Abstract
Background
The finite life of non-rechargeable batteries powering implantable pulse generators (IPG) necessitates their periodic replacement. Children receiving deep brain stimulation (DBS) may require frequent battery changes over their treatment lifetime.
Objectives
We aimed to determine the battery life of IPGs used in pallidal DBS for the treatment of dystonia in children and young people.
Methods
We make use of a review of case notes of all children and young people undergoing DBS surgery at our institution from June 2005 to May 2010.
Results
A total of 54 children and young people underwent surgery on at least one occasion, with a total of 76 IPGs implanted. Replacement IPGs due to battery failure were required in 15 out of 54 (27.8%). The average time to battery failure was 24.5 ± 2.9 months (95% confidence interval), with a range of 13–39 months. Battery life was significantly longer in primary compared to subsequent IPGs. No difference in longevity was seen between different IPG devices.
Conclusions
IPG battery life may be short in children and young people receiving treatment for dystonia. These findings highlight the potential benefits of the recently introduced rechargeable neurostimulators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pallidal deep brain stimulation (DBS) has become well established in the treatment of dystonia and is increasingly being used in the paediatric age group [9, 14, 18, 21–23]. One major limitation of the commonly used Kinetra® and Soletra® implantable pulse generators (IPGs) is their finite lifetime. This necessitates periodic surgical replacement, exposing the patient to the attendant risks this entails. IPG replacement may also lead to a loss of stability of symptom control, with the gradual loss of even unilateral battery power potentially resulting in a gradual worsening of disabling symptoms [1, 8]. In the adult setting, battery lifetimes of around 4 years have been reported in the treatment of Parkinson’s disease (PD) [5, 17]. In the treatment of dystonia, shorter lifetimes of closer to 24 months have been reported, with much greater variability between patients, relating to the higher energy consumption of the settings needed to produce symptomatic relief [7, 13].
Paediatric patients receiving pallidal DBS for dystonia may conceivably require treatments for decades of life. As secondary dystonia is more common than primary dystonia in childhood, higher stimulation parameters and consequently energy delivery may be required in comparison to adult patients with dystonia undergoing pallidal DBS. As a consequence, they are likely to require numerous IPG replacement procedures. We aimed to determine the average length of time from initial insertion to replacement due to battery failure for paediatric patients receiving pallidal stimulation in our centre. We also aimed to determine if a decline in response to stimulation was seen prior to total battery depletion.
Patients and methods
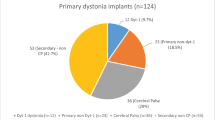
We reviewed the clinical records of all children undergoing pallidal DBS in our centre between June 2005 and May 2010. During this period a total of 54 children and young people underwent DBS surgery on at least one occasion. The mean age of children at the time of surgery was 11.1 years (range 3.3–20). A diagnosis of primary dystonia had been made in 13 children, of secondary dystonia in 30 children and neuronal degeneration with brain iron accumulation (NBIA) in the remaining 11 children. A total of 76 IPGs were implanted, comprising of 16 paired Soletra®, 25 Kinetra® and 35 Activa® RC unit (Fig. 1).
In one case unilateral DBS leads were implanted into the ventralis oralis anterior (VOA) thalamus and globus pallidus interna (GPi). In all other patients bilateral DBS surgery was performed with the posterolateral GPi as the target nucleus (Lead Model 3389; Medtronic, Minneapolis, MN). Surgery was performed under general anaesthesia. Direct MRI-guided targeting was used in all cases using preoperative stereotactic high-resolution MRI. Post-operative stereotactic imaging was performed to verify the position of electrodes in all cases. Intra-operative impedance or microelectrode recordings were also used when targeting the nuclei. We identified those patients requiring replacement IPGs due to battery failure and measured the length of time from initial surgery to this point. For three patients with Soletra® pulse generators, we also plotted serial Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) motor scores over time, comparing scores with battery voltage levels reported by the pulse generators during routine general impedance checks over the same period.
Results
During the study period, 15 out of 54 children (27.8%) required surgery to implant replacement pulse generators due to battery failure (Table 1). Of the 76 surgeries performed to implant IPGs, a total of 22 implant replacement surgeries were performed (28.9% of surgeries). Considering the 15 children requiring replacements, 9 patients required a single battery replacement surgery, 5 patients required two surgeries and 1 patient required three surgeries during the study period. On ten occasions Soletra® (Medtronic) model IPGs were replaced. In all cases both Soletra® IPGs were replaced at the same surgery (i.e. a total of 20 Soletra® IPGs removed and replaced with either two further Soletra® IPGs or a single IPG (capable of dual-channel stimulation). The Kinetra® (Medtronic) model device was replaced on 12 occasions.
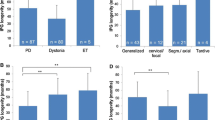
The average time from insertion to replacement due to battery failure was 24.5 ± 2.9 months (95% confidence interval), with a range of 13–39 months (Fig. 2). In calculating this average, twinned Soletra® IPG units replaced due to battery failure have been considered as a single IPG. The mean battery life was longer for the primary compared to subsequent implants [28.1 ± 2.6 months (95% confidence interval) compared to 16.7 ± 1.6 months (95% confidence interval), Student t test p value <0.005]. Comparing the battery life of the Kinetra® and Soletra® implantable pulse generators, no significant difference was seen (mean of 22.5 versus 26.7 months, Student t test p = 0.15).
Length of time (months) from initial insertion of implantable pulse generator to battery failure. Bars represent mean times, with error bars representing 95% confidence interval of the mean. a Comparison of (1) all 22 pulse generators combined, (2) Kinetra® and (3) Soletra® pulse generator. A mean time from insertion to battery failure for all pulse generators combined was 24.5 ± 2.9 months (±95% CI). There was no significant difference between an average time to battery failure for 12 Kinetra® pulse generators of 22.5.0 ± 3.5 months (±95% CI) and 10 Soletra® Pulse generators of 26.7 ± 4.6 months (±95% CI), Student t test, p = 0.15. b A significant difference in battery life between first and subsequent pulse generators was seen patients, 28.1 ± 2.6 months (±95% CI) compared to 16.7 ± 1.6 months (±95% CI), Student t test, p < 0.005
Concentrating on battery longevity in the first 2 years post-insertion, data were pooled from across the 32 patients who initially received non-rechargeable IPG devices. Battery life data were analysed for each battery with a full 24-month follow-up period post-surgery or with battery failure prior to this point. Data were included for both primary and subsequent IPGs. This gave a total number of n = 28 IPGs, 21 primary implants and 7 replacements. Plotting the Kaplan–Meier survival curve for these IPGs over the first 24 months post-implantation (Fig. 3), a marked difference can be seen in the time to failure comparing first and subsequent IPGs. Combining data for all IPGs (n = 28), a 64% survival rate was seen at 24 months. For first IPGs alone (n = 21), this survival rate was 81%, whilst by 21 months post-implantation all subsequent IPGs (n = 7) had required replacement.
Kaplan–Meier curve for battery survival over first 24 months post-DBS insertion. Data included for all IPGs with either a follow-up to 24 months from IPG implantation or a battery failure prior to this time point (n = 28). Combining battery data across primary and replacement IPGs, at 24 months post-insertion, 36% (10 out of 28) of all IPGs had been replaced due to battery failure. Considering primary implants alone (n = 21), only 19% (4 out of 21) of IPGs required replacement for battery failure in this time. For subsequent IPGs (n = 7), all implants had required replacement by 21 months
For patients 7, 8 and 13 [all with a genetic diagnosis of pantothenate kinase-associated neurodegeneration(PKAN)], changes in serial BFMDRS motor scores compared to baseline were plotted on the same time scale as battery voltage output levels from their implanted Soletra® pulse generators (Fig. 4). In all three cases, the BFMDRS improvement is followed by an apparent deterioration in efficacy of pallidal DBS. This loss of dystonia control is apparent even with small drops in battery voltage output, prior to absolute battery failure. This seemed to occur with a decline in battery voltage levels to below 3.6 V from a steady state of 3.7 V.
Comparison of Soletra® maximum battery voltage from right and left GPi over time since stimulation compared to% improvement in Burke–Fahn–Marsden Dystonia rating scale compared to pre-DBS baseline in three patients with pantothenate kinase-associated neurodegeneration. In all three patients, the steady state MBV was ∼3.7 V. Prior to absolute battery failure, a decline in the efficacy of response to DBS appears to occur
Discussion
The overall average battery life of the Soletra® and Kinetra® IPGs in our series was comparable to that previously reported for patients receiving pallidal DBS in the treatment of dystonia. No difference in battery life was seen between the Soletra® and Kinetra® IPGs. Average battery life of the primary IPG was considerably longer than that for subsequent IPGs. This is likely to reflect the slow increase in stimulation parameter settings in the first few months following primary implantation, during which time total energy expenditure will be comparatively low. Following IPG replacement, stimulation parameters are generally set at the pre-replacement settings.
A shorter battery life was again seen in comparison to IPGs used in the treatment of PD patients, presumably due to the higher stimulation settings required to control dystonic symptoms. Traditionally, high-frequency stimulation has been used in the treatment of dystonia, i.e. frequencies >120 Hz. Some authors have reported improvement in dystonic symptoms in patients with primary generalised dystonia receiving lower frequency stimulation at 60–80 Hz, with a corresponding increase in IPG battery life up to 48 months [2, 3, 13]. Only small numbers of reports have demonstrated the benefits of stimulation at this frequency though with conflicting reports of efficacy in cervical dystonia [10, 16]. Some experimental evidence in a small number of patients has suggested an improvement in individual patients with an increase in stimulation frequency from 50 to 130 Hz, with further improvement with increases to 180 and then 250 Hz [15].
It is difficult to predict the treatment battery lifetime for paediatric patients receiving pallidal DBS. In addition to the improvement of dystonic symptoms in both primary and secondary dystonias, pallidal DBS has been shown to improve function, quality of life and also to reduce the development of secondary complications, such as soft tissue contractures [6, 11, 22]. Children receiving pallidal DBS are likely to need treatment for several decades. For a patient beginning stimulation at 6 years of age, an initial mean battery life of 28 months, followed by a mean subsequent battery life of 16 months, could result in the need for 16–17 IPG replacements by 30 years of age.
Pallidal DBS has not yet been shown to improve patient longevity, though it seems reasonable to predict such a benefit in patients with primary dystonias given the reduced contribution of dystonia to respiratory compromise and aspiration events. Even patients with progressive neurodegenerative conditions including PKAN might be expected to benefit in terms of an increase in life span to an albeit lesser extent, though it is important to consider that DBS is unlikely to affect the natural history of the underlying pathologies of these patients. However, children with severe dystonias are vulnerable to life-threatening crises caused by worsening dystonia towards battery end of life as illustrated in our PKAN cases. Recurrently failing batteries obviously multiply such risks and also may cast doubt on the long-term efficacy of DBS if battery failure is not recognized.
Even without connection and implantation, non-rechargeable batteries have a finite lifetime due to internal chemical reaction causing a slow self-discharge. For this reason existing IPG devices have a manufacturer set shelf life prior to implantation that has been defined in order for expected longevity to be achieved. Environmental factors such as temperature may adversely affect this shelf life, and so minimum and maximum temperatures are recommended during transport and storage. For both Soletra® and Kinetra® devices, a shelf life of 18 months has been recommended, as beyond this time battery longevity following implantation may be compromised. For the newer Activa® RC device, a shorter shelf life of 11 months has been advised. For all cases during the course of this study, IPGs were implanted prior to shelf life expiration, and so time from manufacture to surgery would not be expected to have influenced longevity.
The apparent decline in efficacy of pallidal DBS in three patients prior to total battery failure was not anticipated. This may have occurred coincidentally given the natural fluctuation in the severity of dystonic symptoms over time, particularly as all three patients had an underlying diagnosis of PKAN. Our hypothesis, however, is that subtle reductions in battery performance might have caused reduced DBS efficacy suggesting that IPG battery replacements need to be scheduled at the earliest sign of reduced voltage output and well before total battery failure is recorded, to prevent clinical relapse of symptoms. An obvious corollary of this is to suspect battery voltage output failure with deteriorating dystonia, particularly in neurodegenerative disorders when one might implicate a worsening of the natural history of the disease. This policy of early battery change would further reduce the effective lifetime of non-rechargeable IPGs, necessitating an increase in the frequency of battery changes in an attempt to prevent a potential 6-month period of clinical instability attributable to a gradually failing battery.
IPG devices have continued to evolve technically since their introduction more than a decade ago. The original single-channel devices required the implantation of two IPGs to facilitate bilateral pallidal or thalamic DBS. The introduction of dual-channel devices enabled bilateral DBS with just one IPG (though limiting the possibilities of differential unilateral stimulation settings). Newer technologies have enabled the development of rechargeable IPG batteries, such as the Activa® RC, (Medtronic Inc.), potentially greatly prolonging battery lifetimes for IPG devices and reducing the frequency of replacement surgery. One open-label study of a rechargeable IPG (Restore, Medtronic Inc) in 41 patients receiving spinal cord stimulation in the treatment of chronic neuropathic pain refractory to medical treatment reported 100% of patients able to recharge successfully in the first 12 months following implantation [19]. Longer term studies are required to confirm that patients and carers can continue to find the extra burden of time spent recharging the device tolerable. In addition to the potential benefits in reducing the frequency of IPG replacement surgery, with a volume of just 22 cm3, the smaller size of the Activa® RC device may facilitate its implantation in children of a much smaller weight and age in comparison to both the Kinetra® and Soletra® IPGs (Kaminska et al. 2011, unpublished data). Delay from symptom onset to commencement of pallidal DBS has been shown in a number of studies to correlate negatively with outcome, such that patients with a shorter duration of symptoms and younger age at implantation experience better outcomes [4, 12, 20].
In conclusion, we have found a mean battery life of 24 months for IPG in paediatric patients receiving pallidal DBS in the treatment of dystonia. Paediatric patients with traditional non-rechargeable IPGs may require frequent surgery to replace IPGs with depleted batteries over a treatment lifetime that could span decades. Our findings highlight the potential benefits in this age group of the newer generation of rechargeable IPG devices.
References
Allert N, Kirsch H, Weirich W, Karbe H (2009) Stability of symptom control after replacement of impulse generators for deep brain stimulation. J Neurosurg 110:1274–1277
Alterman RL, Miravite J, Weisz D, Shils JL, Bressman SB, Tagliati M (2007) Sixty hertz pallidal deep brain stimulation for primary torsion dystonia. Neurology 69:681–688
Alterman RL, Shils JL, Miravite J, Tagliati M (2007) Lower stimulation frequency can enhance tolerability and efficacy of pallidal deep brain stimulation for dystonia. Mov Disord 22:366–368
Andrews C, Aviles-Olmos I, Hariz M, Foltynie T (2010) Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry 81:1383–1389
Anheim M, Fraix V, Chabardes S, Krack P, Benabid AL, Pollak P (2007) Lifetime of Itrel II pulse generators for subthalamic nucleus stimulation in Parkinson’s disease. Mov Disord 22:2436–2439
Anheim M, Vercueil L, Fraix V, Chabardes S, Seigneuret E, Krack P, Benabid AL, Vidailhet M, Pollak P (2008) Early stimulation of DYT1 primary generalized dystonia prevents from its secondary irreversible complications. Mov Disord 23:2261–2263
Blahak C, Capelle HH, Baezner H, Kinfe TM, Hennerici MG, Krauss JK (2011) Battery lifetime in pallidal deep brain stimulation for dystonia. Eur J Neurol 18:872–875
Chou KL, Siderowf AD, Jaggi JL, Liang GS, Baltuch GH (2004) Unilateral battery depletion in Parkinson’s disease patients treated with bilateral subthalamic nucleus deep brain stimulation may require urgent surgical replacement. Stereotact Funct Neurosurg 82:153–155
Coubes P, Cif L, El Fertit H, Hemm S, Vayssiere N, Serrat S, Picot MC, Tuffery S, Claustres M, Echenne B, Frerebeau P (2004) Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg 101:189–194
Goto S, Mita S, Ushio Y (2002) Bilateral pallidal stimulation for cervical dystonia. An optimal paradigm from our experiences. Stereotact Funct Neurosurg 79:221–227
Halbig TD, Gruber D, Kopp UA, Schneider GH, Trottenberg T, Kupsch A (2005) Pallidal stimulation in dystonia: effects on cognition, mood, and quality of life. J Neurol Neurosurg Psychiatry 76:1713–1716
Isaias IU, Alterman RL, Tagliati M (2008) Outcome predictors of pallidal stimulation in patients with primary dystonia: the role of disease duration. Brain 131:1895–1902
Isaias IU, Alterman RL, Tagliati M (2009) Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol 66:465–470
Krause M, Fogel W, Kloss M, Rasche D, Volkmann J, Tronnier V (2004) Pallidal stimulation for dystonia. Neurosurgery 55:1361–1368
Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider GH, Poewe W, Eisner W, Wolters A, Muller JU, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Naumann M, Volkmann J (2006) Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 355:1978–1990
Moro E, Piboolnurak P, Arenovich T, Hung SW, Poon YY, Lozano AM (2009) Pallidal stimulation in cervical dystonia: clinical implications of acute changes in stimulation parameters. Eur J Neurol 16:506–512
Ondo WG, Meilak C, Vuong KD (2007) Predictors of battery life for the Activa Soletra 7426 Neurostimulator. Parkinsonism Relat Disord 13:240–242
Parr JR, Green AL, Joint C, Andrew M, Gregory RP, Scott RB, McShane MA, Aziz TZ (2007) Deep brain stimulation in childhood: an effective treatment for early onset idiopathic generalised dystonia. Arch Dis Child 92:708–711
Van Buyten JP, Fowo S, Spincemaille GH, Tronnier V, Beute G, Pallares JJ, Naous H, Zucco F, Krauss JK, De Andres J, Buchser E, Costantini A, Lazorthes Y (2008) The restore rechargeable, implantable neurostimulator: handling and clinical results of a multicenter study. Clin J Pain 24:325–334
Vasques X, Cif L, Gonzalez V, Nicholson C, Coubes P (2009) Factors predicting improvement in primary generalized dystonia treated by pallidal deep brain stimulation. Mov Disord 24:846–853
Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, Lagrange C, du Tezenas MS, Dormont D, Grand S, Blond S, Detante O, Pillon B, Ardouin C, Agid Y, Destee A, Pollak P (2005) Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 352:459–467
Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, Bardinet E, Benabid AL, Navarro S, Dormont D, Grand S, Blond S, Ardouin C, Pillon B, Dujardin K, Hahn-Barma V, Agid Y, Destee A, Pollak P (2007) Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol 6:223–229
Vidailhet M, Yelnik J, Lagrange C, Fraix V, Grabli D, Thobois S, Burbaud P, Welter ML, Xie-Brustolin J, Braga MC, Ardouin C, Czernecki V, Klinger H, Chabardes S, Seigneuret E, Mertens P, Cuny E, Navarro S, Cornu P, Benabid AL, Le Bas JF, Dormont D, Hermier M, Dujardin K, Blond S, Krystkowiak P, Destee A, Bardinet E, Agid Y, Krack P, Broussolle E, Pollak P (2009) Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study. Lancet Neurol 8:709–717
Acknowledgments
This work was supported by a grant from the Guy’s and St Thomas’ Charity: Project Number G06070.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lumsden, D.E., Kaminska, M., Tustin, K. et al. Battery life following pallidal deep brain stimulation (DBS) in children and young people with severe primary and secondary dystonia. Childs Nerv Syst 28, 1091–1097 (2012). https://doi.org/10.1007/s00381-012-1728-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-012-1728-6