only the presence of PERV proviruses, but mainly their expression, that is important for the evaluation of risk, RNA studies including high-throughput sequencing should be considered in the future.
References
Wilson CA (2008) Porcine endogenous retroviruses and xenotransplantation. Cell Mol Life Sci 65:3399–33412
Boeke JD, Stoye JP (1997) Retrotransposons, endogenous retroviruses and the evolution of retroelements. In: Coffin JM, Hughes SH, Varmus HE (eds) Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 343–436
Herniou E, Martin J, Miller K, Cook J, Wilkinson M, Tristem M (1998) Retroviral diversity and distribution in vertebrates. J Virol 72:5955–5966
Stoye JP (2001) Endogenous retroviruses: still active after all these years? Curr Biol 11:R914–R916
Denner J (2010) Endogenous retroviruses. In: Kurth R, Bannert N (eds) Retroviruses: molecular biology, genomics and pathogenesis. Caister Academic Press, Norwich, pp 35–69
Akiyoshi DE, Denaro M, Zhu H, Greenstein JL, Banerjee P, Fishman JA (1998) Identification of a full-length cDNA for an endogenous retrovirus of miniature swine. J Virol 72:4503–4507
Takeuchi Y, Patience C, Magre S, Weiss RA, Banerjee PT, Le Tissier P, Stoye JP (1998) Host range and interference studies of three classes of pig endogenous retrovirus. J Virol 72:9986–9991
Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA (1997) Two sets of human-tropic pig retrovirus. Nature 389:681–682
Patience C, Switzer WM, Takeuchi Y, Griffiths DJ, Goward ME, Heneine W, Stoye JP, Weiss RA (2001) Multiple groups of novel retroviral genomes in pigs and related species. J Virol 75:2771–2775
Patience C, Takeuchi Y, Weiss RA (1997) Infection of human cells by an endogenous retrovirus of pigs. Nat Med 3:282–286
Wilson CA, Wong S, VanBrocklin M, Federspiel MJ (2000) Extended analysis of the in vitro tropism of porcine endogenous retrovirus. J Virol 74:49–56
Specke V, Tacke S, Boller K, Schwendemann J, Denner J (2001) Porcine endogenous retroviruses (PERVs): In vitro host range and attempts to establish small animal models. J Gen Virol 82:837–844
Specke V, Rubant S, Denner J (2001) Productive infection of human primary cells and cell lines with porcine endogenous retroviruses. Virology 285:177–180
Lieber MM, Sherr CJ, Benveniste RE, Todaro GJ (1975) Biologic and immunologic properties of porcine type C viruses. Virology 66:616–619
Niebert M, Tönjes RR (2005) Evolutionary spread and recombination of porcine endogenous retroviruses in the suiformes. J Virol 79:649–654
Wood JC, Quinn G, Suling KM, Oldmixon BA, Van Tine BA, Cina R, Arn S, Huang CA, Scobie L, Onions DE, Sachs DH, Schuurman HJ, Fishman JA, Patience C (2004) Identification of exogenous forms of human-tropic porcine endogenous retrovirus in miniature swine. J Virol 78:2494–2501
Bartosch B, Stefanidis D, Myers R, Weiss R, Patience C, Takeuchi Y (2004) Evidence and consequence of porcine endogenous retrovirus recombination. J Virol 78:13880–13890
Harrison I, Takeuchi Y, Bartosch B, Stoye JP (2004) Determinants of high titer in recombinant porcine endogenous retroviruses. J Virol 78(13871):13879
Oldmixon BA, Woods JC, Ericsson TA, Wilson CA, White-Scharf ME et al (2002) Porcine endogenous retrovirus transmission characteristics of an inbred herd of miniature swine. J Virol 76:3045–3048
Dieckhoff B, Puhlmann J, Büscher K, Hafner-Marx A, Herbach N, Bannert N, Büttner M, Wanke R, Kurth R, Denner J (2007) Expression of porcine endogenous retroviruses (PERVs) in melanomas of Munich miniature swine (MMS) Troll. Vet Microbiol 123:53–68
Denner J (2008) Recombinant porcine endogenous retroviruses (PERV-A/C): a new risk for xenotransplantation? Arch Virol 153:1421–1426
Martin SI, Wilkinson R, Fishman JA (2006) Genomic presence of recombinant porcine endogenous retrovirus in transmitting miniature swine. Virol J 3:91–96
Denner J, Specke V, Thiesen U, Karlas A, Kurth R (2003) Genetic alterations of the long terminal repeat of an ecotropic porcine endogenous retrovirus (PERV) during passage in human cells. Virology 314:125–133
Karlas A, Irgang M, Votteler J, Specke V, Ozel M, Kurth R, Denner J (2010) Characterisation of a human cell-adapted porcine endogenous retrovirus PERV-A/C. Ann Transplant 15:45–54
Scheef G, Fischer N, Krach U, Tönjes RR (2001) The number of a U3 repeat box acting as an enhancer in long terminal repeats of polytropic replication-competent porcine endogenous retroviruses dynamically fluctuates during serial virus passages in human cells. J Virol 75:6933–6940
Denner J, Schuurman H, Patience C (2009) The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Chapter 5: Strategies to prevent transmission of porcine endogenous retroviruses. Xenotransplantation 16:239–248
Dieckhoff B, Kessler B, Jobst D, Kues W, Petersen B, Pfeifer A, Kurth R, Niemann H, Wolf E, Denner J (2009) Distribution and expression of porcine endogenous retroviruses in multi-transgenic pigs generated for xenotransplantation. Xenotransplantation 16:64–73
Kaulitz D, Mihica D, Dorna J, Costa MR, Petersen B, Niemann H, Tönjes RR, Denner J (2011) Development of sensitive methods for detection of porcine endogenous retrovirus-C (PERV-C) in the genome of pigs. J Virol Methods 175:60–65
Mang R, Maas J, Chen X, Goudsmit J, van der Kuyl AC (2001) Identification of a novel type C porcine endogenous retrovirus: evidence that copy number of endogenous retroviruses increases during host inbreeding. J Gen Virol 32:1829–1834
Adlhoch C, Wolf A, Meisel H, Kaiser M, Ellerbrok H, Pauli G (2009) High HEV presence in four different wild boar populations in East and West Germany. Vet Microbiol 139:270–278
Adlhoch C, Kaiser M, Ellerbrok H, Pauli G (2010) High prevalence of porcine Hokovirus in German wild boar populations. Virol J 7:171
Preuss T, Fischer N, Boller K, Tonjes RR (2006) Isolation and characterization of an infectious replication-competent molecular clone of ecotropic porcine endogenous retrovirus class C. J Virol 80:10258–10261
Hector RD, Meikle S, Grant L, Wilkinson RA, Fishman JA, Scobie L (2007) Pre-screening of miniature swine may reduce the risk of transmitting human tropic recombinant porcine endogenous retroviruses. Xenotransplantation 14:222–226
Acknowledgment
This work was supported by the Deutsche Forschungsgemeinschaft (DE 729/4-3). We thank Kay-Martin Hanschmann, Paul Ehrlich Institute, Langen, Germany, for the probit analysis. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
705_2012_1490_MOESM1_ESM.ppt
Fig. S1. (A) Estimation of the efficiency of the duplex real-time PCR specific for the new variant (nv) of PERV-C. Dilution of a specific nv PERV-C amplicon (efficiency 107 %), and of a cyclophilin plasmid (efficiency 115 %). (B) Real-time PCR analysis of three different pigs for investigating the presence of PERV-C, PERV-C new variant and porcine cyclophilin. Primers and probes for PERV envC and the new variant of PERV envC were used. Notice that the residual low reactivity for PERV envC in pig 2 is due to the limited difference between the primers and the target. (PPT 1237 kb)
Rights and permissions
About this article
Cite this article
Kaulitz, D., Mihica, D., Adlhoch, C. et al. Improved pig donor screening including newly identified variants of porcine endogenous retrovirus-C (PERV-C). Arch Virol 158, 341–348 (2013). https://doi.org/10.1007/s00705-012-1490-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-012-1490-9