Abstract
Haloperidol (HAL) is a widely used neuroleptic drug for the treatment of acute and chronic psychosis. Tardive dyskinesia (TD) is a complex hyperkinetic syndrome consisting of choreiform and athetoid movements, which persists for months or years after withdrawal. Increased levels of thiobarbituric acid reactive products are found in the cerebrospinal fluid and plasma of patients treated with neuroleptics, especially those with movement disorders. Alpha lipoic acid (ALA), a natural metabolic antioxidant, is effective in both prevention and treatment of numerous types of neurological disorders. It is proposed to study the effect of ALA on TD induced by HAL and to correlate it with oxidative stress by studying total antioxidant status and lipid peroxidation (LP). HAL (1 mg/kg/i.p.) was used to induce vacuous chewing movements in rats. ALA was suspended in 0.2% carboxy methyl cellulose at a dose of 25, 50 and 100 mg/kg and was administered orally by oral gavage 1 h before HAL on 21st day of treatment. ALA supplementation significantly decreased HAL-induced TD at a dose of 100 mg/kg and catalepsy dose dependently. ALA improved TD and catalepsy by decreasing HAL-induced LP. ALA and its metabolite dihydro lipoic acid protect against HAL-induced TD and catalepsy by scavenging reactive oxygen species and reactive nitrogen species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haloperidol (HAL) is a widely used neuroleptic drug for the treatment of acute and chronic psychosis. The use of HAL is limited by its tendency to produce a range of typical antipsychotic extrapyramidal movement disorders such as tardive dyskinesia (TD), akathisia, dystonia and parkinsonism. TD is a complex hyperkinetic syndrome consisting of choreiform, athetoid or rhythmic abnormal involuntary movements. The face, mouth and tongue are most frequently involved (orofacial type), but a variety of less frequent motor abnormalities of the upper, lower limbs and trunk also occur (Kane and Smith 1982). The syndrome has usually a delayed onset and the intensity of the disturbance fluctuates over time. The most serious aspect of TD is that it may persist for months or years after drug withdrawal and in some patients it is irreversible (Jeste et al. 1979; Casey 1985a, b; Glazer et al. 1990) and the syndrome is a major clinical and ethical issue (APA 1992).
Oxidative stress (OST) resulting from increased production of reactive oxygen species (ROS) and a decrease in antioxidant defense damages biological macromolecules, disrupts normal 5HT-1A metabolism and physiology (Sies 1985) and is proposed as a pathogenic mechanism in TD (Cadet et al. 1986; Cadet and Kahler 1994).
HAL is metabolized by an oxidase, generates large quantities of oxyradicals and a potent toxic pyridinium-like metabolite (Subramaniam et al. 1990) and induces OST (Behl et al. 1995; Sagara 1998; Post et al. 1998). Blockade of dopamine D2 receptors by neuroleptics is associated with increased dopamine turnover particularly in catecholamine-rich regions such as basal ganglia (Creese et al. 1976). This supports the hypothesis that typical neuroleptics increase the oxyradicals as a result of increased catecholamine and drug metabolism, and contribute to oxyradical injury in schizophrenic patients (Cadet and Lohr 1989; See 1991).
Increased levels of thiobarbituric acid (TBA) reactive products are found in the cerebrospinal fluid and plasma of patients treated with neuroleptics (Pall et al. 1987), especially those with movement disorders (Lohr et al. 1990; Peet et al. 1993). Amelioration of TD after treatment with Pro-Leu-glycinamide (Sharma et al. 2003) and vitamin E, a lipid soluble antioxidant, confirms (Lohr et al. 1987; Elkashef et al. 1990; Scapicchio et al. 1991; Egan et al. 1992; Adler et al. 1993a, b, 1998) the involvement of OST in HAL-induced TD.
Alpha lipoic acid (ALA) also known as thioctic acid is a naturally occurring compound synthesized by plants and animals. In humans, it is readily synthesized in the body and is well absorbed from the diet through the stomach and intestine. ALA is a medium length (eight carbon atoms) fatty acid containing two sulfur atoms that are oxidized or reduced. This feature allows ALA to function as a potent antioxidant and cofactor for several important enzymes (Cadenas and Packer 1996). ALA is effective in both prevention and treatment of numerous types of neurological disorders, due to its ability to cross the BBB and accumulates in brain tissue and is an ideal therapeutic antioxidant for treatment of brain and neurodegenerative disorders involving free radical processes (Packer et al. 1995). The ability of ALA or its reduced form dihydro lipoic acid (DHLA) to scavenge ROS and chelate transition metals in Fenton reaction restricts the molecular damage by hydroxyl radicals and reduces cellular need for glutathione peroxidase (GPx) and catalase (CAT). ALA or DHLA prevents oxygen-induced DNA damage, exhibits chelating activity, reduces lipid peroxidation (LP) and prevents glycation of proteins (Packer et al. 2001; Biewenga et al. 1997; Maritim et al. 2003; Obrosova et al. 2003a, b; Maddux et al. 2001). The antioxidant responsiveness mediated by ALA has biological significance in eliminating reactive free radicals and restoring antioxidant enzymes that otherwise affect the normal functioning, especially brain.
The most widely used animal model for TD is the rat model in which vacuous chewing movements (VCMs) are induced by chronic neuroleptic treatment (Andreassen and Jorgensen 1995; Clow et al. 1979; Iversen et al. 1980; Gunne et al. 1982, 1986; Waddington 1990; Egan et al. 1995; Hyde et al. 1995; Kaneda et al. 1992; Shirakawa and Tamminga 1994; Andreassen and Jorgensen 1995; Andreassen et al. 1996).
The present investigation is designed to study the effect of ALA on the selected extrapyramidal movement disorders (parkinsonism, TD) produced by HAL in rats. A dose of 25, 50, 100 mg/kg of ALA is selected, as this dose was effective in increasing antioxidant status of brain and peripheral organs in various studies (Muthuswamy et al. 2006; Sethumadhavan and Chinnakannu 2006; Sahin et al. 2006; Arivazhagan et al. 2006).
Materials and methods
Animals
Male albino Wistar rats weighing 150–200 g bred in Central Animal House facility of our university were used. Male rats were chosen to avoid fluctuations due to estrous cycle-induced antioxidant effects (Sugioka et al. 1987). The rats were housed in polypropylene cages (five per cage) under standard laboratory conditions with 12-h light/dark cycle and were fed with standard laboratory chow (Hindustan Lever Ltd, Mumbai) and water ad libitum. Animals were acclimatized to the laboratory conditions prior to experimentation and all the experiments were carried out between 0900 and 1200 hours. The study protocol was approved by the Institutional Animal Ethical Committee and conducted according to the Indian National Science Academy guidelines for the use and care of experimental animals.
Induction of VCMs and catalepsy
Haloperidol (1 mg/kg/i.p.) was administered chronically to rats for a period of 21 days to induce VCMs and catalepsy (Sasaki et al. 1995; Naidu and Kulkarni 2001a, b; Naidu et al. 2003a, b; Naidu and Kulkarni 2004; Thaakur and Jyothi 2007). All the behavioral assessments were carried out 24 h after the last dose of HAL.
Assessment of behavioral parameters
Behavioral assessment of VCMs
On the test day, rats were placed individually in a small (30 × 20 × 30 cm) Plexiglass cage for the assessment of VCMs. The floor and back wall of cage consisted of mirrors so as to permit the observation of VCMs (frequencies) when the animal was facing back and down. VCMs were counted with the help of a hand-operated counter by trained observers. VCMs are referred to as single mouth openings in the vertical plane not directed toward a physical material and were counted for a period of 5 min and in all experiments scorer was unaware of the treatment given to the animal and counting was stopped whenever the rat began grooming and restarted when grooming stopped (Sasaki et al. 1995; Naidu and Kulkarni 2001a, b, 2004; Naidu et al. 2003a, b; Thaakur and Jyothi 2007).
Catalepsy
The catalepsy was measured by standard procedure using 3 and 9 cm wooden blocks (Goyal et al. 2006). The following scores were assigned to the rats: rats move normally when placed on table, score 0; rats move normally when touched/pushed, score 0.5; front paws of rat were placed on 3 cm block and if it fails to correct the posture in 10 s, a score of 0.5 was assigned to each paw (total 1). If the rat fails to correct the posture within 10 s, when placed on 9 cm block, score for each paw was 1 (total 2); thus for a single rat maximum score assigned was 3.5.
Locomotor activity
Locomotor activity or spontaneous motor activity (SMA) was assessed using Photoactometer (Inco, Ambala, India). The total count over 30-min period was recorded for each animal. Increase in count was regarded as central nervous system stimulant activity, whereas decrease in count was regarded as central nervous system depressant activity.
Biochemical measurements
On 21st day, blood samples were collected from retro-orbital plexus under light ether anesthesia for the estimation of total antioxidant status (TAS) and LP.
Estimation of TAS
0.1 ml of serum was deprotonated by the addition of 1 ml of methanol, vortexed for 30 s and centrifuged at 3,000 rpm for 30 min to separate the proteins. To the clear supernatant, 1.5 ml of methanol and 0.5 ml of diphenyl picryl hydrazine solution were added, mixed thoroughly and absorbance was read at 517 nm against blank, which was prepared in an identical way but without the addition of serum (Blios 1958).
Estimation of LP
0.1 ml of plasma was treated with 2 ml (0.37%) of TBA, 0.25 N hydrochloric acid and 15% trichloro acetic acid (1:1:1 ratio) and placed in water bath for 15 min, cooled and centrifuged and then clear supernatant was measured at 535 nm against reference blank (Nichans and Samuelson 1968).
Drugs and study protocol
Haloperidol was dissolved in 0.9% saline and injected intraperitoneally in a constant volume of 0.5 ml/kg body weight. Animals were divided into six groups:
-
Group I: orally received 0.2% carboxy methyl cellulose in a constant volume of 0.5 ml daily by oral gavage.
-
Group II: received HAL 1 mg/kg/i.p. in a constant volume of 0.5 ml/kg body weight for 21 days (0.9% saline).
-
Group III: received ALA 100 mg/kg/p.o. suspended in 0.2% CMC and was administered orally by oral gavage once (1 h before 0.9% saline) on the last day of treatment.
-
Group IV: received ALA 25 mg/kg/p.o. suspended in 0.2% CMC and was administered orally by oral gavage once (1 h before HAL 1 mg/kg/i.p. in 0.9% saline) on the last day of treatment.
-
Group V: received ALA 50 mg/kg/p.o. suspended in 0.2% CMC and was administered orally by oral gavage once (1 h before HAL 1 mg/kg/i.p. in 0.9% saline) on the last day of treatment.
-
Group VI: received ALA 100 mg/kg/p.o. suspended in 0.2% CMC and was administered orally by oral gavage once (1 h before HAL 1 mg/kg/i.p. in 0.9% saline) on the last day of treatment.
Statistical analysis
All values were expressed as mean ± SEM. The data were analyzed using Instat by one-way analysis of variance followed by Dunnett’s t test. P < 0.05 was considered significant.
Results
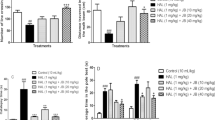
Figure 1 shows the effect of ALA on the HAL-induced VCMs. Significant increase in the number of VCMs was observed on the 21st day of treatment in HAL, ALA 25, 50 and 100 mg/kg treated groups when compared with 1st day of treatment. ALA at a dose of 100 mg/kg significantly decreased the VCMs when compared to HAL treatment group.
Figure 2 shows the effect of ALA on HAL-induced catalepsy. ALA significantly decreased the catalepsy at a dose of 25 mg/kg as compared to control group. When compared to HAL group, ALA significantly decreased the catalepsy at a dose of 25, 50 and 100 mg/kg, at a dose of 100 mg/kg it reversed the catalepsy induced by HAL.
Figure 3 shows the effect of ALA on HAL-induced alterations in locomotor activity. HAL significantly decreased the locomotor activity (P < 0.001) when compared to control group. ALA significantly reversed the HAL-induced decrease in locomotor activity as compared to HAL group at a dose of 25, 50 and 100 mg/kg (P < 0.001).
Figure 4 shows the effect of ALA on HAL-induced changes in TAS. Significant decrease in the total antioxidant levels was observed in HAL, ALA 25, 50, 100 mg/kg treated groups as compared to control group (P < 0.001).
Figure 5 shows the effect of ALA on HAL-induced LP. Significant increase in the lipid peroxide levels was observed in HAL, ALA 25, 50 mg/kg treated groups when compared to control group (P < 0.01), whereas ALA 100 mg/kg reversed the HAL-induced LP.
Discussion
In present study, HAL-treated animals showed increased frequencies of VCMs and catalepsy. Antipsychotic drugs produced two main kinds of motor disturbances in humans, TD and catalepsy, collectively termed as extrapyramidal side effects, which result directly or indirectly from D2-receptor blockade. These effects constitute one of the main disadvantages for the therapeutic use of typical neuroleptics such as HAL.
Numerous reports indicate that an excessive production of free radicals (OST) is associated with chronic neuroleptic use and contributes to the onset of TD and other movement disorders (Cadet et al. 1986; Pall et al. 1987; Lohr et al. 1987, 1990; Peet et al. 1993; Elkashef et al. 1990; Scapicchio et al. 1991; Egan et al. 1992; Adler et al. 1993a, b, 1998). OST is the shift in balance in cellular oxidation–reduction reactions in favor of oxidation, leading to cellular damage and is indicated by oxidized products of lipids, proteins and nucleic acids (Halliwell et al. 1994).
Neuroleptics act by blocking dopamine receptors (Creese et al. 1976), which in turn increase number of D2 receptor sites and increase catecholamine levels, leading to excess production of free radicals and as a consequence, hydrogen peroxide resulting in OST (Cohen and Spina 1989; Elkashef and Wyatt 1999), especially in catecholamine-rich areas such as the basal ganglia. HAL produces ROS in rat primary cortical neurons and the mouse hippocampal cell line HT-2 (Sagara 1998), by decreasing the genetic expression of superoxide dismutase (SOD), GPx and CAT and thus decreases the enzymatic activities and protein content of SOD, GPx, CAT (Parikh et al. 2003; Naidu et al. 2003a, b; Elkashef and Wyatt 1999; Shivakumar and Ravindranath 1993; Thaakur and Jyothi 2007; Von Voigtlander et al. 1990). Among antioxidant enzymes, SOD dismutases superoxide radicals to form hydrogen peroxide, which in turn is decomposed to water and oxygen by GPx, CAT, there by preventing the formation of hydroxyl radicals (Yao et al. 1998), these enzymes act co-operatively at different sites in the metabolic pathway of free radicals. Chronic HAL treatment increases LP (Parikh et al. 2003; Thaakur and Jyothi 2007) and also protein and nucleic acid peroxidation (Esterbauer et al. 1991), which are mutagenic and alters signal transduction and gene expression (Burcham 1998; Keller and Mattson 1998); and cytotoxic (Ravindranath and Reed 1990) neuronal loss in the striatum is reported in animals treated chronically with neuroleptics (Nielson and Lyon 1978; Skoblenick et al. 2006; Ukai et al. 2004).
Surprisingly, TAS was not improved at the given doses of ALA, in recent years there is increasing interest in assessing the total antioxidant capacity because of the difficulty in measuring each antioxidant component separately. Acute dose of ALA used in this study might be sufficient to scavenge-free radicals in specific brain areas such as striatum and protect them from OST and TD but might be insufficient to show systemic change in TAS of blood as observed in this study. However, importance of blood in this research area is increasing since it is rich in antioxidants as well as it reflects changes in the antioxidant activity in other less accessible tissues.
In the present study, lipid peroxides were significantly increased in HAL, ALA 25, 50 mg/kg treated groups, but ALA at a dose of 100 mg/kg significantly reversed the HAL-induced LP. LP is one of the well-established indices of cellular peroxidative membrane injury associated with increased OST and reduced membrane fatty acids (Mahadik et al. 1999). Living organisms developed complex antioxidant systems to reduce oxidative damage that include enzymes such as SOD, CAT, GPx, macromolecules such as albumin, ceruloplasmin, ferritin and an array of small molecules including ascorbic acid, alpha-tocopherol, carotene, uric acid, ubiquinol-10, methionine, reduced glutathione (Esterbaur et al. 1990; Pascand 1993). ALA is water insoluble and its reduced metabolite, DHLA, forms a redox couple. Free ALA is rapidly taken up by cells and reduces to dithiol (Freeman and Capro 1982); DHLA contributes to the antioxidant activity of ALA in vivo and its β oxidation products, bis nor and tetra nor lipoic acid, also contribute to the antioxidant activity (Biewenga et al. 1997). DHLA prevents oxidative damage by interacting with ROS and reactive nitrogen species (RNS) (Packer et al. 2001). ALA scavenges hydroxyl radicals, singlet oxygen and hypochlorous acid, and regenerates other antioxidants such as glutathione, vitamin C, ubiquinol (coenzyme Q10) and indirectly, vitamin E (Cadenas and Packer 1996; Roy and Packer 1998). In addition, DHLA is capable of reducing the oxidized forms of vitamin C, glutathione and coenzyme Q10, which regenerates oxidized alpha-tocopherol (vitamin E), forming an antioxidant network (Kramer and Packer 2001; Scholich et al. 1989). Certain free metal ions such as iron and copper induce oxidative damage by catalyzing reactions that generate highly reactive free radicals. Both ALA and DHLA chelate metal or bind ions in a way that it prevents them from generating free radicals (Zhang and Frei 2001).
HAL-induced TD and OST are effectively prevented by dietary supplementation with antioxidants and essential fatty acids (Mahadik and Gowda 1996; Mahadik and Scheffer 1996; Reddy and Yao 1996; Mahadik et al. 2001). Vitamin E (Adler et al. 1993a, b, 1998; Gupta et al. 1999; Soares and McGrath 2000; Elkashef and Wyatt 1999; Barak et al. 1998), melatonin (Shamir et al. 2000, 2001; Naidu et al. 2003a, b; Faria et al. 2005) and vitamin C are reported to be effective against HAL-induced oral dyskinesia. HAL-induced orofacial dyskinesia is attenuated by inhibiting nitric oxide synthase (Naidu et al. 2003a, b; Chen et al. 2001; Raso et al. 2001) and monoamino oxidase B activity (Lee et al. 2001); nitric oxide- and mono amine-induced free radicals are involved in the pathophysiology of orofacial dyskinesia (Naidu and Kulkarni 2001a). Our findings are in agreement with previous investigations which reported decrease in frequencies of HAL-induced TD by various antioxidants such as vitamin E, melatonin, carvedilol, selegiline, quercetin and faria ebselen.
Neuroleptic-induced catalepsy is used as an animal model for the study of extrapyramidal side effects such as parkinsonism, associated with antipsychotic use in humans (Naidu et al. 2003a, b; Pires et al. 2005). Catalepsy is linked to the blockade of post-synaptic striatal dopamine D1 and D2 receptors (Sanberg et al. 1988). Surprisingly, our results suggest that free radicals are also involved in the incidence of catalepsy; chronic blockade of D2 inhibitory DA receptors localized on glutaminergic terminals in the striatum leads to the persistent enhanced release of glutamate that kills the striatal output neurons (Naidu and Kulkarni 2001a, b).
Casey (1985a, b) proposed that chronic blockade of inhibitory dopamine receptors by HAL enhances catecholamine and/or glutamate release in the striatum, leading to chronic excitotoxic neurodegeneration. The competitive NMDA receptor antagonist SDZ 250-581, LY235959 (Chartoff et al. 1999) and MK-801, a non-competitive NMDA receptor antagonist reduced the cataleptogenic effect of HAL (Verma and Kulkarni 1992; Chartoff et al. 1999). Raised Ca2+ levels increase glutamate release, thereby activation of nitric oxide synthase, while low concentrations of NO are neuroprotective, high concentrations in the presence of ROS generate peroxynitrite and hydroxyl free radicals, which damages many important biomolecules including membrane lipids, proteins and DNA causing neurodegeneration.
Since antipsychotics are the drugs of choice for the treatment of psychiatric disorders, understanding their effects on OST and oxidative cell injury may be very important. In conclusion, ALA improves TD and catalepsy by scavenging hydroxyl radicals, singlet oxygen and hypochlorous acid, and regenerating other antioxidants such as glutathione, vitamin C, ubiquinol (coenzyme Q 10) and indirectly, vitamin E, its metabolite DHLA reduces oxidized forms of vitamin C, glutathione and coenzyme Q10, which regenerates oxidized alpha-tocopherol (vitamin E), forming an antioxidant network scavenges ROS and RNS.
Further research on the effect of chronic administration of ALA in the improvement of TD, catalepsy and its correlation with the indicators of OST in various brain regions may be needed to draw better conclusions.
References
Adler LA, Paselow E, Rotrosen J, Duncan E, Lee M, Rosenthal M, Angrist B (1993a) Vitamin E treatment of tardive dyskinesia. Am J Psychiatry 150:1405–1407
Adler LA, Paselow E, Rotrosen J, Duncan E, Rosenthal M, Angrist B (1993b) Vitamin E in tardive dyskinesia: time course of effect placebo substitution. Psychopharmacol Bull 29:371–374
Adler LA, Edson R, Lavori P, Peselow E, Duncan E, Rosenthal M, Rotrosen J (1998) Long term effects of vitamin E for tardive dyskinesia. Biol Psychiatry 43:868–872
American Psychiatric Association (APA) (1992) Tardive dyskinesia: a task force report of the American Psychiatric Association. APA Press, Washington DC
Andreassen OA, Jorgensen HA (1995) The rat model of tardive dyskinesia: relationship between vacuous chewing movements and gross motor activity during acute and long term haloperidol treatment. Life Sci 57:2263–2272
Andreassen OA, Aam TO, Jorgensen HA (1996) Inhibition by memantine of the development of persistent oral dyskinesias induced by long term haloperidol treatment of rats. Br J Pharmacol 119:751–757
Arivazhagan P, Ayusawa D, Panneerselvam C (2006) Protective efficacy of alpha lipoic acid on acetyl choline esterase activity in aged rat brain regions. Rejuvenation Res 9(2):198–201
Barak Y, Swartz M, Shamir E, Stein D, Weizman A (1998) Vitamin E in the treatment of tardive dyskinesia: a statistical meta-analysis. Ann Clin Psychiatry 10:101–105
Behl C, Rupprecht R, Skutella T, Holsboer F (1995) Haloperidol-induced cell death-mechanism and protection with vitamin E in vitro. Neuroreport 7:360–364
Biewenga GP, Haenen GR, Bast A (1997) The pharmacology of the antioxidant lipoic acid. Gen Pharmacol 29(3):315–331
Blios MS (1958) Antioxidant determination by the use of stable free radical. Nature 26:1199
Burcham PC (1998) Genotoxic lipid peroxidation products: their DNA damaging properties and role in formation of endogenous and role in formation of endogenous DNA adducts. Mutagenesis 13:287–305
Cadenas E, Packer L (1996) Handbook of antioxidants. Marcel Dekker, New York, pp 545–591
Cadet JL, Kahler LA (1994) Free radical mechanisms in schizophrenia and tardive dyskinesia. Neurosci Biobehav Rev 18:457–467
Cadet JL, Lohr JB (1989) Possible involvement of free radicals in neuroleptic-induced movement disorders. Ann N Y Acad Sci 570:176–185
Cadet JL, Lohr JB, Jeste DV (1986) Free radicals and tardive dyskinesia. Trends Neurosci 9:107–108
Casey DE (1985a) Spontaneous and tardive dyskinesias: clinical and laboratory studies. J Clin Psychiatry 46:42–47
Casey DE (1985b) Tardive dyskinesia: reversible and irreversible. Psychopharmacology 2:88–97
Chartoff EH, Ward RP, Dorsa DM (1999) Role of adenosine and NMDA receptors in mediating haloperidol-induced gene expression and catalepsy. J Pharmacol Exp Ther 291(2):591–597
Chen YC, Shen SC, Lee WR, Hou WC, Yang LL, Lee TJ (2001) Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expression by rutin, quercetin and quercetin penta acetate in RAW 264.7 macrophages. J Cell Biochem 82:537–548
Clow A, Jenner P, Marsden CD (1979) Changes in dopamine mediated behaviour during one year’s neuroleptic administration. Eur J Pharmacol 57:365–375
Cohen G, Spina MB (1989) Deprenyl suppresses the oxidant stress associated with increased dopamine turnover. Ann Neurol 26:689–690
Creese I, Burt DR, Snyder SH (1976) Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192:481–483
Egan MT, Hyde TM, Albers GW, Elkashef A, Alexander RC, Reeve A, Blum A, Saez RE, Wyatt RJ (1992) Treatment of tardive dyskinesia with vitamin E. Am J Psychiatry 149:773–777
Egan MF, Hyde TM, Kleinman JE, Wyatt RJ (1995) Neuroleptic-induced vacuous chewing movements in rodents: incidence and effects of long term increases in haloperidol dose. Psychopharmacology 117:74–81
Elkashef AM, Wyatt RJ (1999) Tardive dyskinesia: possible involvement of free radical and treatment with vitamin E. Schizophr Bull 25:731–740
Elkashef AM, Ruskin PE, Bacher N, Barett D (1990) Vitamin E in the treatment of tardive dyskinesia. Am J Psychiatry 147:505–506
Esterbaur H, Zollner H, Schaur RJ (1990) Aldehydes formed by lipid peroxidation mechanism of formation, occurrence and determination. In: Vigopelfrey C (ed) Membrane lipid peroxidation. CRC, Baco Raton
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxy alkenal, malondialdehyde and related aldehydes. Free Radic Biol Med 11:81–128
Faria RR, Abilio VC, Grassl C, Chinen CC, Negrao LTR, Castro JPMV, Fukshiro DF, Rodrigues MSD, Gomes PHZ, Registro S, Carvalho RCC, D’Almeida V, Silva RH, Ribeiro RA, Fussa-Filho R (2005) Beneficial effects of vitamin C and vitamin E on reserpine induced oral dyskinesia in rats: critical role of strital catalase activity. Neuropharmacology 48:993–1001
Freeman BA, Capro JD (1982) Biology of disease: free radicals and tissue injury. Lab Invest 47:412–425; Chem 243:5753–5756
Glazer WM, Morgenstern H, Schooler N, Berkman CS, Moore DC (1990) Predictors of improvement in tardive dyskinesia following discontinuation of neuroleptic medication. Br J Psychiatry 157:585–592
Goyal RK, Patel NM, Bhatt RV, Mehta AA, Prabhakhar MC (2006) Study of catalepsy in animals. In: Practicals in pharmacology, 5th edn. B S Shah Prakashan, Ahmedabad, pp 128
Gunne LM, Growdon J, Glaeser B (1982) Oral dyskinesia in rats following brain lesions and neuroleptic drug administration. Psychopharmacology 77:134–139
Gunne LM, Andersson U, Bondesson U, Johansson (1986) Spontaneous chewing movements in rats during acute and chronic antipsychotic drug administration. Pharmacol Biochem Behav 25:897–901
Gupta S, Monsik D, Black DW, Berry S, Masand PS (1999) Tardive dyskinesia; review of treatments past, present, and future. Ann Clin Psychiatry 11:257–266
Halliwell B, Gutteridge JMC, Carrol EC (1994) Free radicals, antioxidants and human disease: where are we now? J Lab Clin Med 119:598–619
Hyde TM, Egan MF, Wing LL, Wyatt RJ, Weinberger DR, Kleinman JE (1995) Persistent catalepsy associated with severe dyskinesias in rats treated with chronic injections of haloperidol decanoate. Psychopharmacology (Berl) 118:142–149
Iversen SD, Howells RB, Hughes RP (1980) Behavioral consequences of long-term treatment with neuroleptic drugs. Adv Biochem Psychopharmacol 24:305–313
Jeste DV, Potkin SG, Sinha S, Feder S, Wyatt RJ (1979) Tardive dyskinesia-reversible and persistent. Arch Gen Psychiatry 36:585–590
Kane JM, Smith JM (1982) Tardive dyskinesia: prevalence and risk factors, 1959 to 1979. Arch Gen Psychiatry 39:473–481
Kaneda H, Shirakawa O, Dale J, Goodman L, Bachus SE, Tamminga CA (1992) Co-administration of progabide inhibits haloperidol-induced oral dyskinesias in rats. Eur J Pharmacol 212:43–49
Keller JN, Mattson MP (1998) Roles of lipid peroxidation in modulation of cellular signaling pathways, cell dysfunction and death in the nervous system. Rev Neurosci 9:105–116
Kramer K, Packer L (2001) R-alpha lipoic acid. In: Kramer K, Hoppe P, Packer L (eds) Neutraceuticals in health and disease prevention. Marcel Dekker, New York
Lee MH, Lin RD, Shen LY, Yang YY, Yen KY, Hou WC (2001) Monoamino oxidase B and free radical scavenging activities of natural flavonoids in melastoma candidum D. Don J Argic Food Chem 49:5551–5555
Lohr JB, cadet JL, Lohr MA, Jeste DV, Wyatt RJ (1987) Alpha-tocopherol in tardive dyskinesia. Lancet 1:913–914
Lohr JB, Kuczenski R, Bracha HS, Moir M, Joste DV (1990) Increased indices of free radical activity in cerebrospinal fluid of patients with tardive dyskinesia. Biol Psychiatry 28:533–539
Maddux BA, See W, Lawrence JC, Goldfine AL, Goldfine ID, Evans JL (2001) Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by micro molar concentrations of alpha-lipoic acid. Diabetes 50:404–410
Mahadik SP, Gowda S (1996) Antioxidants in the treatment of schizophrenia. Drugs Today 32:553–565
Mahadik SP, Scheffer RE (1996) Oxidative injury and potential use of antioxidants of schizophrenia. Prostaglandins Leukot Essent Fatty Acids 55:45–54
Mahadik SP, Sitasawad V, Mulchandani M (1999) Membrane peroxidation and the neuropathology of schizophrenia. In: Peet M, Glen I, Horrobin DF (eds) Phospholipids spectrum disorders in psychiatry. Marius Press, Lancashire
Mahadik SP, Evans D, Lal H (2001) Oxidative stress and role of antioxidant and ω-3 essential fatty acid supplementation in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 25:463–493
Maritim AC, Sanders RA, Watkins JBIII (2003) The effect of α-lipoic acid on biomarkers of oxidative stress in streptozocin induced diabetic rats. J Nutr Biochem 14:88–294
Muthuswamy AD, Vedagiri K, Ganesan M, Chinnakannu P (2006) Oxidative stress mediated molecular damage and dwindle in antioxidant status in aged rat brain regions: role of l-carnitine and dl-alpha lipoic acid. Clin Chim Acta 368:84–92
Naidu PS, Kulkarni SK (2001a) Excitatory mechanisms in neuroleptic-induced vacuous chewing movements (VCMs): possible involvement of calcium and nitric oxide. Behav Pharmacol 12(3):209–216
Naidu PS, Kulkarni SK (2001b) Possible involvement of prostaglandins in haloperidol-induced orofacial dyskinesia in rats. Eur J Pharmacol 430:295–298
Naidu PS, Kulkarni SK (2004) Quercetin, a bioflavonoid, reverses haloperidol-induced catalepsy. Methods Find Exp Clin Pharmacol 26:323–326
Naidu PS, Singh A, Kaur P, Sandhir R, Kulkarni SK (2003a) Possible mechanism of action in melatonin attenuation of haloperidol-induced orofacial dyskinesia. Pharmacol Biochem Behav 74:641–648
Naidu PS, Singh A, Kulkarni SK (2003b) Quercetin, a bioflavonoid, attenuates haloperidol-induced orofacial dyskinesia. Neuropharmacology 44:1100–1106
Nichans WG, Samuelson D (1968) Formation of malondialdehyde from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130
Nielson EB, Lyon M (1978) Evidence for cell loss in corpus striatum after long term treatment with a neuroleptic drug (flupenthixol) in rats. Psychopharmacology 59:85–89
Obrosova IG, Fathallah L, Edwin L, Nourooz-Zadeh J (2003a) Early changes oxidative stress in the diabetic kidney: effect of dl-α-lipoic acid. Free Radic Boil Med 34(20):186–195
Obrosova IG, Fathallah L, Greene DA (2003b) Early changes in lipid peroxidation and antioxidative defense in diabetic rat retina: effect of dl-α-lipoic acid. Eur J Pharmacol 398:139–146
Packer L, Witt KH, Tristschler HJ (1995) Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med 19:227–250
Packer L, Kraemer K, Rimbach G (2001) Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 17:888–895
Pall HS, Williams AC, Blake DR, Lunec J (1987) Evidence of enhanced lipid peroxidation in the cerebrospinal fluid of patients taking phenothiazines. Lancet 2:596–599
Parikh V, Mohammad Khan M, Sahebarao Mahadik P (2003) Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res 37:43–51
Pascand M (1993) The essential polyunsaturated fatty acids of spirulina and our immune response. Bull Inst Oceanogr 12:49–57
Peet M, Langharne J, Rangarajan N, Renolds GP (1993) Tardive dyskinesia, lipid peroxidation and sustained amelioration with vitamin E treatment. Int Clin Psychopharmacol 8:151–153
Pires JG, Bonikovski V, Furturo-Neto HA (2005) Acute effects of selective serotonin reuptake inhibitors on neuroleptic-induced catalepsy in mice. Braz J Med Biol Res 38:1867–1872
Post A, Holsboer F, Behl C (1998) Induction of NF-kappaB activity during haloperidol-induced oxidative toxicity in clonal hippocampal cells: suppression of NF-kappaB and neuroprotection by antioxidants. J Neurosci 18:8236–8246
Raso GM, Meli R, Di Carlo R (2001) Inhibition of inducible nitric oxide synthase and cyclooxygenase expression by flavonoids in macrophage J773A.I. Life Sci 12:921–931
Ravindranath V, Reed DJ (1990) Glutathione depletion and formation of glutathione protein mixed disulfide following exposure of brain mitochondria to oxidative stress. Biochem Biophys Res Commun 169:150–158
Reddy R, Yao JK (1996) Free radical pathology in schizophrenia: a review. Prostaglandins Leukot Essent Fatty Acids 55:33–43
Roy S, Packer L (1998) Redox regulation of cell function by alpha-lipoate: biochemical and molecular aspects. Biofactors 7:263–267
Sagara Y (1998) Induction of reactive oxygen species in neurons by haloperidol. J Neurochem 71:1002–1012
Sahin M, Sagdic G, Elmas O, Akpinar D, Derin N, Asian M, Agar A, Aliciguzel Y, Yargicoglu P (2006) Effect of chronic resistant stress and alpha lipoic acid on lipid peroxidation and antioxidant enzyme activity in rat peripheral organs. Pharmacol Res 54(3):247–252
Sanberg PR, Bunsey MD, Giordano M et al (1988) The catalepsy test: its ups and downs. Behav Neurosci 102:748–759
Sasaki H, Hashimoto K, Maeda Y, Inada T, Kitao Y, Fukui S, Iyo M (1995) Rolipram, a selective c-AMP phosphodiesterase inhibitor suppresses oro-facial dyskinetic movements in rats. Life Sci 56:443–447
Scapicchio P, Decina P, Mukherjee S, Caracci G (1991) Effects of α-tocopherol on persistent tardive dyskinesia in elderly schizophrenic patients. Ital J Psychiatry Behav Sci 1:111–114
Scholich H, Murphy ME, Sies H (1989) Antioxidant activity of dihydrolipoate against microsomal lipid peroxidation and its dependence on alpha-tocopherol. Biochem Biophys Acta 1001:256–261
See RE (1991) Striatal dopamine metabolism increases during long-term haloperidol administration in rats but shows tolerance in response to acute challenge with raclopride. Neurosci Lett 129:265–268
Sethumadhavan S, Chinnakannu P (2006) l-Carnitine and alpha lipoic acid improve age associated decline in mitochondrial respiratory activity of rat heart muscle. J Gerontol A Biol Sci Med Sci 61(7):650–659
Sies H (1985) Oxidative stress: introductory remarks. In: Sies H (ed) Oxidative stress. Academic, London, pp 1–5
Shamir E, Barak Y, Plopsky I, Zisapel N, Elizur A, Weizman A (2000) Is melatonin treatment effective for tardive dyskinesia? J Clin Psychiatry 61:556–558
Shamir E, Barak Y, Shalman I, Laudon M, Zisapel N (2001) Melatonin treatment for tardive dyskinesia. Arch Gen Psychiatry 58:1049–1052
Sharma S, Paladino P, Gabriele J, Saeedi H, Henry P, Chang M, Mishra RK, Johnson RL (2003) Pro-Leu-glycinamide and its peptidomimetic, PAOPA, attenuate haloperidol induced vacuous chewing movements in rat: a model of human tardive dyskinesia. Peptides 2:313–319
Shirakawa O, Tamminga CA (1994) Basal ganglia GABA and dopamine D1 binding site correlates of haloperidol-induced oral dyskinesias in rat. Exp Neurol 127:62–69
Shivakumar BR, Ravindranath V (1993) Oxidative stress and thiol modification induced by chronic administration of haloperidol. J Pharmacol Exp Ther 265:1137–1141
Skoblenick KJ, Castellano JM, Rogoza RM, Dyck BA, Thomas N, Gabriele JP, Chong VZ, Mishra RK (2006) Translocation of AIF in the human and rat striatum following protracted haloperidol, but not clozapine treatment. Apoptosis 11(5):663–672
Soares KV, McGrath JJ (2000) Vitamin E for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev 2:CD000209
Subramaniam B, Rollema H, Woolf T, Castagnoli NG (1990) Identification of a potentially neurotoxic pyridinium metabolite of haloperidol in rats. Biochem Biophys Res Commun 166:238–244
Sugioka K, Shimosegawa Y, Nakano M (1987) Estrogens as natural antioxidants of membrane phospholipids peroxidation. FEBS Lett 210:37–39
Thaakur SR, Jyothi B (2007) Effect of spirulina maxima on the tardive dyskinesia and oxidative stress induced by haloperidol in rats. J Neural Transm. doi:10.1007/S00702-007-0744
Ukai W, Ozawa H, Tateno M, Hashimoto E, Saito T (2004) Neurotoxic potential of haloperidol in comparison with risperidone: implication of Akt-mediated signal changes by haloperidol. J Neural Transm 111:667–681
Verma A, Kulkarni SK (1992) D1/D2 dopamine and NMDA receptor participation in experimental catalepsy in rats. Psychopharmacology 109(4):477–483
Von Voigtlander PF, Burian MA, Althaus JS, Willams LR (1990) Effects of chronic haloperidol on vitamin E levels and monoamine metabolism in rats fed normal and vitamin E deficient diets. Res Commun Chem Pathol Pharmacol 68(3):343–352
Waddington JL (1990) Spontaneous orofacial movements induced in rodents by very long-term neuroleptic drug administration. Phenomenology, pathophysiology and putative relationship to tardive dyskinesia. Psychopharmacology 101:431–447
Yao JK, Reddy R, Elhinny LG, Vankammen DP (1998) Effects of haloperidol on antioxidant defense system enzymes in schizophrenia. J Psychiatr Res 32:385–391
Zhang WJ, Frei B (2001) Alpha lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J 15(13):2423–2432
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thaakur, S., Himabindhu, G. Effect of alpha lipoic acid on the tardive dyskinesia and oxidative stress induced by haloperidol in rats. J Neural Transm 116, 807–814 (2009). https://doi.org/10.1007/s00702-009-0232-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-009-0232-y