Abstract
The aim of the present study was to investigate the effects of one session of high-frequency repetitive transcranial magnetic stimulation (rTMS) applied over the left dorsal premotor cortex (PMd) and left dorsolateral prefrontal cortex (DLPFC) on choice reaction time in a noise-compatibility task, and cognitive functions in patients with Parkinson’s disease (PD). Clinical motor symptoms of PD were assessed as well. Ten patients with PD entered a randomized, placebo-controlled study with a crossover design. Each patient received 10 Hz stimulation over the left PMd and DLPFC (active stimulation sites) and the occipital cortex (OCC; a control stimulation site) in the OFF motor state, i.e. at least after 12 h of dopaminergic drugs withdrawal. Frameless stereotaxy was used to target the optimal position of the coil. For the evaluation of reaction time, we used a noise-compatibility paradigm. A short battery of neuropsychological tests was performed to evaluate executive functions, working memory, and psychomotor speed. Clinical assessment included a clinical motor evaluation using part III of the Unified Parkinson’s Disease Rating Scale. Statistical analysis revealed no significant effect of rTMS applied over the left PMd and/or DLPFC in patients with PD in any of the measured parameters. In this study, we did not observe any effect of one session of high frequency rTMS applied over the left PMd and/or DLPFC on choice reaction time in a noise-compatibility task, cognitive functions, or motor features in patients with PD. rTMS applied over all three stimulated areas was well tolerated and safe in terms of the cognitive and motor effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Repetitive transcranial magnetic stimulation (rTMS) has been studied as a potential treatment in many neurological disorders. Several studies have demonstrated positive effects of one session of high frequency rTMS applied over the motor cortex (MC) on motor symptoms of patients with Parkinson disease (PD). Nevertheless, there is no evidence to suggest that the motor cortex is the most suitable cortical target for rTMS in those patients (Siebner 2005).
Results from lesion studies in nonhuman primates and brain imaging investigations in humans support the hypothesis that the dorsal premotor cortex (PMd) is involved in integrating external information with motor commands and could potentially serve as an area where a selection process for movement is mediated (Halsband and Passingham 1985; Wessel et al. 1997). Accumulating evidence indicates that the PMd is not only active during demanding motor tasks, but also plays a role in various cognitive tasks (e.g. Jonides et al. 1993; Paulesu et al. 1993; Smith et al. 1998; Marois et al. 2006; Praamstra et al. 1999). These findings are in accordance with results of neuroanatomical studies revealing that PMd has a close relationship with the primary motor cortex as well as with the prefrontal cortex (Barbas and Pandya, 1987; Luppino et al. 1993). Behavioural after-effects of rTMS applied over the PMd were studied on precued-choice reaction times with low frequency stimulation (1 Hz) in healthy subjects. Delayed reaction times were described after stimulation with no significant impact on error rates or movement times (Terao et al. 2007).
The dorsolateral prefrontal cortex (DLPFC) is anatomically connected with the rostal part of supplementary motor area (SMA) (Bates and Goldman-Rakic 1993) and sends projections to the striatum (Selemon and Goldman-Rakic 1985). Using [(11)C] raclopride PET, Strafella and colleagues demonstrated that high frequency rTMS applied over the left DLPFC leads to dopamine release in the ipsilateral caudate nucleus (Strafella et al. 2001). Dopamine is implicated in movement as well as in a range of frontal executive-type cognitive processes (Cropley et al. 2006). It has also been shown that the DLPFC possesses cortico-cortical connections with the parietal and premotor cortices that are involved in visuomotor control of action, and DLPFC has a crucial role in the cognitive control of motor behaviour (Hoshi 2006). Futhermore, several previous studies have demonstrated a positive effect of high frequency rTMS over the left DLPFC on executive functions in patients with depression, Parkinson Disease, and with mild cognitive impairment of vascular etiology (e.g. Triggs et al. 1999; Speer et al. 2001; Moser et al. 2002; Boggio et al. 2005; Rektorova et al. 2005).
High frequency rTMS applied over the motor cortex leads to facilitatory after-effects on corticospinal excitability, while low-frequency stimulation leads to opposite (i.e. inhibitory) after-effects (Siebner and Rothwell 2003). Nevertheless, it is not precisely known what effect (high or low frequency) rTMS applied over the DLPFC and/or PMd has on these cortices specifically. Therefore, the aim of the present study was to primarily investigate whether high frequency rTMS applied over the left PMd and/or the left DLPFC would have any measurable impact on choice reaction time and executive functions in patients with PD. Motor scores prior to and after each stimulation were assessed in addition to our primary outcomes.
Method
Subjects
We studied ten patients (9 males, 1 female) with PD (mean age 63.7 ± 6.7). The patients fulfilled the established criteria for diagnosis of Parkinson’s disease according to the Parkinson’s Disease Society of UK Brain Bank (Gibb and Lees 1988); for patients characteristics, see Table 1. The patients were selected with regard to presence of akinesia and bradykinesia of the upper limbs predominantly expressed on the right side and with minimal tremor. Nine patients were taking L-Dopa/carbidopa (mean dose 802.5 ± 325.5 mg/day); of those, eight were also taking DA-agonist (8 patients- pramipexole with a daily dose of 1.8 ± 0.5 mg; one patient- ropinirole with a daily dose of 15 mg); four of those patients were also on entacapone (mean daily dose 750 ± 192 mg). One patient was given only pramipexole in a daily dose of 2.1 mg; see Table 1. The medication regimen had been stable in all patients for at least 4 months prior to the study. All patients were tested after overnight withdrawal (at least 12 h) of dopaminergic medication at the same time of day. All of the patients were right-handed. The Montgomery-Asberg Depression Rating Scale (Montgomery and Asberg 1979) with a cut-off score of 10 was used to exclude the patients with depression (Silberman et al. 2006). All subjects gave their written informed consent as approved by a local ethics committee.
Transcranial magnetic stimulation
rTMS was applied using the Magstim Super Rapid Magnetic Stimulator (Magstim Company, Whitland, UK) and a figure-eight air-cooled coil (7 cm mean diameter). A crossover design was used. Each patient received three sessions of 10 Hz stimulation in random order: over the left PMd (an active stimulation site), over the left DLPFC (an active stimulation site) and over the left occipital cortex (OCC, serving as a control stimulation site, as this permitted verification of any non-specific effects of rTMS). The sessions were separated from each other by at least 1 day without stimulation. One session of high frequency rTMS (10 Hz) consisted of three rTMS blocks (15 × 30-pulse trains; 100% resting motor threshold intensity, inter-train interval of 10 s, total number of 1,350 stimuli), each separated by a 10 min interval. Frameless stereotaxy (Brainsight Frameless 1.5; Rogue Research Inc., Montreal, Canada) was used to target the optimal position of each stimulated side and to ensure the same cortical location in all patients. The coordinates for the left DLPFC [X = −40, Y = 32, Z = 30] were chosen based on the probable location as revealed by a PET study of verbal working memory (Petrides et al. 1993) and used by other authors (Strafella et al. 2001; Barrett et al. 2004) and corresponding to cytoarchitectonic area 9/46 as defined by Petrides and Pandya (1999). For a location of the left dorsal premotor cortex [X = −21, Y = −2, Z = 52], we used the coordinates as defined in a previous TMS study by Chouinard et al. (2003) and corresponding to the probable location of PMd revealed by a Positron emission tomographic study as being ~20 mm anterior to the location of the primary MC for hand (Fink et al. 1997). Coordinates [X = −56, Y = −58, Z = −3] were chosen for the occipital cortex (Strafella et al. 2001).
A magnetic resonance (MR) image of each subject’s brain was acquired and transformed into the standardized stereotaxic space. The coordinates of the DLPFC, PMd, and OCC were transformed to the subject’s brain coordinate (“native”) space with an inverse version of the native-to-stereotaxic transformation matrix (Paus et al. 1997; Paus 1998). This allowed us to determine where the target region was located in a given subject on the MR images. Using frameless stereotaxy, the coil was placed over an appropriate location, marked on the MR images. For the DLPFC, the coil was oriented tangentially to the scalp with the short axis of the figure-eight coil angled at 45° away from the midline, inducing a posterior–anterior current in the brain. For the PMd, we oriented the coil tangentially to the scalp with the short axis of the figure-eight coil perpendicular to the interhemispheric fissure (Chouinard et al. 2003). In that case, the resulting electric current induced in the brain flowed in lateral-to-medial directions. For the occipital stimulation (OCC), the coil was held tangentially to the skull with the handle pointing back (Bermpohl et al. 2005).
Procedure
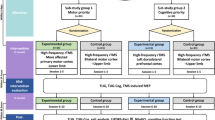
We evaluated the effect of rTMS over different cortical areas by using the reaction time (RT) protocol, a short battery of neuropsychological tests, and neurological examination using the Unified Parkinson’s Disease Rating Scale (UPDRS part III). The whole evaluation after each stimulation lasted approximately 30 min. For a timeline of an rTMS session, see Fig. 1. A previous study in PD patients reported that the effect of a single session of high frequency rTMS over the MC, manifested as an improvement in UPDRS, may last at least 1 h after stimulation (Siebner et al. 2000). The battery of neuropsychological tests was chosen carefully in order to meet our primary endpoints and the total length of examination.
For the evaluation of choice reaction time, we used a noise-compatibility paradigm (as modified by Praamstra) prior to and immediately after each rTMS session. In this task, subjects perform the choice reaction on two different stimuli: compatible and incompatible. For the analysis of the behaviour responses, decision errors were calculated (Praamstra et al. 1998).
Compatible stimuli
Nine arrows were presented on the screen (in three columns and three lines); all of the arrows pointed to the right or left, respectively.
Incompatible stimuli
The target arrow (in the centre) pointed to the right or left, and was surrounded by eight distractor arrows with an opposite orientation.
Stimuli were presented for 100 ms with a 3–4 s interstimulus interval in a 20 min block. Subjects were instructed to move a joystick with their right hand as soon as possible according to the orientation of the arrow in the centre (the target arrow). Before the experimental session, all subjects performed one practice block in order to familiarize themselves with the task.
A short battery of neuropsychological tests was performed to evaluate executive function, working memory and psychomotor speed. The battery included a Verbal Fluency Test (VFT; Halstead 1947), Trail Making Tests (TMT) A and B (Halstead 1947), and the Digit Span subtests of the Wechsler Memory Scale (Wechsler 1975). The tests were performed prior to the first rTMS session and immediately after the completion of the reaction time protocol. TMT A and B were practiced prior to the study to avoid susceptibility to practice effects (Collie et al. 2003). For the Digit Span subtest and the Verbal Fluency Test (animal, food, clothing, occupations), different variants were used. The performance of all the patients but one was within the normal range in all of the evaluated psychological tests. One patient was out of normal range in TMT A prior to an rTMS session on the first day of stimulation.
The neurological evaluation included the Unified Parkinson’s Disease Rating Scale (UPDRS, part III); this was performed before and after each rTMS session.
Results
For effects of rTMS on choice reaction time, UPDRS III and cognitive functions, see Tables 2 and 3. One patient prematurely withdrew from the study, and did not undertake the control stimulation over OCC (the reason for the patient’s withdrawl was unrelated to the study). The statistical analysis was performed using the two-way ANOVA.
Reaction time
The two-way ANOVA did not show any significant effect of the factors “Time” (prior to and after rTMS session) and “Site of Stimulation” (PMd, DLPFC, OCC) for compatible stimuli (F (2,52) = 0.35860, P = 0.700368) and incompatible stimuli (F (2,52) = 0.27499; P = 0.760674). The number of errors was low, and all errors occurred in the incompatible condition (see Table 2). There was no difference in the number of errors and in the reaction time of error responses when the data collected prior to and after each appropriate rTMS session were compared (“data not shown”).
Neurocognitive tests
The data analyses did not reveal any significant effect of the factors “time” and “site of stimulation” for VFT (F (3,36) = 0.23295; P = 0.872820), TMT A (F (3,35) = 0.01570; P = 0.99726), TMT B (F (3,35) = 0.03638; P = 0.99055) and Digit span (F (3,35) = 0.48241; P = 0.69664).
UPDRS (Part III)
The two-way ANOVA did not show any significant effect of the factors “time” and “site of stimulation” for the UPDRS III (F (2,52) = 0.03021; P = 0.97026).
rTMS applied over the PMd, DLPFC, and OCC was well tolerated and safe in terms of the cognitive and motor effects in patients with PD. During the OCC stimulation, no visual phenomena were observed.
Discussion
In the present study, we did not demonstrate any effect of one session of high frequency rTMS applied over the left PMd and/or DLPFC on choice reaction time, executive functions, and motor performance in patients with PD.
We questioned whether the cognitive tests were sensitive to the stimulated cortical areas. For the evaluation of reaction time, we used a noise-compatibility task, in which performance requires subjects to attend to a centrally fixated stimulus while ignoring distractor elements (incompatible stimuli). A consistent finding is that response times are longer with incompatible than with compatible stimuli. A stimulus-response conflict with the necessity of suppression of automatic activation has been proposed to explain this effect (e.g. Eimer et al. 1995; Ridderinkhof 2002). Using visual guidance, PD patients may improve motor performance (Brown and Marsden 1988). It has thus been proposed that patients may be more susceptible to the interfering effects of incompatible stimuli due to distractor elements (Praamstra et al. 1998).
PMd is believed to be engaged in visuomotor control of action (Halsband and Passingham 1985; Wessel et al. 1997). Increased rCBF as measured by H2(15)O PET during complex finger movements was demonstrated in PD patients compared to controls (Samuel et al. 1997). According to the authors of that study, a switch from the use of striato-mesial frontal to parietal-lateral premotor circuits may facilitate the performance in PD patients. The involvement of the PMd in the inhibition of a prepotent motor response was reported in a functional imaging study with a response inhibition task (Sylvester et al. 2003). In accordance with previous results, Praamstra et al. (1999) confirmed the role of the premotor cortex in the inhibitory control of automatic response in a choice reaction task by means of rTMS. It is well documented that the DLPFC is involved in the suppression of automatic responses (Jahanshahi et al. 1998) and in resolving stimulus conflict (Liu et al. 2006; Milham et al. 2003, 2005).
A short neurocognitive battery of tests sensitive to assessing executive functions was used including the Trail Making Test, Verbal Fluency Test, and the Wechsler Digit Span subtest. The results from functional imaging studies and rTMS revealed an engagement of the DLPFC in the Verbal Fluency Test (Frith et al. 1991; Kalbe et al. 2009; Jenkins et al. 2002) and the Digit Span subtest (Hoshi et al. 2000; Gerton et al. 2004). These results are in line with findings in patients with lesions of the DLPFC, who usually present a decreased conceptualization, decreased attention resources (inattention), impaired ability to employ active cognitive strategy (word-finding, impaired memory retrieval), impaired set shifting and set maintenance (distractibility) (Dubois et al. 2008). The accumulating evidence indicates that the premotor cortex plays a role in various cognitive tasks such as temporal maintenance or update of verbal information used for solving non-motor cognitive tasks (e.g. Paulesu et al. 1993; Smith et al. 1998), response selection (Marois et al. 2006; Praamstra et al. 1999), or spatial attention (e.g. Jonides et al. 1993).
Taken together, although both stimulated regions are likely to be to be involved in both of our choice- reaction and cognitive tasks, the acute after-effects of rTMS were not sufficient to induce any measurable behavioural changes in our PD subjects.
One possible explanation of our negative results could be that our patients’ cognitive performance was within the normal range (except for baseline TMT A performance in one patient). Nevertheless, “off-line” rTMS (evaluation after stimulation) has been successfully used in a number of studies of cognition and reaction time tasks even in healthy subjects, and the positive effects of rTMS have been demonstrated (Jahanshahi 2005; Pascual-Leone et al. 1993; Pascual-Leone et al. 1999; Cappa et al. 2002; Evers et al. 2001; Jenkins et al. 2002).
With regard to our negative results, the laterality of rTMS (left-sided stimulation) has to be discussed as well. Could right-sided or bilateral stimulation have induced any measurable effects? We were in favour of the left-sided stimulation since our patients were all right-handed and were selected with regard to the presence of bradykinesia and rigidity predominantly expressed on the right side. Also, it has been proposed that the PMd in the left hemisphere is dominant for the selection of actions (Schluter et al. 1998, 2001; Rushworth et al. 2003) and may be implicated in response selection (Iacoboni et al. 1998). Tasks involving interference resolution (detection/resolution) revealed a network including bilateral DLPFC (Nee et al. 2007). However, both functional imaging and rTMS studies indicate that performance of verbal fluency tasks as well as of tasks that require resolving stimulus conflict or suppression of habitual responses is related predominantly to the left side (Liu et al. 2006; Milham et al. 2003; Milham and Banich 2005; Sylvester et al. 2003; Jahanshahi et al. 1997, 1998; Rektorova et al. 2005).
In terms of rTMS effects on cognitive and motor symptoms of PD, variable results have been described in literature. Better performance in the Stroop test and other tests evaluating executive functions (e.g. Hooper Visual Organization Test, Wisconsin Card Sorting Test) was observed after repeated sessions of high rTMS overt the left DLPFC (Boggio et al. 2005). An improvement in neuropsychological measures and in motor performance (UPDRS part III) was found also by other authors (Epstein et al. 2007). However, in both of these studies PD patients with concurrent depression were involved and improvement in depression was also described. Therefore, the results might reflect the impact of mood changes rather than effects of rTMS per se. Positive cumulative benefits of high frequency rTMS on motor functions (improvement of upper limb bradykinesia and gait speed) were observed in patients with PD after repeated sessions of rTMS applied over the bilateral left and right MC and DLPFC in each session (Lomarev et al. 2006). Fregni et al. (2004) reported improvement in mood and in motor functions again after repeated sessions of high-frequency rTMS as evaluated by Activity of Daily Living scores. Conversely, no clinical benefit on motor functions (as measured by the UPDRS part III) was reported in two other studies after repeated high frequency rTMS applied over the left DLPFC (del Olmo et al. 2007; Rektorova et al. 2007).
The clinical experience with stimulation applied over the PMd in PD is very limited and has been mostly evaluated in terms of changes in cortical excitability of the ipsilateral motor cortex (Buhmann et al. 2004; Bäumer et al. 2009). In a recent study, one session of low rTMS over PMd was applied in patients with advanced PD. Beside changes in cortical excitability (silence period), no clinical relevant effect as measured by UPDRS (part III) was observed (Bäumer et al. 2009). Therefore, the clinical correlate of these changes remains unclear (Buhmann et al. 2004; Bäumer et al. 2009). Indeed, we cannot fully exclude the possibility that repeated sessions might be needed in order to produce any beneficial behavioural effects on motor symptoms of PD in particular. Nevertheless, the change in motor scores was not the primary outcome in our study.
A possible limitation of our study could have been that our patients were stimulated in the OFF state. Other methodological limitations of this study include the small sample size and the heterogenity of patients, especially with respect to PD duration. Our patients had a normal cognitive performance even in their OFF state (except for baseline TMT A performance in one patient). We preferred to stimulate in the OFF state also because we wanted to be able to distinguish between the potential effect of rTMS and the possible effects of dopaminergic treatment. It has been demonstrated that dopaminergic treatment may induce both positive and negative effects on executive functioning in PD patients (Gotham et al. 1988; Kulisevsky et al. 1996).
The main problem regarding the optimal stimulation parameters remains unsolved. Currently, there is no consensus on the stimulation parameters to be used, which is complicated by the fact that the susceptibility of individual subjects to the influence of rTMS is very variable (Tergau et al. 1999; Maeda et al. 2000; Sommer et al. 2002; Siebner et al. 2002). Recently, a meta-analysis evaluating the effect of rTMS on motor signs in PD was conducted including only controlled studies with low or high rTMS applied over MC or DLPFC. This study confirms that only high-frequency rTMS can significantly reduce motor signs in PD patients. No other analysis in terms of site stimulation (MC versus DLPFC), state of patients (ON versus OFF state), and other parameters of stimulation including the number of sessions needed to produced any effect were evaluated (Elahi et al. 2009). It has been suggested that a certain number of magnetic stimuli is needed to induce a beneficial after-effect in PD when rTMS is applied to motor cortex (5 Hz, 90%MT, 2250 pulses, OFF state; Siebner 2005).
Taken together, we did not produce any measurable behavioural effects of rTMS applied over the left DLPFC and/or PMd on cognitive performance in our PD patients. The motor symptoms of PD as measured by the UPDRS III scores did not change either. Although studies on the therapeutic use of rTMS applied over different cortical areas in patients with PD have grown considerably, there are only a limited numbers of studies that have proven a relevant clinical effect of rTMS. The main problem remains unsolved, i.e.: the basic mechanism mediating the effects of rTMS is still poorly understood; there is no consensus on the site of stimulation and stimulation parameters to be used; and the susceptibility of individual subjects to the influence of rTMS is variable.
References
Barbas H, Pandya DN (1987) Architecture and frontal cortical connections of the premotor cortex (are 6) in the rhesus monkey. J Comp Neurol 256:211–228
Barrett J, Della-Maggiore V, Chouinard PA, Paus T (2004) Mechanisms of action underlying the effect of repetitive transcranial magnetic stimulation on mood: behavioural and brain imaging studies. Neuropsychopharmacology 29:1172–1189
Bates JF, Goldman-Rakic PS (1993) Prefrontal connections of medial motor areas in the rhesus monkey. J Comp Neurol 336:211–228
Bäumer T, Hidding U, Hamel W, Buhmann C, Moll CK, Gerloff C, Orth M, Siebner HR, Münchau A (2009) Effects of DBS, premotor rTMS, and levodopa on motor function and silent period in advanced Parkinson’s disease. Mov Disord 24(5):672–676
Bermpohl F, Fregni F, Boggio PS, Thut G, Northoff G, Otachi PT, Rigonatti SP, Marcolin MA, Pascual-Leone A (2005) Left prefrontal repetitive transcranial magnetic stimulation impairs performance in affective go/no-go task. Neuroreport 16:615–619
Boggio PS, Fregni F, Bermpohl F, Mansur CG, Rosa M, Rumi DO, Barbosa ER, Odebrecht Rosa M, Pascual-Leone A, Rigonatti SP, Marcolin MA, Araujo Silva MT (2005) Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov Disord 20:1178–1184
Brown RG, Marsden CD (1988) Internal versus external cues and the control of attention in Parkinson’s disease. Brain 111:323–345
Buhmann C, Gorsler A, Bäumer T, Hidding U, Demiralay C, Hinkelmann K, Weiller C, Siebner HR, Münchau A (2004) Abnormal excitability of premotor-motor connections in de novo Parkinson’s disease. Brain 127:2732–2746
Cappa SF, Sandrini M, Rossinin PM, Sosta K, Minuissi C (2002) The role of the left frontal lobe in action naming: rTMS evidence. Neurology 59:720–723
Chouinard PA, Van Der Werf YD, Leonard G, Paus T (2003) Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol 90:1071–1083
Collie A, Maruff P, Darby DG, McStephen M (2003) The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test–retest intervals. J Int Neuropsychol Soc 9:419–428
Cropley VL, Fujita M, Innis RB, Nathan PJ (2006) Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol Psychiatry 59(10):898–907
del Olmo MF, Bello O, Cudeiro J (2007) Transcranial magnetic stimulation over dorsolateral prefrontal cortex in Parkinson’s disease. Clin Neurophysiol 118:131–139
Dubois B, Anrade K, Levy R (2008) Executive dysfunction and neurocognitive testing. In: Duyckaerts C, Litvan I (eds) Dementias: handbook of clinical neurology (Series Editors: Aminoff MJ, Boller F, Swaab DF). Elsevier, Amsterdam, pp 35–52
Eimer M, Hommel B, Prinz W (1995) S-R compatibility and response selection. Acta Psychol 90:301–313
Elahi B, Elahi B, Chen R (2009) Effect of transcranial magnetic stimulation on Parkinson motor function—systematic review of controlled clinical trials. Mov Disord 24(3):357–363
Epstein CM, Evatt ML, Funk A, Girard-Siqueira L, Lupei N, Slaughter L, Athar S, Green J, McDonald W, DeLong MR (2007) An open study of repetitive transcranial magnetic stimulation in treatment-resistant depression with Parkinson’s disease. Clin Neurophysiol 118:2189–2194
Evers S, Bockermann I, Nyhuis PW (2001) The impact of transcranial magnetic stimulation on cognitive processing: an event-related potential study. Neuroreport 17:2915–2918
Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE (1997) Multiple nonprimary motor areas in the human cortex. J Neurophysiol 77:2164–2174
Fregni F, Santos CM, Myczkowski ML, Rigolino R, Gallucci-Neto J, Barbosa ER, Valente KD, Pascual-Leone A, Marcolin MA (2004) Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J Neurosurg Psychiatry 75:71–1174
Frith D, Friston KJ, Liddle P, Frackowiak RSJ (1991) A PET study of word finding. Neuropsychologie 29:1137–1148
Gerton BK, Brown TT, Meyer-Lindenberg A, Kohn P, Holt JL, Olsen RK, Berman KF (2004) Shared and distinct neurophysiological components of the digits forward and backward tasks as revealed by functional neuroimaging. Neuropsychologia 42:1781–1787
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Gotham AM, Brown RG, Marsden CD (1988) Frontal cognitive function in patients with Parkinson’s disease ‘on’ and ‘off’ levodopa. Brain 2:299–321
Halsband U, Passingham RE (1985) Premotor cortex and the conditions for movement in monkey (Macaca fascicularis). Behav Brain Res 18:269–277
Halstead WC (1947) Brain and intelligence: a quantitative study of the frontal lobes. University of Chicago Press, Chicago
Hoshi E (2006) Functional specialization within the dorsolateral prefrontal cortex: a review of anatomical and physiological studies of non-human primates. Neurosci Res 54:73–84
Hoshi Y, Oda I, Wada Y, Ito Y, Yamashita Yutaka, Oda M, Ohta K, Yamada Y, Tamura Mamoru (2000) Visuospatial imagery is a fruitful strategy for the digit span backward task: a study with near-infrared optical tomography. Brain Res Cogn Brain Res 9:339–342
Iacoboni M, Woods RP, Mazziotta JC (1998) Bimodal (auditory and visual) left frontoparietal circuitry for sensorimotor integration and sensorimotor learning. Brain 121:2135–2143
Jahanshahi M (2005) Other cognitive functions. In: Hallett M, Chokroverty S (eds) Magnetic stimulation in clinical neurophysiology, 2nd edn. Elsevier, Philadelphia, pp 281–302
Jahanshahi M, Dirnberger G, Fulle R, Firth CD (1997) The functional anatomy of random number generation studied with PET. J Cereb Blood Flow Metab 17(1):S643
Jahanshahi M, Profice P, Brown RG, Mike C, Ridding MC, Dirnberger G, Rothwell JC (1998) The effects of transcranial magnetic stimulation over the dorsolateral prefrontal cortex on suppression of habitual counting during random number generation. Brain 121:1533–1544
Jenkins J, Shajahan PM, Lappin JM, Ebmeier KP (2002) Right and left prefrontal transcranial magnetic stimulation at 1 Hz does not affect mood in healthy volunteers. BMC Psychiatry 2:1
Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA (1993) Spatial working memory in humans as revealed by PET. Nature 363:623–625
Kalbe E, Voges J, Weber T, Haarer M, Baudrexel S, Klein JC, Kessler J, Sturm V, Heiss WD, Hilker R (2009) Frontal FDG-PET activity correlates with cognitive outcome after STN-DBS in Parkinson disease. Neurology 72:42–49
Kulisevsky J, Avila A, Barbano M, Antonijoan R, Berthier M, Gironelli A (1996) Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson’s disease patients at different levodopa plasma levels. Brain 119:2121–2132
Liu X, Banich MT, Jacobson BL, Tanabe JL (2006) Functional dissociation of attentional selection within PFC: response and non-response related aspects of attentional selection as ascertained by fMRI. Cereb Cortex 16:827–834
Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M (2006) Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord 12:325–331
Luppino G, Matelli M, Camarda R, Rizzolatti G (1993) Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol 338:114–140
Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A (2000) Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 133:425–430
Marois R, Larson JM, Chun MM, Shima D (2006) Response-specific sources of dual-task interference in human pre-motor cortex. Psychol Res 70:436–447
Milham MP, Banich MT (2005) Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Hum Brain Mapp 25:328–335
Milham MP, Banich MT, Barad V (2003) Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: an event-related fMRI study of the Stroop task. Brain Res Cogn Brain Res 17:212–222
Montgomery SA, Asberg MA (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Moser DJ, Jorge RE, Manes F, Paradiso S, Benjamin BS, Robinson RG (2002) Improved executive functioning following repetitive transcranial magnetic stimulation. Neurology 58:1288–1290
Nee DE, Wager TD, Jonides J (2007) Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7:1–17
Pascual-Leone A, Houser CM, Reese K, Shotland LI, Grafman J, Sato S, Valls-Solé J, Brasil-Neto JP, Wassermann EM, Cohen LG et al (1993) Safety of rapid-rate transcranial magnetic stimulation in normal volunteers. Electroencephalogr Clin Neurophysiol 89:120–130
Pascual-Leone A, Valls-Solé J, Brasil-Neto JP, Cammarota A, Grafman J, Hallett M (1994) Akinesia in Parkinson’s disease. II. Effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology 44:892–898
Pascual-Leone A, Tarazona F, Keenan J, Tormos JM, Hamilton R, Catala MD (1999) Transcranial magnetic stimulation and neuroplasticity. Neuropsychologia 37:207–217
Paulesu E, Frith CD, Frackowiak RS (1993) The neural correlates of the verbal component of working memory. Nature 362(6418):342–345
Paus T (1998) Imaging the brain before, during, and after transcranial magnetic stimulation. Neuropsychologia 37:219–224
Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC (1997) Transcranial magnetic stimulation during positron emission tomography: a new method of studying connectivity of the human cerebral cortex. J Neurosci 17:3178–3184
Petrides M, Pandya DN (1999) Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 11:1011–1036
Petrides M, Alivisatos B, Meyer E, Evans AC (1993) Functional activation of the human frontal cortex during the performance of verbal working memory task. Proc Natl Acad Sci USA 90:878–882
Praamstra P, Stegman DF, Cools AR, Horstink MW (1998) Reliance on external cues for movement initiation in Parkinson’s disease. Brain 121:167–177
Praamstra P, Kleine B, Schnitzler A (1999) Magnetic stimulation of the dorsal premotor cortex modulates the Simon effect. Neuroreport 10:3671–3674
Rektorova I, Megova S, Bares M, Rektor I (2005) Cognitive functioning after repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia: a pilot study of seven patients. J Neurol Sci 229–230:157–161
Rektorova I, Sedlackova S, Telecka S, Hlubocky A, Rektor I (2007) Repetitive transcranial stimulation for freezing of gait in Parkinson’s disease. Mov Disord 22:1518–1519
Ridderinkhof KR (2002) Activation and suppression in conflict tasks: empirical clarification through distributional analyses. In: Prinz W, Hommel B (eds) Common mechanisms in perception and action: attention and performance XIX. Oxford University Press, Oxford
Rushworth MF, Johansen-Berg H, Gobel SM, Delvin JT (2003) The left parietal and premotor cortices: motor attention and selection. Neuroimage 20:89–100
Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE et al (1997) Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study Brain 120:963–976
Schluter ND, Rushworth MF, Passingham RE, Mills KR (1998) Temporary interference in human lateral premotor cortex suggests dominance for the selection of movement: a study using transcranial magnetic stimulation. Brain 121:785–799
Selemon LD, Goldman-Rakic PS (1985) Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci 5:776–794
Siebner HR (2005) Treatment of Movement Disorders. In: Hallett M, Chokroverty S (eds) Magnetic stimulation in clinical neurophysiology, 2nd edn. Elsevier, Philadelphia
Siebner HR, Rothwell J (2003) Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 148:1–16
Siebner HR, Siebner HR, Rossmeier C, Mentschel C, Peinemann A, Conrad B (2000) Short-term motor improvement after sub-threshold 5-Hz repetitive transcranial magnetic stimulation of the primary motor hand area in Parkinson’s disease. J Neurol Sci 178:91–94
Siebner HR, Loeer C, Mentschel C, Weindl D, Conrad B (2002) Repetitive transcranial magnetic stimulation in Parkinson’s disease and focal dystonia. Clin Neurophysiol Suppl 54:399–409
Silberman CD, Laks J, Capitão CF, Rodrigues CS, Moreira I, Engelhardt E (2006) Recognizing depression in patients with Parkinson’s disease: accuracy and specificity of two depression rating scale. Arq Neuropsiquiatr 64:407–411
Smith EE, Jonides J, Marshuetz C, Koeppe RA (1998) Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci USA 95:876–882
Sommer M, Wu T, Tergau F, Paulus W (2002) Intra- and interindividual variability of motor responses to repetitive transcranial magnetic stimulation. Clin Neurophysiol 113:265–269
Speer AM, Repella JD, Figueras S, Demian NK, Kimbrell TA, Wasserman EM, Post RM (2001) Lack of adverse cognitive effects of 1 Hz and 20 Hz repetitive transcranial magnetic stimulation at 100% of motor threshold over left prefrontal cortex in depression. J ECT 17:259–263
Strafella AP, Paus T, Barrett J, Dagher A (2001) Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 21:1–4
Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J (2003) Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia 41:357–370
Terao Y, Furubayashi T, Okabe S, Mochizuki H, Arai N, Kobayashi S, Ugawa Y (2007) Modifying the cortical processing for motor preparation by repetitive transcranial magnetic stimulation. J Cogn Neurosci 19:1556–1573
Tergau F, Naumann U, Paulus W, Steinhoff BJ (1999) Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet 353:2209
Triggs WJ, McCoy KJ, Greer R, Rossi F, Bowers D, Kortenkamp S, Nadeau SE, Heilman KM, Goodman WK (1999) Effects of left frontal transcranial magnetic stimulation on depressed mood, cognition, and corticomotor threshold. Biol Psychiatry 45:1440–1446
Wechsler D (1975) Wechsler memory scale. Psychological Corporation, New York
Wessel K, Zeffiro T, Toro C, Hallett M (1997) Self-paced versus metronome-paced finger movements. A positron emission tomography study. J Neuroimaging 7:145–151
Acknowledgments
Supported by research program of the Ministry of Education of the Czech Republic MSM 0021622404.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sedláčková, S., Rektorová, I., Srovnalová, H. et al. Effect of high frequency repetitive transcranial magnetic stimulation on reaction time, clinical features and cognitive functions in patients with Parkinson’s disease. J Neural Transm 116, 1093–1101 (2009). https://doi.org/10.1007/s00702-009-0259-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-009-0259-0