Abstract
Phospholipase A2 (PLA2) is involved in important aspects of dementia, for example neurotransmission and memory processing, membrane function, choline availability, and antioxidative defense. Reduced PLA2-activity has been reported so far in blood samples and postmortem neuronal tissue in Alzheimer disease. For the first time, we studied PLA2 in cerebrospinal fluid (CSF) in Alzheimer disease (AD), vascular (VD), and mixed Alzheimer/vascular dementia (MD). Intracellular PLA2 was assessed in CSF of 16 AD, 12 VD, 15 MD patients, and 19 healthy control subjects. A fluorometric assay was applied using the PLA2-specific substrate NBDC6-HPC. Significantly reduced PLA2 activity was not only found in AD, but also in VD and MD. This finding was independent of demographic co-variates and medication. PLA2 results in CSF corroborate previous findings of impaired PLA2 function in Alzheimer’s disease and extend these to patients with VD. They are likely to reflect an involvement of PLA2 impairment in a variety of pathomechanisms crucial in different dementia subtypes, in which disruption of cholinergic neurotransmission and disturbance of intact membrane function appear to be the key mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of patients suffering from dementia is constantly rising, in Germany from an estimated 935,000 in the year 2000 to approximately 2.3 million by 2050 (Bickel 2000). Therefore, research into parameters relevant to diagnosis and therapy of dementia is of great interest. Activity of intracellular phospholipases of the A2-type (PLA2), acting as key enzymes of membrane repair and remodeling [“housekeeping” enzymes (McLean et al. 1993)], is an interesting target of this research, as it allows the characterization of biologically active processes at cell membrane level. Generally, PLA2 enzymes catalyze the hydrolysis of the middle ester bond of membrane phospholipids, to which a polyunsaturated fatty acid, in turn acting as a second messenger, is often bound (Six and Dennis 2000). Due to this important cell-metabolic function, enzymes of the PLA2 family are involved in numerous processes known to be disturbed in dementia, for example, exocytosis as one aspect of neurotransmission (Bloch-Shilderman et al. 2002; Ray et al. 1999), generation of acetylcholine (Blusztajn et al. 1987; Farooqui et al. 1992), induction of memory by long term potentiation (Fujita et al. 2001; Wolf et al. 1995), memory processing (Hölscher and Rose 1994; Hölscher et al. 1995; Fujita et al., 2000; Sato et al. 2007; Schaeffer and Gattaz 2005, 2007), maintenance of membrane fluidity with influence on receptor function (Farooqui et al. 2004), and antioxidative defense mechanisms (Farooqui et al. 2000).
The group of phospholipase A2 enzymes constitutes a so called “superfamily”, involving three major groups: (1) secretory (extracellular) Ca2+-dependent PLA2 (sPLA2); (2) cytosolic Ca2+-dependent PLA2 (cPLA2); and (3) intracellular Ca2+-independent PLA2 (iPLA2) (Dennis 1994; Sun et al. 2004; Balsinde et al. 1999; Taketo and Masahiro 2002). Previous studies on changes of intracellular PLA2 activity in cases of dementia focused on platelets or post mortem brain tissue of patients with Alzheimer disease (AD), showing a decrease in enzyme activity in almost all studies (Gattaz et al. 1996b, 2004; Ross et al. 1998; Talbot et al. 2000). This is of interest, as other psychiatric disorders were associated with different PLA2 findings, such as increased PLA2 activity in schizophrenia (Gattaz et al. 1987, 1990, 1995; Lasch et al. 2003; Noponen et al. 1993; Ross et al. 1997, 1999; Smesny et al. 2005; Tavares et al. 2003), and no PLA2 alteration in depression or bipolar disorder (Albers et al. 1993; Gattaz et al. 1987, 1990, 1995; Katila et al. 1997; Noponen et al. 1993; Ross et al. 1999). In schizophrenia, increased PLA2 activity is interpreted as reflecting an ongoing regenerative process compensatory to neurotoxic effects of the acute psychotic state (Law et al. 2006). An understanding of PLA2 decrease in AD is still lacking. Furthermore, studies on specificity of PLA2 alterations among different dementia disorders, including patients with non-Alzheimer dementia, are still not available. To our knowledge, PLA2 activity has also not been investigated in CSF as yet. Therefore, we investigated PLA2 activity for the first time in CSF, including not only patients with AD, but also patients with vascular dementia (VD) and mixed dementia (MD means AD and VD pathology) in order to detect the alterations of PLA2 in the CNS compartment and also in different dementia subtypes.
Methods and materials
Subjects
A total of 101 subjects were screened between 2004 and 2007 for participation in this study. Patients with inflammatory or infectious diseases (n = 17) or those who did not allow a doubtless diagnosis of dementia (n = 22) were excluded, leaving a total of 62 subjects for whom cerebrospinal fluid (CSF) was investigated (demographical data in table 1). All subjects of the patient group were included from consecutive admissions to the geriatric psychiatry unit at the Department of Psychiatry, University of Jena (inclusion criteria: Mini Mental Status Test score ≤23; Clock drawing test score ≥3; see also Table 2). Subjects of the control group were included at the Departments of Neurology and Anesthesiology of the University of Jena. Thus in patients, CSF was taken as part of the routine diagnostics for dementia. In controls, CSF was taken in the context of epidural anesthesia or to exclude neurological disorders. For the healthy control group, we only included subjects found to be free of any CNS inflammatory/infectious disease. While co-variance and correlation analysis to control for effects of age, gender, or medication (statins, acetylsalicylic acid, cholinesterase inhibitors) did not show any influence of these co-variates on the CSF-PLA2-activity, the mean age of patients and controls was significantly different. Neither patients nor controls were given any medication other than mentioned above.
CSF samples were acquired in the morning by lumbar puncture using atraumatic cannula, and were immediately divided into two or more sub-samples. One sub-sample was used for routine diagnostics (cell count, protein content, microbiology, tau-Protein, β-Amyloid etc.), the other sub-sample underwent immediate centrifugation to remove cell debris and was stored at −80°C until PLA2 analysis.
To assure the clinical and screening diagnosis of dementia, all patients underwent an extensive diagnostic program (procedures and cut-off values are given in Table 2).
AD and VD were differentiated by standardized criteria taking into account the history, clinical presentation and structural abnormalities [assessed according to the National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l′Enseignement en Neuroscience (NINDS–ARIEN) criteria (Roman et al. 1993), and the Alzheimer Disease Diagnostic and Treatment Center (ADDTC) criteria, the latter also proposing the definition of “mixed” categories, as used in this study (MD)(Chui et al. 1992)], neurocognitive testing (consortium to establish a registry for Alzheimer’s Disease (CERAD)-series (Morris 1997; Morris et al., 1989), the Nuremberg Aging Inventory (NAI: Nürnberger Altersinventar) (Oswald and Fleischmann 1999), and established diagnostic CSF parameters (phosphorylated Tau-Protein, β-Amyloid 42/40 ratio). Intending to investigate a naturalistic population, a group of patients with MD was established, including patients with features of both AD and VD (e.g. increased Tau-Protein and decreased β-Amyloid-Ratio and SAE, vascular risk factors etc.).
The study was approved by the Research Ethics Committee of Friedrich-Schiller-University Jena. All subjects or their legal guardians gave written informed consent to participate in the study.
Analysis of PLA2 activity
Most of the intracellular PLA2 enzymes investigated here need calcium in micromolar concentrations at most (cPLA2) or are completely independent of calcium (iPLA2). Therefore, according to the most recent genetically defined classification, PLA2 activity investigated in this study comprises most likely activity of group IV and group VI type isoenzymes (Sun et al. 2004). This classification of our target enzyme activity is based on previous methodical investigations (Lasch et al. 2003) and the actual adaptation of the serum PLA2 assay on CSF. This research showed an almost complete (more than 90%) inhibitory effect of calcium ions on enzyme activity and a 70% inhibition of the enzyme activity by bromoenole lactone (BEL), a suicide inhibitor of iPLA2 (Jenkins et al. 2002; Lucas et al. 2005; Song et al. 2006; White and McHowat 2007). The use of selective antibodies could reveal that PLA2 activity in blood serum and CSF results from identical enzyme proteins. Previous research also revealed that our results do not reflect the activity of PAF-Hydrolases, as PAF-Hydrolases do not cleave the used commercial substrate. There was also no reaction with PAF-Hydrolase antibodies.
Thus, the PLA2 subtypes and PLA2 assay were basically the same as has been already established for our investigations in schizophrenia patients (Lasch et al. 2003; Smesny et al. 2005). However, the reaction stock was now adapted to the requirements of measurements in CSF. Briefly, it included 80 μl undiluted CSF, 10 μl HEPES buffer (N-2-hydroxyethylpiperazine-N-2-ethane-sulfonic acid, pH 7.4, 0.4 M) and 10 μl of the commercial fluorogenic substrate NBDC6-HPC (2-(6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine, Molecular Probes, Europe BV Leiden, The Netherlands). The reaction batch was then incubated for 60 min at a temperature of 37°C. Finally, a separation of the reaction products using thin-layer chromatography and digital image scanning for signal detection followed as described in more detail by Lasch and colleagues (Lasch et al. 2003). Storage time of CSF samples before PLA2 analysis differed between 4 and 73 days (mean ± standard deviation: 38 ± 25). In both patients and controls, there was no association between the storage interval and enzyme activity (patients: r = −2.3, n.s.; controls: r = −1.4, n.s.).
Data analysis
Statistical procedures included analyses of variance for general effects and post hoc tests (significance level α = 0.05). Possible effects of gender and medication were investigated by co-variate analysis and (if possible) subgroup analysis. Possible effects of age or duration of CSF storage were investigated by calculating correlation coefficients. For post hoc analysis, the double sample t-Test was used when variance was equal, and the Welch Test (a more robust version of the t-Test) when variances differed (significance level α = 0.05).
Results
Comparison of groups
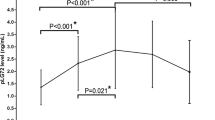
Initial ANOVA revealed a main effect of GROUP, indicating differences of CSF-PLA2 activity between patients with AD, VD, MD, and healthy controls (F 3;61 = 3.399; P = 0.024). Post hoc analyses showed significantly smaller PLA2 values in patients with AD (t 27;0.975 = 2.332; P = 0.027), patients with VD (t 27;0.975 = 2.67; P = 0.013, excluding one extreme value), and in patients with MD (t 27;0.975 = 2.575; P = 0.016) as compared to controls (Fig. 1). Also, there was significantly reduced PLA2 activity comparing a merged sample of AD and MD patients with controls (t 23;0.975 = 2.62; P = 0.015).
Effects of age and gender
Age: There was no correlation between age and PLA2 values in either the total sample (r = −0.44; n.s.) or healthy subjects (r = −0.13; n.s.).
Gender: ANOVA with GROUP as between-subject factor and GENDER as co-variate revealed no significant effect of the factor GENDER on PLA2 activity (F1;61 = 3.315; n.s.) and no significant GROUP × GENDER interaction (F3;61 = 0.564; n.s.).
Effects of medication
We tested the potential effects of statins, acetylsalicylic acid (max. 100 mg/day), and cholinesterase inhibitors by comparing medicated and non-medicated subjects in each case. ANOVA did not indicate any significant influence of either statins (F 1;55 = 2.317; n.s.), aspirin (F 1;43 = 1.526; n.s.), or cholinesterase inhibitors (F 1;61 = 0.361; n.s.) on PLA2 values.
Discussion
The study revealed two main findings: (1) decreased activity of intracellular PLA2 in CSF of patients with AD. (2) Decreased activity of intracellular PLA2 in CSF of patients with MD and VD. As in previous studies, PLA2 findings were not significantly affected by either age or gender (Smesny et al. 2005), nor by concurrent medication with acetylcholinesterase inhibitors (Gattaz et al. 2004), statins or acetylsalicylic acid (aspirin). There was also no correlation of PLA2 activity with any of the cognitive or routine clinical parameters (among others CSF tau-protein and beta-amyloid).
The first result of decreased PLA2 activity in CSF of patients suffering from AD is in good agreement with results of other groups investigating platelets and postmortem brain tissue of patients with AD (Gattaz et al. 1996a, 2004; Ross et al. 1998; Talbot et al. 2000). This is to our knowledge the first study directly showing PLA2 abnormalities in the CNS compartment of patients suffering from AD. Considering that in total 31 patients (AD + MD) were identified with features of AD, the study also included one of the largest samples being investigated in this field so far.
We are not aware of any study till date, investigating PLA2 activity in dementia other than AD. In our study, decreased PLA2 activity was not exclusively associated with Alzheimer-type pathology. Including patients with non-Alzheimer disease, we were also able to show decreased PLA2 activity in CSF of patients suffering from VD. Decreased PLA2 activity in non-Alzheimer dementia is suggestive of an underlying pathomechanism, common to these different dementias. Indeed, shared pathophysiology of AD and VD has been also repeatedly discussed in the literature (Hentschel et al. 2005; Jellinger 2002; Kalaria and Ballard 1999). Some of them require intact PLA2 function and are therefore of interest here, especially with regards to cholinergic dysfunction, oxidative stress, and disturbances of membrane function.
Wide agreement exists about the substantial role of a cholinergic deficit and a disruption of cholinergic neurotransmission in AD (Gsell et al. 1996, 2004) as well as in VD (Pratt and Perdomo 2002; Tomimoto et al. 2005), representing a major correlate of cognitive deficits (Bierer et al. 1995). The formation of free choline triggered by PLA2-catalyzed hydrolysis of phosphatidylcholine (Blusztajn et al. 1987; Farooqui et al. 1992) is an important molecular pathway for de novo synthesis of acetylcholine in cholinergic neurons. This is in accordance with findings of induction of phosphatidylcholine synthesis followed by increased PLA2 activity (Barbour et al. 1999). Hence, reduced activity of intracellular PLA2 could aggravate or even cause the cholinergic deficit seen in AD and VD. In addition, impaired function of PLA2 enzymes has also general effects on neurotransmission, for example both the cPLA2 and the iPLA2 subtype are involved in exocytosis (Bloch-Shilderman et al. 2002), whereas the Ca2+ activated cPLA2 is involved in the release of neurotransmitters (Ray et al. 1999).
The most striking clinical feature of dementia is memory impairment, often starting with impaired short-term memory function. Changes in short term memory in particular can be traced back to the interference of long-term potentiation (Chapman et al. 1999; Dawson et al. 1999; Morton et al. 2002), which is also impaired in patients with AD (Battaglia et al. 2007). Interestingly, five different animal studies were able to show a direct degradation of memory functions through the intra-cerebral inhibition of PLA2 (Fujita et al. 2000; Hölscher and Rose 1994; Schaeffer and Gattaz 2005, 2007, Sato et al. 2007), presumably also explained by the involvement of PLA2 in long-term potentiation (Fujita et al. 2001; Wolf et al. 1995).
Thirdly, intracellular PLA2 subtypes are crucially involved in membrane repair and remodeling processes. Inhibition of cPLA2 and iPLA2 was shown to result in reduction of membrane fluidity (Schaeffer et al., 2005), which in turn was associated with memory deficits in animal models (Clarke et al. 1999; Hong 1995) and in patients with AD (Eckert et al. 2000; Mecocci et al. 1996, 1997).
Reduced availability of choline-containing compounds, disturbance of exocytosis, interference of long-term potentiation, and impaired membrane fluidity are inter-related through their dependency on intact membrane lipid metabolism. As nervous tissue is naturally highly vulnerable to lipid peroxidation (due to high number of double bounds in membrane phospholipids), one important reason for membrane lipid alterations is increased oxidative stress. Increased lipid peroxidation was repeatedly found in both AD (Mattson 2002; Nunomura et al. 2001; Perry et al. 2002; Sayre et al. 1997), and VD (Ihara et al. 1997; Paragh et al. 2002). Acting as a “housekeeping enzyme” of membrane reconstruction, intracellular PLA2 counteracts increased oxidative stress in hydrolyzing peroxidized fatty acids (Baba et al. 1993; McLean et al. 1993; Salgo et al. 1993; van den Berg et al. 1993). In the case of reduced PLA2 activity, restricted neuronal regeneration mechanisms might promote damages resulting from lipid peroxidation processes.
Our finding of decreased PLA2 activity in both AD and VD indicates PLA2 deregulation, possibly not to be specifically associated with Alzheimer-type pathology. Considering the finding of decreased PLA2 activity as a secondary effect of cellular changes, two different mechanisms are plausible: (1) the functional capacity of PLA2 is exceeded due to increased need for membrane repair processes associated with the disorder. This assumption would also explain the finding of increasing loss of PLA2 function with severity of illness, reported by other groups (Gattaz et al. 1996a, 2004). (2) Decreased activity of PLA2 could also be caused by primary disturbance of enzyme protein function or enzyme regulation. In that case, one would expect decreased PLA2 activity already in the early state of dementia (mild cognitive impairment) or even before clinical manifestation. However, this aspect has not been investigated in detail.
In summary, this first study of intracellular PLA2 activity in CSF corroborates the finding of impaired PLA2 function in AD and extends these to patients with VD. Taken together, our results are likely to reflect an involvement of PLA2 impairment in a variety of pathomechanisms crucial in different dementia subtypes, in which disturbance of intact membrane function appears to be a key mechanism. Membrane function in turn is markedly susceptible to increased oxidative stress, which is counteracted by PLA2. Further studies might characterize the potential of PLA2 activity to serve as marker of the individual capacity to resist to oxidative damage and the related pathology occurring in dementia. This research in the prodromal or early phase of disorder could initiate new preventative, diagnostic, and therapeutic approaches, which, especially against the backdrop of ever increasing proportions of older people in our society, would be of immense importance.
References
Albers M, Meurer H, Marki F, Klotz J (1993) Phospholipase A2 activity in serum of neuroleptic-naive psychiatric inpatients. Pharmacopsychiatry 26:94–98
Baba N, Nikami Y, Shigeta Y, Nakajima S, Kaneko T, Matsuo M (1993) Hydrolysis of glycerophosphocholine hydroperoxide by phospholipase A2. Biosci Biotechnol Biochem 57:2200–2201
Balsinde J, Balboa MA, Insel PA, Dennis EA (1999) Regulation and inhibition of phospholipase A2. Annu Rev Pharmacol Toxicol 39:175–89
Barbour SE, Kapur A, Deal CL (1999) Regulation of phosphatidylcholine homeostasis by calcium-independent phospholipase A2. Biochim Biophys Acta 1439:77–88
Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E, Nixon RA (2007) Cortical plasticity in Alzheimer’s disease in humans and rodents. Biol Psychiatry 62:1405–1412
Bickel H (2000) Dementia syndrome and Alzheimer disease: an assessment of morbidity and annual incidence in Germany. Health Care 62:211–218
Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, Perl DP, Schmeidler J, Kanof P, Davis KL (1995) Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem 64:749–760
Bloch-Shilderman E, Abu-Raya S, Trembovler V, Boschwitz H, Gruzman A, Linial M, Lazarovici P (2002) Pardaxin stimulation of phospholipases A2 and their involvement in exocytosis in PC–12 cells. J Pharmacol Exp Ther 301:953–962
Blusztajn JK, Liscovitch M, Richardson UI (1987) Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc Natl Acad Sci USA 84:5474–5477
Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2:271–276
Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R (1992) Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology 42:473–480
Clarke MS, Prendergast MA, Terry AV Jr (1999) Plasma membrane ordering agent pluronic F–68 (PF–68) reduces neurotransmitter uptake and release and produces learning and memory deficits in rats. Learn Mem 6:634–649
Cohen CA, Gold DP, Shulman KI, Wortley JT, McDonald G, Wargon M (1993) Factors determining the decision to institutionalize dementing individuals: a prospective study. Gerontologist 33:714–720
Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O’Dowd G, Bowery BJ, Boyce S, Trumbauer ME, Chen HY, Van der Ploeg LH, Sirinathsinghji DJ (1999) Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience 90:1–13
Dennis EA (1994) Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem 269:13057–13060
Eckert GP, Cairns NJ, Maras A, Gattaz WF, Muller WE (2000) Cholesterol modulates the membrane-disordering effects of beta-amyloid peptides in the hippocampus: specific changes in Alzheimer’s disease. Dement Geriatr Cogn Disord 11:181–186
Farooqui AA, Hirashima Y, Horrocks LA (1992) Brain phospholipases and their role in signal transduction. In: Bazan NG, Toffano G, Murphy M (eds) Neurobiology of essential fatty acids. Plenum Press, New York, pp 11–25
Farooqui AA, Horrocks LA, Farooqui T (2000) Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem Phys Lipids 106:1–29
Farooqui AA, Ong WY, Horrocks LA (2004) Biochemical aspects of neurodegeneration in human brain: involvement of neural membrane phospholipids and phospholipases A2. Neurochem Res 29:1961–1977
Folstein MF, Folstein SE, Hugh PR (1975) “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinican. J Psychiatr Res 12:189–198
Fujita S, Ikegaya Y, Nishikawa M, Nishiyama N, Matsuki N (2001) Docosahexaenoic acid improves long-term potentiation attenuated by phospholipase A2 inhibitor in rat hippocampal slices. Br J Pharmacol 132:1417–1422
Fujita S, Ikegaya Y, Nishiyama N, Matsuki N (2000) Ca2+-independent phospholipase A2 inhibitor impairs spatial memory of mice. Jpn J Pharmacol 83:277–278
Gattaz WF, Kollisch M, Thuren T, Virtanen JA, Kinnunen PK (1987) Increased plasma phospholipase-A2 activity in schizophrenic patients: reduction after neuroleptic therapy. Biol Psychiatry 22:421–426
Gattaz WF, Hubner CV, Nevalainen TJ, Thuren T, Kinnunen PK (1990) Increased serum phospholipase A2 activity in schizophrenia: a replication study. Biol Psychiatry 28:495–501
Gattaz WF, Schmitt A, Maras A (1995) Increased platelet phospholipase A2 activity in schizophrenia. Schizophr Res 16:1–6
Gattaz WF, Cairns NJ, Levy R, Forstl H, Braus DF, Maras A (1996a) Decreased phospholipase A2 activity in the brain and in platelets of patients with Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 246:129–131
Gattaz WF, Levy R, Cairns NJ, Forstl H, Braus DF, Maras A (1996b) Relevance of metabolism of membrane phospholipids for Alzheimer dementia. Prog Neurol Psychiatry 64:8–12
Gattaz WF, Forlenza OV, Talib LL, Barbosa NR, Bottino CM (2004) Platelet phospholipase A2 activity in Alzheimer’s disease and mild cognitive impairment. J Neural Transm 111:591–601
Gsell W, Strein I, Riederer P (1996) The neurochemistry of Alzheimer type, vascular type and mixed type dementias compared. J Neural Transm 47:73–101
Gsell W, Jungkunz G, Riederer P (2004) Functional neurochemistry of Alzheimer’s disease. Curr Pharm Des 10:265–293
Hentschel F, Supprian T, Frölich L (2005) Alzheimer’s dementia versus vaskular dementia—dichotomia or interaction? Prog Neurol Psychiatry 73:317–326
Hölscher C, Rose SP (1994) Inhibitors of phospholipase A2 produce amnesia for a passive avoidance task in the chick. Behav Neural Biol 61:225–232
Hölscher C, Canevari L, Richter-Levin G (1995) Inhibitors of PLA2 and NO synthase cooperate in producing amnesia of a spatial task. Neuroreport 6:730–2
Hong A (1995) The neural basis of learning and memory declines in aged rats. Sheng Li Ke Xue Jin Zhan 26:240–242
Ihara Y, Hayabara T, Sasaki K, Fujisawa Y, Kawada R, Yamamoto T, Nakashima Y, Yoshimune S, Kawai M, Kibata M, Kuroda S (1997) Free radicals and superoxide dismutase in blood of patients with Alzheimer’s disease and vascular dementia. J Neurol Sci 9:76–81
Jellinger KA (2002) Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm 109:813–836
Jenkins CM, Han X, Mancuso DJ, Gross RW (2002) Identification of calcium-independent phospholipase A2 (iPLA2) beta, and not iPLA2gamma, as the mediator of arginine vasopressin-induced arachidonic acid release in A–10 smooth muscle cells. Enantioselective mechanism-based discrimination of mammalian iPLA2 s. J Biol Chem 277:32807–14
Kalaria RN, Ballard C (1999) Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord 13:115–123
Katila H, Appelberg B, Rimon R (1997) No differences in phospholipase-A2 activity between acute psychiatric patients and controls. Schizophr Res 26:103–105
Lasch J, Willhardt I, Kinder D, Sauer H, Smesny S (2003) Fluorometric assays of phospholipase A2 activity with three different substrates in biological samples of patients with schizophrenia. Clin Chem Lab Med 41:908–914
Law MH, Cotton RG, Berger GE (2006) The role of phospholipases A2 in schizophrenia. Mol Psychiatry 11:547–556
Lucas KK, Svensson CI, Hua XY, Yaksh TL, Dennis EA (2005) Spinal phospholipase A2 in inflammatory hyperalgesia: role of group IVA cPLA2. Br J Pharmacol 144:940–952
Mattson MP (2002) Oxidative stress, perturbed calcium homeostasis, and immune dysfunction in Alzheimer’s disease. J Neurovirol 8:539–550
McLean LR, Hagaman KA, Davidson WS (1993) Role of lipid structure in the activation of phospholipase A2 by peroxidized phospholipids. Lipids 28:505–509
Mecocci R, Cherubini A, Beal MF, Cecchetti R, Chionne E, Polidori MC, Romano G, Senin U (1996) Altered mitochondrial membrane fluidity in AD brain. Neurosci Lett 207:129–132
Mecocci P, Beal MF, Cecchetti R, Polidori MC, Cherubini A, Chionne F, Avellini L, Romano G, Senin U (1997) Mitochondrial membrane fluidity and oxidative damage to mitochondrial DNA in aged and AD human brain. Mol Chem Neuropathol 31:53–64
Morris JC (1997) Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 9:173–176
Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C (1989) The consortium to establish a registry for alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39:1159–1165
Morton RA, Kuenzi FM, Fitzjohn SM, Rosahl TW, Smith D, Zheng H, Shearman M, Collingridge GL, Seabrook GR (2002) Impairment in hippocampal long-term potentiation in mice under-expressing the Alzheimer’s disease related gene presenilin-1. Neurosci Lett 319:37–40
Noponen M, Sanfilipo M, Samanich K, Ryer H, Ko G, Angrist B, Wolkin A, Duncan E, Rotrosen J (1993) Elevated PLA2 activity in schizophrenics and other psychiatric patients. Biol Psychiatry 34:641–649
Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA (2001) Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 60:759–767
Oswald WD, Fleischmann UM (1999) Nürnberger-Alters-Inventar (NAI). Testmanual und Textband, Hogrefe, Göttingen
Paragh G, Balla P, Katona E, Seres I, Egerhazi A, Degrell I (2002) Serum paraoxonase activity changes in patients with Alzheimer’s disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci 252:63–67
Perry G, Cash AD, Smith MA (2002) Alzheimer's disease and oxidative stress. J Biomed Biotechnol 2:120–123
Pratt RD, Perdomo CA (2002) Donepezil-treated patients with probable vascular dementia demonstrate cognitive benefits. Ann NY Acad Sci 977:513–522
Ray P, Ishida H, Millard CB, Petrali JP, Ray R (1999) Phospholipaise A2 and arachidonic acid-mediated mechanism of neuroexocytosis: a possible target of botidinum neurotoxin A other then SNAP-25. J Appl Toxicol 19:27–28
Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43:250–260
Ross BM, Hudson C, Erlich J, Warsh JJ, Kish SJ (1997) Increased phospholipid breakdown in schizophrenia. Evidence for the involvement of a calcium-independent phospholipase A2. Arch Gen Psychiatry 54:487–494
Ross BM, Moszczynska A, Erlich J, Kish SJ (1998) Phospholipid-metabolizing enzymes in Alzheimer’s disease: increased lysophospholipid acyltransferase activity and decreased phospholipase A2 activity. J Neurochem 70:786–793
Ross BM, Turenne S, Moszczynska A, Warsh JJ, Kish SJ (1999) Differential alteration of phospholipase A2 activities in brain of patients with schizophrenia. Brain Res 821:407–413
Salgo MG, Corongiu FP, Sevanian A (1993) Enhanced interfacial catalysis and hydrolytic specificity of phospholipase A2 toward peroxidized phosphatidylcholine vesicles. Arch Biochem Biophys 304:123–132
Sato T, Ishida T, Irifune M, Tanaka K, Hirate K, Nakamura N, Nishikawa T (2007) Effect of NC-1900, an active fragment analog of arginine vasopressin, and inhibitors of arachidonic acid metabolism on performance of a passive avoidance task in mice. Eur J Pharmacol 560:36–41
Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA (1997) 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem 68:2092–2097
Schaeffer EL, Gattaz WF (2005) Inhibition of calcium-independent phospholipase A2 activity in rat hippocampus impairs acquisition of short- and long-term memory. Psychopharmacology 181:392–400
Schaeffer EL, Gattaz WF (2007) Requirement of hippocampal phospholipase A2 activity for long-term memory retrieval in rats. J Neural Transm 114:379–385
Schaeffer EL, Bassi F Jr, Gattaz WF (2005) Inhibition of phospholipase A2 activity reduces membrane fluidity in rat hippocampus. J Neural Transm 112:641–647
Seigerschmidt E, Mösch E, Siemen M, Förstl H, Bickel H (2002) The clock drawing test and questionable dementia: Reliability and validity. Int J Geriatr Psychiatr 17:1048–1054
Shulman KI (2000) Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry 15:548–561
Six DA, Dennis EA (2000) The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta 1488:1–19
Smesny S, Kinder D, Willhardt I, Rosburg T, Lasch J, Berger G, Sauer H (2005) Increased Calcium-independent phospholipase A2 activity in first but not in multi-episode chronic schizophrenia. Biol Psychiatry 57:399–405
Song H, Ramanadham S, Bao S, Hsu FF, Turk J (2006) A bromoenol lactone suicide substrate inactivates group VIA phospholipase A2 by generating a diffusible bromomethyl keto acid that alkylates cysteine thiols. Biochemistry 45:1061–73
Sun GY, Xu J, Jensen MD, Simonyi A (2004) Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. Lipid Res 45:205–213
Talbot K, Young RA, Jolly-Tornetta C, Lee VM, Trojanowski JQ, Wolf BA (2000) A frontal variant of Alzheimer’s disease exhibits decreased calcium-independent phospholipase A2 activity in the prefrontal cortex. Neurochem Int 37:17–31
Taketo MM, Masahiro S (2002) Phospholipase A2 and apoptosis. Biochim Biophys Acta 1585:72–76
Tavares H, Yacubian J, Talib LL, Barbosa NR, Gattaz WF (2003) Increased phospholipase A2 activity in schizophrenia with absent response to niacin. Schizophr Res 61:1–6
Tomimoto H, Ohtani R, Shibata M, Nakamura N, Ihara M (2005) Loss of cholinergic pathways in vascular dementia of the Binswanger type. Dement Geriatr Cogn Disord 19:282–288
van den Berg JJ, Op den Kamp JA, Lubin BH, Kuypers FA (1993) Conformational changes in oxidized phospholipids and their preferential hydrolysis by phospholipase A2: a monolayer study. Biochemistry 32:4962–4967
White MC, McHowat J (2007) Protease activation of calcium-independent phospholipase A2 leads to neutrophil recruitment to coronary artery endothelial cells. Thromb Res 120:597–605
Wolf MJ, Izumi Y, Zorumski CF, Gross RW (1995) Long-term potentiation requires activation of calcium-independent phospholipase A2. FEBS Lett 377:358–362
Acknowledgments
Dr Stefan Smesny was supported by the German Research Foundation (DFG), grant Sm 68/1-1. Prof Jürgen Lasch was getting financial support by the German Research Foundation (DFG), grant LA 759/5-1. The financial support by the DFG was helpful and necessary to prepare and perform the chemical analysis of PLA2 activity in CSF. The authors would also like to thank the staff of the Department of Psychiatry and the Department of Neurology of the University of Jena for their extensive support in performing the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smesny, S., Stein, S., Willhardt, I. et al. Decreased phospholipase A2 activity in cerebrospinal fluid of patients with dementia. J Neural Transm 115, 1173–1179 (2008). https://doi.org/10.1007/s00702-008-0081-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-008-0081-0