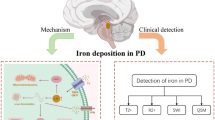
Abstract
Although the exact cause of Parkinson’s disease (PD) is still unknown, recent interest has been focused on the role of iron in the nigral cell death in PD. Several studies have shown that a selective and significant elevation in iron occurs in the substantia nigra of patients with PD. However, the mechanisms involved in iron accumulation also remain unclear. In this article, we describe recent findings regarding the mechanisms and potential toxic effects of iron accumulation in hereditary and sporadic PD and animal models of PD, including our genetic mouse model of PD. The review provides an opportunity to revisit the possible roles of iron accumulation in the pathogenic cascade(s) of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several previous studies have reported the relationship between iron accumulation and dopaminergic cell death in the substantia nigra (SN) of the postmortem brains of patients with Parkinson’s disease (PD; Earle 1968; Sofic et al. 1988; Dexter et al. 1987, 1989). However, whether the high iron content represents the initiation process or merely the result of nigral degeneration remains to be elucidated. Here, we review recent status regarding the mechanisms and potential toxic effects of iron accumulation in hereditary and sporadic PD and animal models of PD, including our data.
Iron toxicity
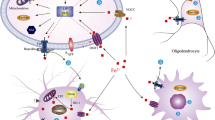
Since iron is a redox-active metal and can facilitate the formation of cytotoxic hydroxyl radicals, superoxide anions, and hydrogen peroxide, accumulation of iron in the SN of patients with PD could be involved in neuronal degeneration. However, in vitro studies demonstrated that the toxic effects of iron are not limited to dopaminergic neurons; for example, treatment with large amount (>50 μM) of iron results in the death of tyrosine hydroxylase (TH)-negative non-dopaminergic cells. Hence, high concentration of free iron seems to be injurious to all types of neurons. On the other hand, significantly moderate amount (~25 μM) of iron induces preferential damage to dopaminergic neurons through interaction with dopamine inside the cells (Mochizuki et al. 1993). Dopamine can be a major source of reactive oxygen species (ROS) within the nigral cells, since oxidation of dopamine by monoamine oxidase releases hydrogen peroxide, which could in turn produce more toxic hydroxyl radicals through Fenton’s reaction mediated through the action of iron (Halliwell 1989). Neuromelanin (NM) is the major iron storage in the substantia nigra dopaminergic neurons (Zecca et al. 1996, 2001, 2004a, b). Since neuromelanin chelates large amounts of iron, it prevents the hydroxyl radical production by Fenton’s reaction (Zecca et al. 2008a, b). These data demonstrated that when neuromelanin is inside neurons, it is neuroprotective. On the contrary, increased tissue iron found in the parkinsonian SN may saturate iron-chelating sites on NM, and a looser association between iron and NM may result in an increased, rather than decreased, production of free radical species. It is hypothesized that this redox-active iron could be released and involved in a Fenton-like reaction leading to an increased production of oxidative radicals (Gerlach et al. 2003). Once neuromelanin is released from dying neurons in the extracellular environment, it is able to activate microglia, increasing neuroinflammation and leading to the neuronal death (Zecca et al. 2008a, b; Zhang et al. 2011). Furthermore, overload of iron in neuromelanin typically occurs in PD where an increase of reactive/toxic iron bound to neuromelanin has been reported (Jellinger et al. 1992; Faucheux et al. 2003).
α-Synuclein plays a central role in the pathogenic cascades in hereditary and sporadic cases of PD. Indeed, α-synuclein is the major component of Lewy bodies (Spillantini et al. 1997), the pathological hallmark of PD, and point mutations in the α-synuclein gene (PARK1). Duplication or triplication of α-synuclein locus (PARK4) is potentially pathogenetic in rare cases of familial PD (Dawson and Dawson 2003; Singleton et al. 2003). On the other hand, low concentrations of certain metals, such as iron, can directly induce α-synuclein fibril formation (Uversky et al. 2001). Ostrerova-Golts et al. (2000) also reported that iron and free radical generators, such as dopamine and hydrogen peroxide, can stimulate the production of intracellular aggregates that contain α-synuclein and ubiquitin. In addition to stimulating aggregate formation, α-synuclein also appears to be neurotoxic. Iron may act in concert with α-synuclein and dopamine to induce the formation of Lewy body pathology and cell death in PD. In this regard, α-synuclein phosphorylation, which is also caused by iron, is due to CK2 upregulation (Takahashi et al. 2007).
Several studies have demonstrated the presence of brain inflammation in PD patients, with marked proliferation of reactive microglial cells (McGeer et al. 1988). Moreover, the loss of dopaminergic neurons is also associated with high levels of cytokines, ROS and nitric oxide (NO). These findings suggest that inflammatory reaction and infection can potentially be involved in the pathogenesis of PD (Furuya et al. 2004). Such inflammation reaction can also result in increased iron contents in dopaminergic neurons of PD. In addition to these neurons, the proinflammatory cytokines expressed in PD brains can also have profound and divergent effects on iron homeostasis in astrocytes and microglia (Rathore et al. 2012). In particular, proinflammatory TNF-α caused an increase in iron uptake and retention by both astrocytes and microglia, while anti-inflammatory cytokine TGF-β1 promoted iron efflux from astrocytes but caused iron retention in microglia (Rathore et al. 2012).
Iron accumulation in PD and PD animal models
Whether iron accumulation is a primary event in PD has been controversial. Several autopsy and radiological studies have reported iron storage in the SN of PD (Dexter et al. 1994; Berg 2006; Wypijewska et al. 2010). Other groups reported an increase in iron content in the early stages of PD and incidental Lewy body disease (Becker et al. 1995; Zecca et al. 2004a, b). Furthermore, locus coeruleus, a catecholaminergic brain region which degenerates in PD, has very low iron levels compared to SN (Zecca et al. 2004a, b). On the other hand, other works have shown the lack of such changes in nigral iron in pre-symptomatic PD or incidental Lewy body disease (Uitti et al. 1989; Galazka-Friedman et al. 1996). These results suggest that iron storage is not a primary event in PD. At this stage, it is difficult to determine whether excess iron is a primary cause of PD by clinical examination of PD patients. Moreover, it is possible that increased iron levels in certain brain regions could result from the altered vascularization that is observed in patients with PD (Faucheux et al. 1999).
Experimental animal models of PD
Several studies have examined iron contents in various experimental models of PD in order to determine whether iron accumulation in the SN is an early or late event. We also used a hemi-parkinsonism model in monkeys, which was prepared by unilateral injection of l-methyl-4-phenyl-l,2,3,6-tetrahydropyridine (MPTP) into the caudate or putamen, and compared iron content in the SN and other basal ganglia by immunohistochemistry (Mochizuki et al. 1994). The results showed that injection of MPTP into the caudate or putamen resulted in marked increase in ferric iron-reaction products in the ipsilateral SN pars compacta. The results indicated that injury to the nigrostriatal system following MPTP injection can induce iron accumulation in the SN. We also confirmed the expression of ferritin in the same model by immunohistochemistry using antibody against l-ferritin (Goto et al. 1996). Interestingly, there was no significant difference in the immunostaining for ferritin in the pars compacta of the SN between the injected and non-injected sides. The normal ferritin immunostaining on the MPTP-injected side suggests that iron accumulation is not related to altered metabolism of l-ferritin in this model. Temlett et al. (1994) measured the total free iron concentration using unilaterally MPTP-treated African green monkeys, which showed obvious contralateral hemiparkinsonism. They confirmed the excess iron accumulation in damaged dopaminergic neurons in MPTP-treated monkeys. He et al. (2003) also investigated changes in iron content in the SN at day 1 to month 18 after MPTP injection, and the relationship between iron accumulation and dopaminergic cell death progression in monkeys with parkinsonism induced by injection of MPTP. They demonstrated the presence of apoptosis in the ipsilateral SN at 1 day after MPTP injection, and a significant decrease in the number of TH-positive cells from 1 week onward. However, iron content was significantly increased in the ipsilateral SN from 4.5 to 18 months after MPTP injection, and the iron increase correlated significantly with the extent of dopaminergic cell death. Dopaminergic cell death induced by MPTP administration might lead to iron accumulation in the monkey SN, and increased iron might contribute to the progression of nigral degeneration.
Iron accumulation in familial Parkinson’s disease
Various genetic causes of parkinsonism have been identified. Iron accumulation in SN has been reported in several postmortem studies. Our group has also demonstrated the presence of more intense iron staining in parkin-deficit PD, PARK2, than in control subjects and sporadic cases of PD, as well as the presence of differences in the pattern of distribution of iron staining between PARK2 and sporadic PD (Takanashi et al. 2001). What is the mechanism of iron accumulation in the presence of parkin deficit? The major transport protein responsible for iron uptake is divalent metal transporter 1 (DMT1). Recent studies demonstrated that the 1B species is regulated post-translationally by degradation via the proteasomal pathway. Roth et al. (2010) demonstrated that parkin is the E3 ligase responsible for ubiquitination of the 1B species of DMT1. Parkin deficit may increase iron entry into neurons through an increase of DMT1. Jimenez Del Rio et al. (2004) also confirmed that the parkin mutation from PARK2 increases the susceptibility to dopamine and iron-mediated apoptosis in lymphocytes, probably due to its failure to dispose unfolded proteins provoked by oxidative stress.
PLA2G6 was reported to be the causative gene of early-onset PARK14-linked dystonia-parkinsonism. PLA2G6 encodes group VIA phospholipase A2 (calcium-independent phospholipase A2β; iPLA2β). The affected patients had parkinsonism, mental retardation/dementia, psychosis, dystonia, and hyperreflexia. Magnetic resonance images showed iron accumulation in the SN and striatum. PLA2G6 mutations have been detected in nearly all cases of classic infantile neuroaxonal dystrophy (INAD), but in only a small group of cases of idiopathic neurodegeneration with brain iron accumulation (Morgan et al. 2006). INAD is a severe psychomotor disorder with early onset and rapid progression of hypotonia, hyperreflexia, and tetraparesis. Spheroids are found in both the central and peripheral nervous systems in INAD, and iron accumulation in the brain is found in a subset of these patients. Beck et al. (2011) already established PLA2G6−/− mice as a model of INAD, and reported the presence of motor disturbances in these mice. They confirmed the presence of mitochondrial damage and spheroid formation in the motor neurons of the spinal cord in the INAD model. We also find iron accumulation in the SN of the same mice (manuscript in preparation). Genetic models of PD may enhance our understanding of the relationship between iron accumulation and dopaminergic cell death.
Conclusion
Several findings have provided a potential involvement of iron accumulation in the nigral cell death in PD. However, it has been controversial whether the iron accumulation is a primary causative event or merely a secondary change related to the dopaminergic neuronal degeneration. Several animal models including familial PD may provide mechanistic aspects of iron accumulation in the SN and pave a new way for clinical interventions.
References
Beck G, Sugiura Y, Shinzawa K, Kato S, Setou M, Tsujimoto Y, Sakoda S, Sumi-Akamaru H (2011) Neuroaxonal dystrophy in calcium-independent phospholipase A2β deficiency results from insufficient remodeling and degeneration of mitochondrial and presynaptic membranes. J Neurosci 31:11411–11420
Becker G, Seufert J, Reichmann H, Reiners K (1995) Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real time sonography. Neurology 45:443–454
Berg D (2006) In vivo detection of iron and neuromelanin by transcranial sonography—a new approach for early detection of substantia nigra damage. J Neural Transm. 113:775–780
Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302:819–822
Dexter DT, Wells FR, Agid F, Agid Y, Lees AJ, Jenner P, Marsden CD (1987) Increased nigral iron content in postmortem parkinsonian brain. Lancet 2:1219–1220
Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD (1989) Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52:1830–1836
Dexter DT, Sian J, Rose S et al (1994) Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol. 35:38–44
Earle KM (1968) Studies on Parkinson’s disease including X-ray fluorescent spectroscopy of formalin fixed brain tissue. J Neuropathol Exp Neurol 27:1–14
Faucheux BA, Bonnet AM, Agid Y, Hirsch EC (1999) Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet 353:981–982
Faucheux BA, Martin ME, Beaumont C, Hauw JJ, Agid Y, Hirsch EC (2003) Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s disease. J Neurochem 86:1142–1148
Furuya T, Hayakawa H, Yamada M, Yoshimi K, Hisahara S, Miura M, Mizuno Y, Mochizuki H (2004) Caspase-11 mediates inflammatory dopaminergic cell death in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. J Neurosci 24:1865–1872
Galazka-Friedman J, Bauminger ER, Friedman A (1996) Iron in parkinsonian and control substantia nigra: a Mossbauer spectroscopy study. Mov Disord 11:8–16
Gerlach M, Double KL, Ben-Shachar D, Zecca L, Youdim MB, Riederer P (2003) Neuromelanin and its interaction with iron as a potential risk factor for dopaminergic neurodegeneration underlying Parkinson’s disease. Neurotox Res 5:35–44
Goto K, Mochizuki H, Imai H, Akiyama H, Mizuno Y (1996) An immuno-histochemical study of ferritin in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced hemiparkinsonian monkeys. Brain Res 724:125–128
Halliwell B (1989) Oxidants and the central nervous system: some fundamental questions. Is oxidant damage relevant to Parkinson’s disease, Alzheimer’s disease, traumatic injury or stroke? Acta Neurol Scand Suppl 126:23–33
He Y, Thong PS, Lee T, Leong SK, Mao BY, Dong F, Watt F (2003) Dopaminergic cell death precedes iron elevation in MPTP-injected monkeys. Free Radic Biol Med. 35:540–547
Jellinger K, Kienzl E, Rumpelmair G, Riederer P, Stachelberger H, Ben-Shachar D, Youdim MB (1992) Iron-melanin complex in substantia nigra of parkinsonian brains: an X-ray microanalysis. J Neurochem 59:1168–1171
Jimenez Del Rio M, Moreno S, Garcia-Ospina G, Buritica O, Uribe CS, Lopera F, Velez-Pardo C (2004) Autosomal recessive juvenile parkinsonism Cys212Tyr mutation in parkin renders lymphocytes susceptible to dopamine- and iron-mediated apoptosis. Mov Disord 19:324–330
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 38:1285–1291
Mochizuki H, Nishi K, Mizuno Y (1993) Iron-melanin complex is toxic to dopaminergic neurons in a nigro-striatal co-culture. Neurodegeneration 2:1–7
Mochizuki H, Imai H, Endo K, Yokomizo K, Murata Y, Hattori N, Mizuno Y (1994) Iron accumulation in the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced hemiparkinsonian monkeys. Neurosci Lett 168:251–253
Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, Sonek S, Cangul H, Coryell J, Canham N, Nardocci N, Zorzi G, Pasha S, Rodriguez D, Desguerre I, Mubaidin A, Bertini E, Trembath RC, Simonati A, Schanen C, Johnson CA, Levinson B, Woods CG, Wilmot B, Kramer P, Gitschier J, Maher ER, Hayflick SJ (2006) PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet 38:752–754
Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B (2000) The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 20:6048–6054
Rathore KI, Redensek A, David S (2012) Iron homeostasis in astrocytes and microglia is differentially regulated by TNF-α and TGF-β1. Glia. 60:738–750
Roth JA, Singleton S, Feng J, Garrick M, Paradkar PN (2010) Parkin regulates metal transport via proteasomal degradation of the 1B isoforms of divalent metal transporter 1. J Neurochem 113:454–464
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K (2003) Alpha-synuclein locus triplication causes Parkinson’s disease. Science 302:841
Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G, Youdim MB (1988) Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm. 74:199–205
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840
Takahashi M, Ko LW, Kulathingal J, Jiang P, Sevlever D, Yen SH (2007) Oxidative stress-induced phosphorylation, degradation and aggregation of alpha-synuclein are linked to upregulated CK2 and cathepsin D. Eur J Neurosci 26:863–874
Takanashi M, Mochizuki H, Yokomizo K, Hattori N, Mori H, Yamamura Y, Mizuno Y (2001) Iron accumulation in the substantia nigra of autosomal recessive juvenile parkinsonism (ARJP). Parkinsonism Relat Disord. 7:311–314
Temlett JA, Landsberg JP, Watt F, Grime GW (1994) Increased iron in the substantia nigra compacta of the MPTP-lesioned hemiparkinsonian African green monkey: evidence from proton microprobe elemental microanalysis. J Neurochem 62:134–146
Uitti RJ, Rajput AH, Rozdilsky B, Bickis M, Wollin T, Yuen WK (1989) Regional metal concentrations in Parkinson’s disease, other chronic neurological disease, and control brains. Can J Neurol Sci 16:310–314
Uversky VN, Li J, Fink AL (2001) Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J Biol Chem 276:44284–44296 (Epub 2001 Sep 11)
Wypijewska A, Galazka-Friedman J, Bauminger ER, Wszolek ZK, Schweitzer KJ, Dickson DW, Jaklewicz A, Elbaum D, Friedman A (2010) Iron and reactive oxygen species activity in parkinsonian substantia nigra. Parkinsonism Relat Disord. 16:329–333
Zecca L, Shima T, Stroppolo A, Goj C, Battiston GA, Gerbasi R, Sarna T, Swartz HM (1996) Interaction of neuromelanin and iron in substantia nigra and other areas of human brain. Neuroscience 73:407–415
Zecca L, Gallorini M, Schünemann V, Trautwein AX, Gerlach M, Riederer P, Vezzoni P, Tampellini D (2001) Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. J Neurochem 76:1766–1773
Zecca L, Stroppolo A, Gatti A, Tampellini D, Toscani M, Gallorini M, Giaveri G, Arosio P, Santambrogio P, Fariello RG, Karatekin E, Kleinman MH, Turro N, Hornykiewicz O, Zucca FA (2004a) The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc Natl Acad Sci USA 101:9843–9848
Zecca L, Youdim MBH, Riederer P, Connor JR, Crichton RR (2004b) Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5:863–873
Zecca L, Casella L, Albertini A, Bellei C, Zucca FA, Engelen M, Zadlo A, Szewczyk G, Zareba M, Sarna T (2008a) Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J Neurochem 106:1866–1875
Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R (2008b) Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson’s disease. Acta Neuropathol 116:47–55
Zhang W, Phillips K, Wielgus AR, Liu J, Albertini A, Zucca FA, Faust R, Qian SY, Miller DS, Chignell CF, Wilson B, Jackson-Lewis V, Przedborski S, Joset D, Loike J, Hong JS, Sulzer D, Zecca L (2011) Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson’s disease. Neurotox Res 19:63–72
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mochizuki, H., Yasuda, T. Iron accumulation in Parkinson’s disease. J Neural Transm 119, 1511–1514 (2012). https://doi.org/10.1007/s00702-012-0905-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-012-0905-9