Abstract
The systemic administration of nitroglycerine induces attacks in migraineurs and is able to activate and sensitize the trigeminal system in animals involving glutamate and α7-nicotinic acetylcholine receptors, among others. Kynurenic acid is one of the endogenous glutamate receptor antagonists, and exerts inhibitory action on the α7-nicotinic acetylcholine receptors. Since kynurenic acid penetrates the blood–brain barrier poorly, therefore a newly synthesized kynurenic acid amide, N-(2-N-pyrrolidinylethyl)-4-oxo-1H-quinoline-2-carboxamide hydrochloride (KYNAa) was used with such a side-chain substitution to facilitate brain penetration in our study. We evaluated its modulatory effect on kynurenic acid concentration in the cervical part of trigemino-cervical complex (C1–C2) and in the model of nitroglycerine-induced trigeminal activation using male Sprague–Dawley rats. One hour after 1 mmol/kg bodyweight KYNAa administration, the kynurenic acid level increased significantly in C1–C2, which returned to the basal level at 300 min measured by high-performance liquid chromatography. KYNAa pre-treatment had dose-dependent, mitigating action on nitroglycerine-induced decrease in calcitonin gene-related peptide and increase in c-Fos, neuronal nitric oxide synthase and calmodulin-dependent protein kinase II alpha expression in the C1–C2. KYNAa also mitigated the behavioural changes after nitroglycerine. Thus, in this model KYNAa is able to modulate in a dose-dependent manner the changes in neurochemical markers of activation and sensitization of the trigeminal system directly and indirectly—via forming kynurenic acid, possibly acting on peripheral and central glutamate or α7-nicotinic acetylcholine receptors. These results suggest that application of kynurenic acid derivatives could be a useful therapeutic strategy in migraine headache in the future with a different mechanism of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is an intensively researched, very common neurological disease, with a partially known pathomechanism that includes activation and sensitization of the trigeminal system (Moskowitz 2008; D’Andrea and Leon 2010).
In most migraineurs, but not in healthy people (Sicuteri et al. 1987), systemic administration of the nitric oxide donor nitroglycerine triggers a delayed migraine-like attack, which is similar to a spontaneous attack and is usually associated with symptoms that include nausea and photophobia (Christiansen et al. 1999). Moreover, nitroglycerine is able to sensitize the trigeminal system in humans (Di Clemente et al. 2009).
Various animal experiments have revealed that nitroglycerine can activate the trigeminal system, e.g. it is able to decrease the calcitonin gene-related peptide (CGRP) content of the primary trigeminal nociceptors in the cervical part of trigemino-cervical complex (C1–C2), suggesting the activation and transmitter release of first-order neurones (Pardutz et al. 2002). Nitroglycerine can also stimulate the second-order trigeminal neurones, increasing the c-Fos expression in the same area (Tassorelli and Joseph 1995). Moreover, nitroglycerine administration increases the neuronal nitric oxide synthase (nNOS) and calmodulin-dependent protein kinase II alpha (CaMKIIα) immunoreactivity in the C1–C2 of the rat (Pardutz et al. 2000, 2007), which may suggest a self-amplifying mechanism at the level of second-order trigeminal neurones relevant in the central sensitization process (Chacur et al. 2010; Fang et al. 2002). Taken together, these results indicate that the systemic administration of nitroglycerine is a valuable tool used to evaluate the activation and sensitization of the trigeminal system in animals.
Kynurenic acid is an endogenous end-product formed from l-kynurenine during the tryptophan metabolism. It is assumed that kynurenic acid plays an important role in the pathomechanism of several neurological disorders (Vecsei et al. 2013) and exerts a neuroprotective effect in certain neurodegenerative diseases (Nemeth et al. 2006; Fuvesi et al. 2012). There is evidence that it can also affect nociception (Mecs et al. 2009; Nasstrom et al. 1992; Pardutz et al. 2012), probably due to an antagonistic effect on N-methyl-d-asparate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainate and α7-nicotinic acetylcholine receptors (Pereira et al. 2002; Birch et al. 1988; Kessler et al. 1989) or an agonistic action on G-protein-coupled receptor-35 (Wang et al. 2006). Due to the poor ability of kynurenic acid to cross the blood–brain barrier (Fukui et al. 1991), its various derivatives with a better central nervous system action and a suggested similarity regarding their pharmacological effect were synthesized and successfully used under experimental conditions (Demeter et al. 2012; Gellert et al. 2012; Zadori et al. 2011). An earlier synthesized kynurenic acid derivative was more effective than the parent compound reducing the second-order trigeminal activation, as previously shown (Knyihar-Csillik et al. 2008) and experimental evidence suggests that this analogue has a similar pharmacological action as kynurenic acid with a presumed better blood–brain barrier penetrance (Marosi et al. 2010).
In the present study, we used a different, newly synthesized kynurenic acid amide (KYNAa) which was prepared directly from kynurenic acid resulting in an altered side-chain.
Our first aim was to determine how peripheral KYNAa administration affects kynurenic acid concentrations in the peripheral blood and the central nervous system, in the cervical trigeminal complex by using high-performance liquid chromatography (HPLC).
Our next objective was to test whether the pre-treatment with this new KYNAa exerts a modulatory effect on the nitroglycerine-induced changes in CGRP, c-Fos, nNOS and CamKIIα expression. We also wanted to test if these morphological alterations reflecting trigeminal activation and sensitization are paralleled with behavioural changes of rats in the open field test.
Materials and methods
Animals
The procedures utilized in this study followed the guidelines for the Use of Animals in Research of the International Association for the Study of Pain and the directives of the European Economic Community (86/609/ECC). They were approved by the Committee of Animal Research at the University of Szeged (I-74-12/2012) and the Scientific Ethics Committee for Animal Research of the Protection of Animals Advisory Board (XI./352/2012). Ninety adult male Sprague–Dawley rats weighing 200–250 g were used. The animals were maintained on a 12-h dark–12-h light cycle under standard laboratory conditions, with tap water and regular rat chow available ad libitum.
Drugs
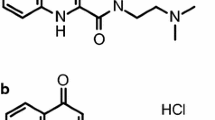
The new kynurenic acid amide (Patent number #P0900281/PCT/HU2010/00050), N-(2-N-pyrrolidinylethyl)-4-oxo-1H-quinoline-2-carboxamide hydrochloride (KYNAa) (Fig. 1), was synthesized in the Department of Pharmaceutical Chemistry, University of Szeged by reacting kynurenic acid with 2-pyrrolidinoethylamine resulting in a side-chain containing tertiary nitrogen in pyrrole ring system with regard to the following structural properties: (1) the presence of a water-soluble side-chain, (2) the inclusion of a new cationic centre, and (3) side-chain substitution to facilitate brain penetration (Fulop et al. 2012).
Nitroglycerine (Nitrolingual®) was purchased from Pohl-Boskamp and was administered intraperitoneally in a dosage of 10 mg/kg bodyweight (bw) as described before (Pardutz et al. 2000). Nitrolingual placebo was obtained from Pohl-Boskamp.
For the HPLC measurements The animals were divided into two groups. In the control group, the animals received only the intraperitoneal (i.p.) vehicle solution (physiological saline) as pre-treatment (n = 4), without drug. In the second group (n = 10), the rats were pre-treated with an i.p. injection of 1 mmol/kg bw KYNAa (diluted to 2 ml, pH 7.4) (Fig. 2a).
For immunohistochemistry The animals were divided into four groups (n = 10 per group). The animals of the control group received only the i.p. vehicle solution (physiological saline) as pre-treatment. In the remaining 3 groups, the rats were pre-treated with an i.p. injection of KYNAa at the dose of 0.1, 0.5 and 1 mmol/kg bw, respectively (diluted to 2 ml, pH 7.4) (Fig. 2b). One hour later in each group, half of the animals (n = 5) received an i.p. injection of nitroglycerine, while the other half of the rats (n = 5) received an i.p. injection of the placebo of nitroglycerine (Fig. 2b).
For Western blot The animals were divided into two groups (n = 10 per group). The animals in the control group received only the i.p. vehicle solution (physiological saline) as pre-treatment. In the second group, the rats were pre-treated with an i.p. injection of 1 mmol/kg bw KYNAa (Fig. 2c). The administration of nitroglycerine was performed as described above (Fig. 2c).
For the Open Field Test The animals were separated into two groups (n = 20 per group). In the control group, the animals received i.p. vehicle solution (physiological saline) as pre-treatment, without drug. In the second group, the rats were pre-treated with an i.p. injection of KYNAa at the dose of 1 mmol/kg bw (Fig. 2d). The administration of nitroglycerine was performed as described above (Fig. 2d).
Kynurenic acid detection with high-performance liquid chromatography (HPLC)
Sampling and sample preparation
At set time points (60 and 300 min, n = 5 per group) following the i.p. injection with the KYNAa, the rats were deeply anaesthetized with chloral hydrate (0.4 g/kg bw, Sigma-Aldrich) and transcardially perfused with phosphate-buffered saline for 5 min. The animals of the control group (n = 4) underwent a similar procedure with one measurement point at 60 min. Then the brain samples with the corresponding upper cervical spinal cord segments (C1–C2) were removed. After dissection, the samples of C1–C2 were stored at −70 °C until analysis. The samples were weighed and then sonicated in an ice-cooled solution (250 μl) comprising perchloric acid (2.5 % w/w, Scharlau), internal standard (3-nitro-l-tyrosine, 2 μM, Sigma-Aldrich,) and distilled water for 1.5 min in an Eppendorf tube. The content of the Eppendorf tube was centrifuged at 12,000 RPM for 10 min at 4 °C. From the supernatant 100 μl was transferred to a test vial.
Chromatographic conditions
The kynurenic acid concentrations of the samples were quantified based on the slightly modified method of Herve et al. (1996), with an Agilent 1100 HPLC system (Agilent Technologies). The system was equipped with a fluorescent and an UV detector, the former was applied for the determination of kynurenic acid and the latter for the determination of the internal standard. Chromatographic separations were performed on an Onyx Monolithic C18 column, 100 mm × 4.6 mm I.D. (Phenomenex Inc.) after passage through a Hypersil ODS pre-column, 20 × 2.1 mm I.D., 5 μm particle size (Agilent Technologies) with a mobile phase composition of 0.2 M zinc acetate (Sigma-Aldrich)/acetonitrile (Scharlau) = 95/5 (v/v), the pH of which was adjusted to 6.2 with acetic acid (VWR International), applying isocratic elution. The flow rate and the injection volume were 1.5 ml/min and 50 μl, respectively. The fluorescent detector was set at excitation and emission wavelengths of 344 and 398 nm. The UV detector was set at 365 nm wavelength.
HPLC method validation
Calibration curve and linearity Calibrants were prepared at six different concentration levels, from 1 to 100 nM and 0.5 to 5 μM for kynurenic acid and the internal standard, respectively. Three parallel injections of each solution were made under the chromatographic conditions described above. The peak area responses were plotted against the corresponding concentration, and the linear regression computations were carried out by the least square method with the freely available R program (R Foundation for Statistical Computing, R Development Core Team). Very good linearity was observed throughout the investigated concentration ranges for kynurenic acid and the internal standard when either fluorescence or UV detection was applied.
Selectivity The selectivity of the method was checked by comparing the chromatograms of kynurenic acid and the internal standard for a blank central nervous system sample and those for a spiked sample. All compounds could be detected in their own selected chromatograms without any significant interference.
LOD and LLOQ Limit of detection (LOD) and lower limit of quantitation (LLOQ) were determined via signal-to-noise ratio with threshold 3, according to the ICH guidelines (Validation of Analytical Procedures: Text and Methodology Q2 (R1), International Conference on Harmonization). The LOD was 0.4 nM, while the LLOQ was 1 nM for kynurenic acid, respectively.
Precision Replicate HPLC analysis showed that the relative standard deviation was ≤3.1 % for the concentration and ≤0.1 % for the retention time.
Recovery The relative recoveries were estimated by measuring spiked samples of kynurenic acid at two different concentrations with three replicates of each. No significant difference was observed for the lower and higher concentrations. The recoveries ranged from 82 to 92 % for kynurenic acid, respectively.
Immunohistochemistry
Histological procedure
Immunohistochemical procedures were performed as described earlier (Vamos et al. 2009, 2010) supplemented with the immunohistochemistry for c-Fos. Briefly, 4 h after treatment with placebo or nitroglycerine, the rats, anaesthetized with chloral hydrate, were perfused and fixed transcardially and the C1–C2 segment of the cervical spinal cord was removed, this is the area where most of trigeminal afferents involved in headache reach the second-order neurones (Strassman et al. 1994). After postfixation and cryoprotection, 30 μm cryostat sections were cut and treated as free-floating sections. After suppression of the endogenous peroxidase activity and several rinses in phosphate-buffered saline containing 1 % Triton X-100 (PBST, VWR International), sections were incubated in PBST containing (1) rabbit anti-rat CGRP polyclonal antibody (Sigma-Aldrich, C-8198, dilution: 1:20,000, incubation: overnight at room temperature) or (2) rabbit anti-rat c-Fos (H-125) polyclonal antibody (Santa Cruz Biotechnology, sc-7202, dilution: 1:1,000, incubation: overnight at room temperature) or (3) rabbit anti-rat nNOS polyclonal antibody (EuroProxima, 2263B220-1, dilution: 1:5,000, incubation: two nights at 4 °C) or (4) mouse anti-rat CaMKIIα monoclonal antibody (Sigma-Aldrich, C-265, dilution: 1:2,000, incubation: four nights at 4 °C). The immunohistochemical reaction was visualized by using Vectastain Elite avidin–biotin kits (Vector Laboratories; PK6101 for c-Fos, nNOS and CGRP; PK6102 for CaMKIIα) and stained with nickel ammonium sulphate (VWR International)-intensified 3,3′-diaminobenzidine (Sigma-Aldrich). The specificity of the immune reactions was checked by omitting the primary antiserum.
Data evaluation
All evaluations were performed by an observer blind to the experimental groups. The detailed methodologies were described previously (Vamos et al. 2009, 2010).
In brief, the area covered by CGRP-immunoreactive fibres was determined using Image Pro Plus 6.2® image analysis software (Media Cybernetics). Photomicrographs of sections were taken under the 10× objective of a Nikon Optiphot-2 light microscope fitted with an Olympus DP70 CCD camera (Olympus Corporation). After image acquisition, the laminae I–II in dorsal horn were determined manually as area of interest and a threshold grey level was established with the Image Pro Plus 6.2® image analysis software, as described in earlier studies (Vamos et al. 2009, 2010). The program expressed the area innervated by the immunoreactive fibres as the number of pixels with densities above the threshold. For the calibration, we measured formations with known area. The area covered by immunoreactive fibres per dorsal horn was calculated as the multiplication of the average number of pixels for the individual dorsal horn and the area of one pixel.
The c-Fos-, nNOS- and CaMKIIα-immunoreactive cells were counted under the 10× objective of a Nikon Optiphot-2 light microscope (Nikon Instruments) in laminae I–II of the C1–C2. The c-Fos-positive neurones were scored as those with obvious specific nuclear staining; the nNOS-immunoreactive neurones as those exhibiting cytoplasmic and dendritic staining and a nucleus; and the CaMKIIα-labelled cells with a clearly increased immunoreactivity relative to the background. The number of immunoreactive neurones per sections was calculated, i.e. the numbers of immunopositive neurones present on the two dorsal horns were summed. Thereafter, the results on the individual sections were averaged for each animal.
Photomicrographs were taken under the 40x objective of a Zeiss Axio Imager M2 Upright Microscope (Carl Zeiss MicroImaging) fitted with an AxioCam MRc camera (Carl Zeiss MicroImaging) and Adobe Photoshop CS2 9.0 graphics program was used to create the artwork.
Western blot
Sampling and sample preparation
In both groups 4 h after the nitroglycerine or placebo injections, the rats were deeply anaesthetized with chloral hydrate as described above, perfused transcardially with 100 ml ice-cold phosphate-buffered saline and the cervical (C1–C2) spinal cord was removed. Until measurements, the samples were stored at −70 °C. The C1–C2 segments were sonicated in ice-cold lysis buffer containing 50 mM Tris–HCl, 150 mM NaCl, 0.1 % igepal, 0.1 % cholic acid, 2 μg/ml leupeptin, 2 mM phenylmethylsulphonyl fluoride (PMSF), 1 μg/ml pepstatin, 2 mM EDTA and 0.1 % sodium dodecyl sulphate (SDS) (all chemicals were from Sigma-Aldrich). The lysates were cleared from cellular debris by centrifugation at 12,000 RPM for 10 min at 4 °C and supernatants were aliquoted and stored at −20 °C. Protein concentration was measured according to BCA protein assay method with BCA Protein Assay Kit (Novagen) using bovine serum albumin as a standard. Samples were cooled on ice during the whole procedure. Prior to loading, each sample was mixed with sample buffer, and denaturated by boiling for 3 min. Equal amounts of protein samples (20 μg/lane) were separated by standard SDS polyacrylamide gel electrophoresis (SDS-PAGE) on 10 % (for nNOS and β-actin) and 15 % (for CGRP) Tris–Glycine gel and electrotransferred onto Amersham Hybond-ECL nitrocellulose membrane (0.45 and 0.2 μm pore size selectively, GE Healthcare). The Page Ruler Prestained Protein Ladder (Fermentas, 10–170 kDa) was used to determine approximate molecular weights. Following the transfer, membranes were blocked for 1 h at room temperature in Tris-buffered saline containing Tween 20 (TBST, MP Biomedicals) and 5 % non-fat dry milk powder and incubated in TBST containing 1 % non-fat dry milk and (1) rabbit anti-rat CGRP polyclonal antibody (Sigma-Aldrich, C-8198, dilution: 1:2,000, incubation: overnight at room temperature) or (2) mouse anti-rat nNOS monoclonal antibody (BD Biosciences, 610308, dilution: 1:2,500, incubation: overnight at room temperature) or (3) mouse anti-rat β-actin monoclonal antibody (Calbiochem, CP01, dilution: 1:10,000, incubation: overnight at room temperature). Next day after several rinses, membranes were incubated in TBST containing 1 % non-fat dry milk and horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (Santa Cruz Biotechnology; sc-2030 and sc-2031) for 1 h at room temperature. Protein bands were visualized after incubation of membranes with the SuperSignal West Pico Chemiluminescent Substrate (Pierce) using Carestream Kodak BioMax Light film (Kodak).
Data evaluation
For densitometric analyses, films were scanned and quantified using Java ImageJ 1.47v analysis software (National Institutes of Health). The results were normalized to the absolute control animals. β-actin served as a control to ensure loading of equivalent amounts of sample proteins.
Open field test
Testing procedure
Before the open field test, animals were habituated in the dimly lit, quiet test room for at least 30 min. The experiments were performed between 11 a.m. and 4 p.m. during the light cycle of rats. To avoid misinterpretation related to the acute cardiovascular effects of nitroglycerine and in parallel with earlier studies showing delayed behavioural alterations after systemic nitroglycerine in mice (Markovics et al. 2012), we tested the rats 3 h and 40 min after the nitroglycerine or placebo injections. The animals were placed in the open field box (48 × 48 × 40 cm, Experimetria Ltd., Hungary) and ambulation time, ambulation distance, local time and the number of rearings were registered for 15 min, and were evaluated using the Conducta 1.0 behaviour analysis program (Experimetria Ltd., Hungary). The rats did not receive any food or water during the observation period. The test box was cleaned and decontaminated after each animal.
Statistical analysis
Statistical analysis of the measurement data was carried out with IBM SPSS Statistics software (Version 20, IBM), using one-way analysis of variance followed by the Tukey or Tamhane post hoc test (except for the data of HPLC analyses), depending on the variances of the data, with p < 0.05 taken as statistically significant. Group values are reported as mean ± SEM. As in the treated groups the data of HPLC analysis showed non-Gaussian distribution, the non-parametric Kruskal–Wallis test was applied for group comparisons followed by Mann–Whitney U test for post hoc analysis, with p < 0.05 taken as statistically significant. The group values of HPLC analyses were reported as median and interquartile range.
Results
Kynurenic acid detection with HPLC
The HPLC measurements clearly indicated a significant (*p < 0.05), more than twofold increase in kynurenic acid level in the C1–C2 60 min after 1 mmol/kg bw KYNAa administration (25.38 pmol/g wet weight, interquartile range: 23.26–40.80 pmol/g wet weight) compared to vehicle-treated samples (11.48 pmol/g wet weight, interquartile range: 8.85–15.00 pmol/g wet weight) (Table 1), while the concentration of the kynurenic acid decreased to baseline at 300 min (9.02 pmol/g wet weight, interquartile range: 6.43–30.92 pmol/g wet weight) (Table 1). Although it needs different validation methods, we screened the kynurenic acid concentration on the blood samples too. Preliminary results suggest a robust increase (approximately 50×) in the peripheral kynurenic acid levels reaching μmolar concentrations (data not shown).
Immunohistochemistry
The transverse sections of the cervical spinal cord (C1–C2) demonstrated abundant CGRP-positive fibres, and c-Fos-, nNOS- and CaMKIIα-immunoreactive neurones in the superficial layers (laminae I–II) of the dorsal horn. The area covered by immunoreactive fibres and the number of immunoreactive cells did not differ significantly between sections located at the various levels along rostrocaudal axis or between the right and left dorsal horns of the cervical segments.
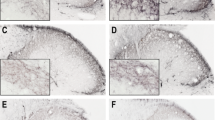
In the control group (pre-treated with saline, n = 10), reduced CGRP staining can be observed on the sections in I–II laminae after nitroglycerine treatment compared to placebo-treated rats (Fig. 3). This decrease is reflected in area covered by CGRP as well, i.e. significant difference can be measured in nitroglycerine-treated rats as compared with placebo-treated animals (**p < 0.01; Fig. 7a). In the same group, increased c-Fos, nNOS and CaMKIIα immunoreactivity (Figs. 4, 5, 6) can be observed in the superficial layers of the C1–C2 after treatment with nitroglycerine compared to placebo. The differences in the numbers of immunoreactive neurones proved to be statistically significant for all three immunostainings (**p < 0.01, ***p < 0.001; Fig. 7b–d).
Calcitonin gene-related peptide (CGRP) immunoreactivity. Representative photos of the cervical part of trigemino-cervical complex (C1–C2) in the rat after CGRP immunohistochemistry. In the control group (a, b), the immunoreactive staining of the CGRP fibres is decreased after the nitroglycerine injection (b) as compared with the placebo-treated animals (a). After 1 mmol/kg bodyweight KYNAa pre-treatment (c, d), the change otherwise induced by nitroglycerine cannot be observed (d) relative to the animals treated with placebo (c). Scale bar 100 μm
c-Fos immunoreactivity. Representative photos of c-Fos-immunoreactivity in the cervical part of trigemino-cervical complex (C1–C2) in the rat. In the control group (a, b), more c-Fos-immunoreactive cells can be observed in the superficial laminae of the C1–C2 after nitroglycerine injection (b) as compared with the placebo-treated animals (a). This nitroglycerine-induced change is not observed after 1 mmol/kg bodyweight KYNAa pre-treatment (c, d). The black arrow in “b” indicates a c-Fos-positive cell. Scale bar 100 μm
Neuronal nitric oxide synthase (nNOS) immunoreactivity. Histological photos of nNOS-stained neurones in the cervical part of trigemino-cervical complex (C1–C2) in the rat. In the control group (a, b), an increased nNOS immunoreactivity can be observed in the superficial laminae of the C1–C2 after nitroglycerine injection (b) as compared with the placebo-treated animals (a). In the group pre-treated with 1 mmol/kg bodyweight KYNAa (c, d), the nitroglycerine-induced change is not observed. The black arrow in “b” points to an nNOS-immunoreactive neurone. Scale bar 100 μm
Calmodulin-dependent protein kinase II alpha (CaMKIIα) immunoreactivity. Typical photos showing CaMKIIα-immunoreactive cells in the cervical part of trigemino-cervical complex (C1–C2) in the rat. Increased CaMKIIα staining can be observed in the superficial laminae of the C1–C2 in the control group (a, b) after nitroglycerine injection (b) as compared with the placebo-treated animals (a). In the 1 mmol/kg bodyweight KYNAa pre-treated group (c, d), this enhancement induced by nitroglycerine is not visible after nitroglycerine injection (d) relative to the placebo treatment (c). The black arrow in “b” points to a CaMKIIα-immunopositive neurone. Scale bar 100 μm
Diagrams illustrating the measurement data in the control, 0.1, 0.5 and 1 mmol/kg bodyweight (bw) KYNAa groups after various immunohistochemical stainings. Within the groups, the differently coloured columns relate to the rats treated with placebo (white bars) or nitroglycerine (black bars) (means + SE of mean, n = 5 per column). a The mean area covered by calcitonin gene-related peptide (CGRP)-immunoreactive fibres. In the control group, nitroglycerine injection significantly decreased the mean area covered by CGRP-immunoreactive fibres in the cervical part of trigemino-cervical complex (C1–C2) as compared with the placebo-treated animals (**p < 0.01). 0.1 mmol/kg bw dose of KYNAa did not significantly reduce the nitroglycerine-induced changes, while 0.5 and 1 mmol/kg bw doses of KYNAa were able to significantly decrease the effect of nitroglycerine (# p < 0.05; ### p < 0.001). b The mean number of c-Fos-immunoreactive neurones. Nitroglycerine injection significantly increased the mean number of c-Fos-immunoreactive cells in the C1–C2 in the control group as compared with the placebo-treated rats (***p < 0.001). We could not detect significant difference between nitroglycerine-treated rats of control group and of group receiving 0.1 mmol/kg bw dose of KYNAa, while there was significant difference between nitroglycerine-treated animals of control group and of groups pre-treated with 0.5 and 1 mmol/kg bw dose of KYNAa (## p < 0.01; ### p < 0.001). c The average number of neuronal nitric oxide synthase (nNOS)-immunoreactive cells. Nitroglycerine injection significantly enhanced the mean number of nNOS-immunoreactive neurones in the C1–C2 in the controls as compared with the rodents treated with placebo (***p < 0.001). There was no difference in results of animals receiving nitroglycerine between the control and 0.1 mmol/kg bw KYNAa group. Both 0.5 and 1 mmol/kg bw dose of KYNAa reduced the effect of nitroglycerine as compared with the rats of the control group (## p < 0.01; ### p < 0.001). d The mean number of calmodulin-dependent protein kinase II alpha (CaMKIIα)-immunoreactive neurones. The mean number of CaMKIIα-immunoreactive cells was significantly increased after nitroglycerine injection in the C1–C2 in the control group as compared with the placebo treatment (***p < 0.001). 0.1 mmol/kg bw dose of KYNAa was not, while 0.5 and 1 mmol/kg bw dose of KYNAa were able to decrease the nitroglycerine-induced changes in CaMKIIα-expression as compared with the animals of the control group (## p < 0.01; ### p < 0.001)
The pre-treatment with KYNAa affected dose-dependently the nitroglycerine-induced changes in the immunoreactivity of all studied markers. The administration of KYNAa in a dosage of 0.1 mmol/kg bw did not influence any of the alterations caused by nitroglycerine, whereas the pre-treatment with higher dosages of KYNAa (0.5 and 1 mmol/kg bw) was able to significantly reduce the effects of nitroglycerine on the CGRP-, c-Fos-, nNOS- and CaMKIIα-related changes in the C1–C2 (# p < 0.05, ## p < 0.01, ### p < 0.001; Figs. 3, 4, 5, 6, 7a–d).
Western blot
Western blot analysis of the C1–C2 region confirmed the results obtained by CGRP and nNOS immunohistochemistry. A band characteristic of the CGRP peptide was identified at 15 kDa and nNOS protein was identified at 155 kDa (Fig. 8a, c). Densitometric analyses confirmed that the CGRP bands were significantly decreased (*p < 0.05) and nNOS bands were significantly enhanced (***p < 0.001) in segments C1–C2 after nitroglycerine administration as compared with the placebo-treated animals (Fig. 8b, d). This effect of nitroglycerine on CGRP and nNOS was attenuated by pre-treatment with 1 mmol/kg bw KYNAa (Fig. 8a–d).
Western blot of calcitonin gene-related peptide (CGRP) and neuronal nitric oxide synthase (nNOS) in the C1–C2 segments of the spinal cord. Density of CGRP band decreased (a–b) and density of nNOS band increased (c–d) significantly after nitroglycerin administration compared to the placebo in the control group (*p < 0.05; ***p < 0.001), which was attenuated by pre-treatment with 1 mmol/kg bodyweight (bw) KYNAa
Open field test
Treatment with nitroglycerine significantly decreased the ambulation distance of the animals compared to placebo-treated rats (*p < 0.05; Fig. 9). Pre-treatment with 1 mmol/kg bw KYNAa attenuated this difference but a tendency for a lower ambulation distance was observed (Fig. 9). There were no significant changes in ambulation time, local time or in the number of rearing between the subgroups (data not shown).
Diagram showing the results of open field test. In the control group, treatment with nitroglycerine decreased the ambulation distance significantly compared to placebo-treated rats (*p < 0.05). Pre-treatment with 1 mmol/kg bodyweight (bw) KYNAa attenuated this effect of nitroglycerine, however, a tendency for a lower ambulation distance can be observed as compared with placebo-treated rats of control group as well
Discussion
In the present work, we confirmed earlier experimental findings showing that systemic administration of nitroglycerine exerts a stimulatory effect on the trigeminal neurones, inducing the reduction of CGRP and the up-regulation of c-Fos, nNOS and CaMKIIα in the C1–C2. The nitroglycerine-induced decrease in the area covered by CGRP-immunoreactive fibres and in the density of Western blot bands in cervical segments 1–2 of spinal cord indicates the release of CGRP from the central terminals of the primary trigeminal afferents, which is a general event after the activation of first-order trigeminal neurones (Zhang et al. 1994), and is in accordance with our own previous observations (Pardutz et al. 2002). Nitroglycerine-related activation also involves the second-order trigeminal neurones, as reflected by the elevated c-Fos expression observed now and previously by other researchers (Tassorelli and Joseph 1995). Furthermore, it can lead to a sensitization process in the C1–C2, marked by the enhancement of nNOS and CaMKIIα, both of these enzymes playing an important role in the development of pain-related central sensitization (Chacur et al. 2010; Fang et al. 2002).
The nitroglycerine-related activation of the C1–C2 neurones probably involves indirect, central action. The nitric oxide generated from nitroglycerine activates the Aδ and C fibres of the primary trigeminal neurones (Pardutz et al. 2000) which causes activation of the second-order trigeminal neurones leading to an increase in the c-Fos, nNOS and CaMKIIα expressions there. Supporting this theory, the increased c-Fos expression after nitroglycerine in the second-order trigeminal neurones can be blocked by the destruction of capsaicin-sensitive fibres (Tassorelli et al. 1997). The relevance of the nitroglycerine model is also underlined by observations that these effects appear to be selective for the trigeminal system, as no changes were detected in most of these markers of activation and sensitization of the trigeminal system, i.e. in the number of nNOS- and CaMKIIα-immunoreactive neurones as well as in the area innervated by CGRP and serotonin immunoreactive fibres at the level of upper thoracic segments (Pardutz et al. 2000, 2002, 2007). In humans, administration of nitroglycerine induces a delayed migraine-like attack in migraineurs (Sicuteri et al. 1987) with typical migraine symptoms (Christiansen et al. 1999), which cannot be observed in healthy volunteers and is able to sensitize the trigeminal system (Di Clemente et al. 2009). Based on results from animal experiments, glutamate and α7-nicotinic acetylcholine receptors can play important role in this process. The activation of the first-order trigeminal neurones leads to an increased level of glutamate in the caudal part of spinal trigeminal nucleus, which correlates with the changes in sensory thresholds on the face of the rat (Oshinsky and Luo 2006). Probably via the NMDA receptors (Wang and Mokha 1996), the activation of the first-order trigeminal neurones leads to activation of the second-order neurones (Burstein et al. 1998). Experimental results suggest that NMDA receptors are involved in the nitric oxide synthesis (Entrena et al. 2005) and that there is an interaction between the NMDA and the nitric oxide/cGMP system via the activation of nNOS (Bredt et al. 1990). Additionally, the NMDA-induced excitotoxicity depends on the excess nitric oxide produced by the activation of nNOS (Garthwaite et al. 1988). The role of NMDA receptors in trigeminal pain processing is also supported by human data, whereas ketamine, an NMDA receptor antagonist was able to decrease migraine pain (Nicolodi and Sicuteri 1995). Furthermore, other glutamate receptors are also present in the caudal part of spinal trigeminal nucleus (Tallaksen-Greene et al. 1992), and can also contribute to this process. This concept is confirmed by that their antagonists are able to block the enhancement in the number of c-Fos-immunoreactive neurones (Mitsikostas et al. 1999) and the evoked potential responses (Storer and Goadsby 1999) in the caudal part of spinal trigeminal nucleus. The activation of second-order neurones can be modified by α7-nicotinic acetylcholine receptors as well, which presynaptically can influence the transmission of nociceptive information to the central nervous system (McGehee et al. 1995; Gray et al. 1996).
The results of the open field testing also showed a marked effect of nitroglycerine. The ambulation distance of the treated rats decreased significantly which may also reflect a pain condition (Denenberg 1969).
In the present experiments, the KYNAa proved to be able to attenuate dose-dependently the effects of nitroglycerine. 0.1 mmol/kg bw dose of KYNAa has not, while 0.5 and 1 mmol/kg bw doses of KYNAa have significantly reduced the decrease in CGRP immunoreactivity and the increases in c-Fos, nNOS and CaMKIIα expression, suggesting a blockade of trigeminal activation and sensitization. This effect was also supported by the Western blot results for CGRP and nNOS showing that employing the maximal dosage pre-treatment with KYNAa attenuated the nitroglycerine-induced changes. HPLC measurements suggesting a robust increase in kynurenic acid concentration in the blood and a more than twofold increase in central nervous system 1 h after KYNAa treatment supports the theory that KYNAa is transformed, at least partially, to kynurenic acid which might be also responsible for the observed effects. Since the increase of kynurenic acid in the central nervous system was observed in a much lesser extent, although the central effect cannot be fully excluded, the witnessed modulatory effects might be related to the peripheral action of kynurenic acid derived from KYNAa. Meanwhile the direct effect of the KYNAa on the peripheral and central arm of the trigeminal system is also possible with a theoretical effect similar to kynurenic acid. This is underlined by the similar pharmacological action of an older kynurenic acid derivative with a presumed better blood–brain barrier penetrance (Marosi et al. 2010).
Attenuation of CGRP release from activated primary nociceptors by KYNAa also suggests a marked peripheral effect, an inhibition of the receptors involved in trigeminal activation. Similarly, peripheral, topical intra-articular administration of kynurenic acid reduced mechanical allodynia dose-dependently during pain conditions (Mecs et al. 2009). Glutamate receptors are present on the peripheral arm of the primary trigeminal nociceptors (Quartu et al. 2002; Watanabe et al. 1994) and their inhibition reduces the release of CGRP (Garry et al. 2000). The α7-nicotinic acetylcholine receptors are also present in the trigeminal ganglion (Liu et al. 1998). Their blockade may contribute to the reduction of glutamate release (Carpenedo et al. 2001), and it mitigates the facial vasodilatation induced by CGRP released from the primary nociceptors (Just et al. 2005). G-Protein-coupled receptor-35 is expressed within nociceptive pathways, including the dorsal root ganglion and spinal cord, at the mRNA and protein levels (Ohshiro et al. 2008), and is negatively coupled to adenylate cyclase—cyclic adenosine monophosphate signalling in the dorsal root ganglion neurones, which can modulate nociceptive signalling (Ohshiro et al. 2008).
The modulatory effect of KYNAa on the activation of second-order trigeminal neurones, reflected by c-Fos changes, may be a consequence of inhibition of the peripheral nociceptors, but it may also arise from the action on receptors located in the central part of trigeminal system. Glutamate receptors are present postsynaptically on the second-order trigeminal neurones (Tallaksen-Greene et al. 1992) playing an important role in the pathomechanism of trigeminal activation related to noxious stimuli. Likewise, the inhibition of glutamate receptors reduces the increase in c-Fos expression in pain conditions (Mitsikostas et al. 1998, 1999). α7-Nicotinic acetylcholine receptors are located on primary trigeminal nociceptors (Liu et al. 1998) and their inhibition at the presynaptic level with kynurenic acid may contribute to the reduction of glutamate release (Carpenedo et al. 2001) and reduce the activation of second-order trigeminal neurones (Carstens et al. 2000). The possibility of central action of kynurenic acid and its derivatives is supported by results demonstrating that intrathecally injected kynurenic acid and 7-chlorokynurenic acid display dose-dependent and reversible analgesic effects in the hot-plate, tail-flick and formalin tests in mice (Nasstrom et al. 1992) and in the hot-plate and tail-flick tests in rats (Kristensen et al. 1993). Intrathecally administered 5,7-dichlorokynurenic acid dose-dependently reversed the hyperalgesia in hyperalgesic Mg-deficient rats (Begon et al. 2001). Moreover, in the nitroglycerine model of trigeminal activation, the i.p. administration of another kynurenic acid derivative and combined pre-treatment with l-kynurenine and probenecid prevented the activation of second-order trigeminal neurones (Knyihar-Csillik et al. 2007, 2008), whereas kynurenic acid alone was less effective in this model, probably because of its inferior level of blood–brain barrier penetrance (Knyihar-Csillik et al. 2008).
The inhibition of nNOS and CaMKIIα by KYNAa in the present study and by another kynurenic acid derivative in earlier works (Vamos et al. 2009, 2010) points to the attenuation of the nitroglycerine-induced central sensitization phenomena, both nNOS and CaMKIIα appearing to be essential in this process (Chacur et al. 2010; Fang et al. 2002). The central sensitization is a consequence of changes ongoing in the central nervous system, during which the neurones undergo anatomical and functional plastic changes after strong noxious stimulation, primarily the activation of AMPA and NMDA receptors being involved (Latremoliere and Woolf 2009). Both AMPA and NMDA receptor antagonists effectively reduce the established long-term potentiation of the C-fibre-mediated response in wide dynamic range neurones in the lumbar dorsal horn, which is thought to be related to central sensitization (Svendsen et al. 1998). In a behavioural study, another competitive NMDA antagonist, LY235959, administered either intrathecally or subcutaneously, significantly reduced the number of formalin-induced flinches in phase 2, i.e. the central sensitization (Davis and Inturrisi 2001). Taken together these results suggest that besides acting on the periphery, KYNAa might attenuate the increases in expression of nNOS and CaMKIIα, i.e. the nitroglycerine-induced central sensitization, probably through the inhibition of AMPA and/or NMDA receptors. However, it is also possible that blockade of the α7-nicotinic acetylcholine receptors could contribute to the inhibition of central sensitization indirectly, since glutamate release can be reduced by the inhibition of these receptors by kynurenic acid at the presynaptic level (Carpenedo et al. 2001).
The results of open field behavioural test show that the nitroglycerine- and placebo-treated group in KYNAa pre-treated animals did not differ from each other significantly, which may suggest antinociceptive effect of KYNAa. However, the KYNAa pre-treated groups showed a tendency of lower ambulation distance, which may refer to a direct central action of KYNAa. This concept is also supported by the fact that only glycine-site NMDA antagonists passing the blood–brain barrier had modulatory effect on ambulation distance in open field test (Christoph et al. 2005).
In conclusion, the new kynurenic acid derivative used in the present study, KYNAa, mitigated the trigeminal activation at the level of the peripheral branches and second-order neurones. Moreover, at the level of the C1–C2, it abolished the alterations related to central sensitization. Besides an effect related to the transformation of KYNAa to kynurenic acid in the periphery, direct and indirect central actions might be responsible for this phenomenon. Since all of these events play a key part in certain headache conditions, KYNAa may have a possible future role in the treatment of these disorders with a different mechanism of action.
References
Begon S, Pickering G, Eschalier A, Mazur A, Rayssiguier Y, Dubray C (2001) Role of spinal NMDA receptors, protein kinase C and nitric oxide synthase in the hyperalgesia induced by magnesium deficiency in rats. Br J Pharmacol 134(6):1227–1236. doi:10.1038/sj.bjp.0704354
Birch PJ, Grossman CJ, Hayes AG (1988) Kynurenate and FG9041 have both competitive and non-competitive antagonist actions at excitatory amino acid receptors. Eur J Pharmacol 151(2):313–315 (pii:0014-2999(88)90814-X)
Bredt DS, Hwang PM, Snyder SH (1990) Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347(6295):768–770. doi:10.1038/347768a0
Burstein R, Yamamura H, Malick A, Strassman AM (1998) Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 79(2):964–982
Carpenedo R, Pittaluga A, Cozzi A, Attucci S, Galli A, Raiteri M, Moroni F (2001) Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci 13(11):2141–2147 (pii: ejn1592)
Carstens E, Simons CT, Dessirier JM, Carstens MI, Jinks SL (2000) Role of neuronal nicotinic-acetylcholine receptors in the activation of neurons in trigeminal subnucleus caudalis by nicotine delivered to the oral mucosa. Exp Brain Res 132(3):375–383. doi:10.1007/s002210000351
Chacur M, Matos RJ, Alves AS, Rodrigues AC, Gutierrez V, Cury Y, Britto LR (2010) Participation of neuronal nitric oxide synthase in experimental neuropathic pain induced by sciatic nerve transection. Braz J Med Biol Res 43(4):367–376. doi:10.1590/S0100-879X2010007500019
Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J (1999) Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia 19 (7):660–667. doi:10.1046/j.1468-2982.1999.019007660.x (discussion 626)
Christoph T, Reissmuller E, Schiene K, Englberger W, Chizh BA (2005) Antiallodynic effects of NMDA glycine(B) antagonists in neuropathic pain: possible peripheral mechanisms. Brain Res 1048(1–2):218–227. doi:10.1016/j.brainres.2005.04.081
D’Andrea G, Leon A (2010) Pathogenesis of migraine: from neurotransmitters to neuromodulators and beyond. Neurol Sci 31(Suppl 1):S1–S7. doi:10.1007/s10072-010-0267-8
Davis AM, Inturrisi CE (2001) Attenuation of hyperalgesia by LY235959, a competitive N-methyl-D-aspartate receptor antagonist. Brain Res 894(1):150–153 (pii:S0006-8993(00)03325-4)
Demeter I, Nagy K, Gellert L, Vecsei L, Fulop F, Toldi J (2012) A novel kynurenic acid analog (SZR104) inhibits pentylenetetrazole-induced epileptiform seizures. An electrophysiological study: special issue related to kynurenine. J Neural Transm 119 (2):151–154. doi:10.1007/s00702-011-0755-x
Denenberg VH (1969) Open-field bheavior in the rat: what does it mean? Ann N Y Acad Sci 159(3):852–859. doi:10.1111/j.1749-6632.1969.tb12983.x
Di Clemente L, Coppola G, Magis D, Gerardy PY, Fumal A, De Pasqua V, Di Piero V, Schoenen J (2009) Nitroglycerin sensitises in healthy subjects CNS structures involved in migraine pathophysiology: evidence from a study of nociceptive blink reflexes and visual evoked potentials. Pain 144(1–2):156–161. doi:10.1016/j.pain.2009.04.018
Entrena A, Camacho ME, Carrion MD, Lopez-Cara LC, Velasco G, Leon J, Escames G, Acuna-Castroviejo D, Tapias V, Gallo MA, Vivo A, Espinosa A (2005) Kynurenamines as neural nitric oxide synthase inhibitors. J Med Chem 48(26):8174–8181. doi:10.1021/jm050740o
Fang L, Wu J, Lin Q, Willis WD (2002) Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci 22(10):4196–4204 (pii: 2002634322/10/4196)
Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR (1991) Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 56(6):2007–2017. doi:10.1111/j.1471-4159.1991.tb03460.x
Fulop F, Szatmari I, Toldi J, Vecsei L (2012) Modifications on the carboxylic function of kynurenic acid. J Neural Transm 119(2):109–114. doi:10.1007/s00702-011-0721-7
Fuvesi J, Rajda C, Bencsik K, Toldi J, Vecsei L (2012) The role of kynurenines in the pathomechanism of amyotrophic lateral sclerosis and multiple sclerosis: therapeutic implications. J Neural Transm 119(2):225–234. doi:10.1007/s00702-012-0765-3
Garry MG, Walton LP, Davis MA (2000) Capsaicin-evoked release of immunoreactive calcitonin gene-related peptide from the spinal cord is mediated by nitric oxide but not by cyclic GMP. Brain Res 861(2):208–219 (pii: S0006-8993(99)02448-8)
Garthwaite J, Charles SL, Chess-Williams R (1988) Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336(6197):385–388. doi:10.1038/336385a0
Gellert L, Varga D, Ruszka M, Toldi J, Farkas T, Szatmari I, Fulop F, Vecsei L, Kis Z (2012) Behavioural studies with a newly developed neuroprotective KYNA-amide. J Neural Transm 119(2):165–172. doi:10.1007/s00702-011-0692-8
Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA (1996) Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature 383(6602):713–716. doi:10.1038/383713a0
Herve C, Beyne P, Jamault H, Delacoux E (1996) Determination of tryptophan and its kynurenine pathway metabolites in human serum by high-performance liquid chromatography with simultaneous ultraviolet and fluorimetric detection. J Chromatogr B Biomed Appl 675(1):157–161 (pii: 037843479500341X)
Just S, Arndt K, Doods H (2005) The role of CGRP and nicotinic receptors in centrally evoked facial blood flow changes. Neurosci Lett 381(1–2):120–124. doi:10.1016/j.neulet.2005.02.012
Kessler M, Terramani T, Lynch G, Baudry M (1989) A glycine site associated with N-methyl-d-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem 52(4):1319–1328. doi:10.1111/j.1471-4159.1989.tb01881.x
Knyihar-Csillik E, Toldi J, Mihaly A, Krisztin-Peva B, Chadaide Z, Nemeth H, Fenyo R, Vecsei L (2007) Kynurenine in combination with probenecid mitigates the stimulation-induced increase of c-fos immunoreactivity of the rat caudal trigeminal nucleus in an experimental migraine model. J Neural Transm 114(4):417–421. doi:10.1007/s00702-006-0545-z
Knyihar-Csillik E, Mihaly A, Krisztin-Peva B, Robotka H, Szatmari I, Fulop F, Toldi J, Csillik B, Vecsei L (2008) The kynurenate analog SZR-72 prevents the nitroglycerol-induced increase of c-fos immunoreactivity in the rat caudal trigeminal nucleus: comparative studies of the effects of SZR-72 and kynurenic acid. Neurosci Res 61(4):429–432. doi:10.1016/j.neures.2008.04.009
Kristensen JD, Post C, Gordh TJ, Svensson BA (1993) Spinal cord morphology and antinociception after chronic intrathecal administration of excitatory amino acid antagonists in the rat. Pain 54(3):309–316 (pii: 0304-3959(93)90030-S)
Latremoliere A, Woolf CJ (2009) Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10(9):895–926. doi:10.1016/j.jpain.2009.06.012
Liu L, Chang GQ, Jiao YQ, Simon SA (1998) Neuronal nicotinic acetylcholine receptors in rat trigeminal ganglia. Brain Res 809(2):238–245 (pii: S0006-8993(98)00862-2)
Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, Szabadfi K, Tuka B, Tajti J, Szolcsanyi J, Pinter E, Hashimoto H, Kun J, Reglodi D, Helyes Z (2012) Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis 45(1):633–644. doi:10.1016/j.nbd.2011.10.010
Marosi M, Nagy D, Farkas T, Kis Z, Rozsa E, Robotka H, Fulop F, Vecsei L, Toldi J (2010) A novel kynurenic acid analogue: a comparison with kynurenic acid. An in vitro electrophysiological study. J Neural Transm 117(2):183–188. doi:10.1007/s00702-009-0346-2
McGehee DS, Heath MJ, Gelber S, Devay P, Role LW (1995) Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 269(5231):1692–1696. doi:10.1126/science.7569895
Mecs L, Tuboly G, Nagy E, Benedek G, Horvath G (2009) The peripheral antinociceptive effects of endomorphin-1 and kynurenic acid in the rat inflamed joint model. Anesth Analg 109(4):1297–1304. doi:10.1213/ane.0b013e3181b21c5e
Mitsikostas DD, Sanchez del Rio M, Waeber C, Moskowitz MA, Cutrer FM (1998) The NMDA receptor antagonist MK-801 reduces capsaicin-induced c-fos expression within rat trigeminal nucleus caudalis. Pain 76(1–2):239–248 (pii: S0304-3959(98)00051-7)
Mitsikostas DD, Sanchez del Rio M, Waeber C, Huang Z, Cutrer FM, Moskowitz MA (1999) Non-NMDA glutamate receptors modulate capsaicin induced c-fos expression within trigeminal nucleus caudalis. Br J Pharmacol 127(3):623–630. doi:10.1038/sj.bjp.0702584
Moskowitz MA (2008) Defining a pathway to discovery from bench to bedside: the trigeminovascular system and sensitization. Headache 48(5):688–690. doi:10.1111/j.1526-4610.2008.01110.x
Nasstrom J, Karlsson U, Post C (1992) Antinociceptive actions of different classes of excitatory amino acid receptor antagonists in mice. Eur J Pharmacol 212(1):21–29 (pii: 0014-2999(92)90067-E)
Nemeth H, Toldi J, Vecsei L (2006) Kynurenines, Parkinson’s disease and other neurodegenerative disorders: preclinical and clinical studies. J Neural Transm Suppl 70:285–304. doi:10.1007/978-3-211-45295-0_45
Nicolodi M, Sicuteri F (1995) Exploration of NMDA receptors in migraine: therapeutic and theoretic implications. Int J Clin Pharmacol Res 15(5–6):181–189
Ohshiro H, Tonai-Kachi H, Ichikawa K (2008) GPR35 is a functional receptor in rat dorsal root ganglion neurons. Biochem Biophys Res Commun 365(2):344–348. doi:10.1016/j.bbrc.2007.10.197
Oshinsky ML, Luo J (2006) Neurochemistry of trigeminal activation in an animal model of migraine. Headache 46(Suppl 1):S39–S44. doi:10.1111/j.1526-4610.2006.00489.x
Pardutz A, Krizbai I, Multon S, Vecsei L, Schoenen J (2000) Systemic nitroglycerin increases nNOS levels in rat trigeminal nucleus caudalis. NeuroReport 11(14):3071–3075. doi:10.1097/00001756-200009280-00008
Pardutz A, Multon S, Malgrange B, Parducz A, Vecsei L, Schoenen J (2002) Effect of systemic nitroglycerin on CGRP and 5-HT afferents to rat caudal spinal trigeminal nucleus and its modulation by estrogen. Eur J Neurosci 15(11):1803–1809 (pii: 2031)
Pardutz A, Hoyk Z, Varga H, Vecsei L, Schoenen J (2007) Oestrogen-modulated increase of calmodulin-dependent protein kinase II (CamKII) in rat spinal trigeminal nucleus after systemic nitroglycerin. Cephalalgia 27(1):46–53. doi:10.1111/j.1468-2982.2006.01244.x
Pardutz A, Fejes A, Bohar Z, Tar L, Toldi J, Vecsei L (2012) Kynurenines and headache. J Neural Transm 119(2):285–296. doi:10.1007/s00702-011-0665-y
Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX (2002) Unconventional ligands and modulators of nicotinic receptors. J Neurobiol 53(4):479–500. doi:10.1002/neu.10146
Quartu M, Serra MP, Ambu R, Lai ML, Del Fiacco M (2002) AMPA-type glutamate receptor subunits 2/3 in the human trigeminal sensory ganglion and subnucleus caudalis from prenatal ages to adulthood. Mech Ageing Dev 123(5):463–471 (pii: S004763740100358X)
Sicuteri F, Del Bene E, Poggioni M, Bonazzi A (1987) Unmasking latent dysnociception in healthy subjects. Headache 27(4):180–185. doi:10.1111/j.1526-4610.1987.hed2704180.x
Storer RJ, Goadsby PJ (1999) Trigeminovascular nociceptive transmission involves N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors. Neuroscience 90(4):1371–1376 (pii: S0306-4522(98)00536-3)
Strassman AM, Mineta Y, Vos BP (1994) Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci 14(6):3725–3735 (pii: 0270-6474/94/143725-l1$05.00/O)
Svendsen F, Tjolsen A, Hole K (1998) AMPA and NMDA receptor-dependent spinal LTP after nociceptive tetanic stimulation. NeuroReport 9(6):1185–1190. doi:10.1097/00001756-199804200-00041
Tallaksen-Greene SJ, Young AB, Penney JB, Beitz AJ (1992) Excitatory amino acid binding sites in the trigeminal principal sensory and spinal trigeminal nuclei of the rat. Neurosci Lett 141(1):79–83 (pii: 0304-3940(92)90339-9)
Tassorelli C, Joseph SA (1995) Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res 682(1–2):167–181 (pii: 0006-8993(95)00348-T)
Tassorelli C, Joseph SA, Nappi G (1997) Neurochemical mechanisms of nitroglycerin-induced neuronal activation in rat brain: a pharmacological investigation. Neuropharmacology 36(10):1417–1424 (pii: S0028390897001226)
Vamos E, Pardutz A, Varga H, Bohar Z, Tajti J, Fulop F, Toldi J, Vecsei L (2009) L-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology 57(4):425–429. doi:10.1016/j.neuropharm.2009.06.033
Vamos E, Fejes A, Koch J, Tajti J, Fulop F, Toldi J, Pardutz A, Vecsei L (2010) Kynurenate derivative attenuates the nitroglycerin-induced CamKIIalpha and CGRP expression changes. Headache 50(5):834–843. doi:10.1111/j.1526-4610.2009.01574.xHED1574
Vecsei L, Szalardy L, Fulop F, Toldi J (2013) Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov 12(1):64–82. doi:10.1038/nrd3793nrd3793
Wang XM, Mokha SS (1996) Opioids modulate N-methyl-d-aspartic acid (NMDA)-evoked responses of trigeminothalamic neurons. J Neurophysiol 76(3):2093–2096
Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L (2006) Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem 281(31):22021–22028. doi:10.1074/jbc.M603503200
Watanabe M, Mishina M, Inoue Y (1994) Distinct gene expression of the N-methyl-D-aspartate receptor channel subunit in peripheral neurons of the mouse sensory ganglia and adrenal gland. Neurosci Lett 165(1–2):183–186. doi:10.1016/0304-3940(94)90740-4
Zadori D, Nyiri G, Szonyi A, Szatmari I, Fulop F, Toldi J, Freund TF, Vecsei L, Klivenyi P (2011) Neuroprotective effects of a novel kynurenic acid analogue in a transgenic mouse model of Huntington’s disease. J Neural Transm 118(6):865–875. doi:10.1007/s00702-010-0573-6
Zhang RX, Mi ZP, Qiao JT (1994) Changes of spinal substance P, calcitonin gene-related peptide, somatostatin, Met-enkephalin and neurotensin in rats in response to formalin-induced pain. Regul Pept 51(1):25–32. doi:10.1016/0167-0115(94)90131-7
Acknowledgments
This work was supported by OTKA KI05077, TÁMOP-4.2.2.A-11/1/KONV-2012-0052 and the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4.A/2-11-1-2012-0001 “National Excellence Program”. Dr. Árpád Párdutz was supported by the Bolyai Scholarship Programme of the Hungarian Academy of Sciences. We acknowledge Mrs. Valéria Vékony for the histotechnical assistance, Péter Klivényi and István Ilisz for their valuable technical advices and David Durham for the linguistic correction of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Á. Párdutz, L. Vécsei contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fejes-Szabó, A., Bohár, Z., Vámos, E. et al. Pre-treatment with new kynurenic acid amide dose-dependently prevents the nitroglycerine-induced neuronal activation and sensitization in cervical part of trigemino-cervical complex. J Neural Transm 121, 725–738 (2014). https://doi.org/10.1007/s00702-013-1146-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-013-1146-2