Abstract
This was a retrospective pooled analysis of data from four comparably designed, double-blind, placebo-controlled, Phase III studies and their long-term open-label extensions. Patients on levodopa and a dopa decarboxylase inhibitor (DDCI) were randomized to entacapone or to placebo in the 6-month, double-blind phase, with all patients subsequently receiving entacapone in the extension phase. UPDRS III motor scores improved by −2.1 points during the first 6 months of levodopa/DDCI and entacapone therapy, and remained below baseline for up to 2 years. Increased daily ‘ON’ time, together with response duration to a single morning dose of levodopa and clinical global evaluation, also supported the long-term efficacy of levodopa/DDCI and entacapone. The mean daily dose of levodopa did not increase over the 5-year follow-up period. Long-term therapy with levodopa/DDCI and entacapone was well-tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Levodopa remains the most effective and best-tolerated treatment for Parkinson’s disease (PD) (Olanow et al. 2004), and is the only agent with a proven positive impact on the mortality rates of PD patients (Rajput 2001). A number of double-blind, levodopa-controlled trials of early dopamine agonist monotherapy have consistently shown that levodopa provides superior symptomatic efficacy as assessed by Unified Parkinson’s Disease Rating Scale (UPDRS) II (activities of daily living [ADL]), III (motor) and total scores. However, chronic treatment with levodopa is associated with an increased risk of motor complications, such as wearing off and dyskinesia (Bracco et al. 2004; Holloway et al. 2004; Oertel et al. 2006; Rascol et al. 2000).
Longitudinal studies of levodopa in PD patients demonstrate a gradual decline in patient function (assessed by UPDRS scores) over time and the continual need to adjust dopaminergic medications (Alves et al. 2005; Goetz et al. 2000). With advancing disease, PD patients are often prescribed increasingly higher and more frequent levodopa doses in an attempt to provide them with sustained levodopa bioavailability and to maintain symptom control over the course of the day. However, although these modification strategies can be useful in the short-term, there is general agreement that they are insufficient in the long-term (Stocchi 2006; Olanow et al. 2006). For example, dose fractionation may lead to suboptimal levodopa exposure, which is often associated with wearing-off symptoms. On the other hand, continually increasing the size of individual levodopa doses usually leads to increased severity of dyskinesias without marked prolongation of therapeutic benefit (Stocchi 2006).
Four placebo-controlled, randomized, double-blind, 6-month, Phase III studies have demonstrated that levodopa/dopa decarboxylase inhibitor (DDCI) and entacapone therapy is an effective management strategy for PD patients experiencing re-emergence of symptoms due to wearing-off, providing significant benefits in terms of symptom control compared with conventional levodopa/DDCI therapy (Brooks et al. 2003; Poewe et al. 2002; PSG 1997; Rinne et al. 1998). In these studies, daily ‘ON’-time increased by up to 1.4 h compared with levodopa/DDCI and placebo, with a corresponding reduction in ‘OFF’-time. In some instances this was accompanied by up to three-point reductions in UPDRS III scores measured during the best ‘ON’ (Rinne et al. 1998). These clinically relevant improvements were seen despite efforts to optimize levodopa and other antiparkinsonian therapy prior to randomization, and subsequent reductions in mean daily levodopa dose during the trials.
As part of the design of these double-blind Phase III trials, open-label safety and efficacy extension periods were included. To date, safety and efficacy data have been published from only one of these open-label extension studies covering a period of 3 years (Larsen et al. 2003). Here we present a pooled analysis of the four double-blind Phase III studies and their open-label extensions, in order to evaluate the efficacy and safety of long-term levodopa/DDCI and entacapone therapy in patients with PD.
Materials and methods
Study design
Pooled data of four comparably designed, randomized, double-blind, placebo-controlled Phase III studies (Brooks et al. 2003; Poewe et al. 2002; PSG 1997; Rinne et al. 1998) and their long-term, open-label extensions (up to 5 years), were analysed. An overview of the design of these four studies in presented in Table 1. The protocols of the original studies were all designed to ensure that results were reflective of a broad range of PD patients experiencing wearing-off whilst treated with conventional levodopa and other antiparkinsonian therapies. All four studies had a 6-month, double-blind phase when patients on levodopa/DDCI were randomized to entacapone or to placebo. Following a washout period of approximately 2 weeks, all patients consenting for the extension phase subsequently received open-label levodopa/DDCI and entacapone treatment for up to 5 years. The open-label extension phase was administratively terminated country-by-country, when marketing approval for the study medication (entacapone, Orion Pharma, Espoo, Finland) was granted. Written informed consent was obtained from each patient. The studies followed the guidelines of the Declaration of Helsinki and were approved by the Ethics Committees of the participating centres.
Patient population and study treatment
Details of the inclusion and exclusion criteria, similar across the four studies, have been reported elsewhere (Brooks et al. 2003; Poewe et al. 2002; PSG 1997; Rinne et al. 1998). Briefly, levodopa-responsive patients with idiopathic PD aged 30–80 years were included. Patients were also required to have PD symptoms that were not adequately controlled by their individually tailored levodopa/DDCI therapy, and other antiparkinsonian therapies. Both carbidopa and benserazide preparations were allowed. Controlled-release (CR) levodopa was permitted in two of the double-blind studies (Brooks et al. 2003; Poewe et al. 2002) and all safety extension studies (Larsen et al. 2003).
Concomitant treatment with amantadine, anticholinergics, the MAO-B inhibitor selegiline and dopamine agonists (except apomorphine) was permitted. The doses of all these antiparkinsonian drugs along with levodopa/DDCI were to be kept constant for at least 4 weeks prior to randomization. The use of neuroleptics, neuroleptic antiemetics, catechol-structured drugs, MAO-A inhibitors or non-selective MAO inhibitors was prohibited.
The levodopa regimen (and other individual antiparkinsonian medications) was optimized according to the treating physicians’ normal clinical practice prior to study entry. Entacapone 200 mg (or placebo in double-blind studies) was given concomitantly with each daily dose of levodopa. During each study, the levodopa dose could be individually adjusted, when clinically indicated, by either changing the levodopa dose or dosing frequency.
Efficacy assessments
Long-term efficacy was assessed by comparing follow-up data with baseline values. Baseline for the patient population in this pooled analysis was defined as the first visit prior to the start of levodopa/DDCI and entacapone treatment (i.e. at the start of the double-blind phase for patients on levodopa/DDCI initially randomized to receive entacapone, and at the start of open-label phase for patients on levodopa/DDCI initially randomized to receive placebo). All patients enrolled to the open-label studies were included in the efficacy analysis until the time point when only ten or fewer patients were available for follow up (average of 56 months).
Here we present the results of the efficacy measures which were evaluated during both the double-blind and open-label extension phases. UPDRS III motor scores (n = 485 at baseline) were available from three of the studies (Poewe et al. 2002; PSG 1997; Rinne et al. 1998). UPDRS scores were recorded on average 1.7 h after intake of levodopa when patients were at their best ‘ON’-state. Global clinical impression scores (n = 647 at baseline) and mean daily levodopa doses (n = 649 at baseline) were recorded in all studies (Poewe et al. 2002; PSG 1997; Rinne et al. 1998). The response duration of the first single morning dose of levodopa/DDCI and entacapone (n = 129) was measured in one double-blind study and its open-label extension phase (Larsen et al. 2003; Rinne et al. 1998).
Safety and tolerability
All patients having received at least one dose of levodopa/DDCI and entacapone were included in the safety analysis. Safety and tolerability were assessed at every visit by monitoring laboratory safety parameters, vital signs, ECG and adverse events (AEs). Particular attention was paid to hepatic safety, including analyses of SGOT/AST (aspartate amino transferase), SGPT/ALT (alanine amino transferase), GGT (gamma-glutamyl transferase), alkaline phosphatase and bilirubin. Potential clinically significant increases in these parameters were defined as levels three times above upper normal limits. Adverse events were classified by System Organ Classes and by Preferred Term using the WHO coding system.
Statistical analysis
The current analysis includes all patients enrolled in the double-blind and open-label extension periods of the four large-scale Phase III trials. All data were included in all the efficacy analyses with no selection of patients. All patients enrolled to the open-label studies were included in the efficacy analysis and all patients who received at least one dose of study medication, regardless of their participation into the extension study, were included in the safety analysis. Only observed cases were used, with no imputations for missing data.
Results
Baseline data
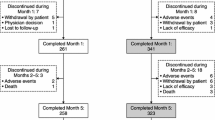
Of the 806 patients who completed the double-blind period, 649 patients (402 male, 247 women) continued in the open-label extension period of the studies, and were included in the efficacy analysis (Fig. 1). At baseline, the mean age (SD) of the patients was 63.1 (±9.4) years and the duration of PD was 9.5 (±5.2) years. The mean daily dose of levodopa was 678 (±363) mg. More than half of patients (54.5%) were using dopamine agonists and 41.4% were using selegiline. A total of 83.9% of patients showed Hoehn and Yahr staging of 2–3. The mean UPDRS part III (motor) score was 24.2 (±12.8) points at baseline and the mean response duration to a morning dose of levodopa was 2.2 (±0.7) hours (Table 2).
Efficacy
Pooled analysis of the UPDRS III scores showed an initial mean improvement of −2.1 (±7.3) points during the first 6 months of levodopa/DDCI and entacapone therapy. Thereafter, UPDRS III scores deteriorated at a rate of approximately 1.0 point/year, remaining below baseline for up to 2 years (Fig. 2).
The duration of response to the first single morning dose of levodopa/DDCI and entacapone treatment increased from 2.2 ± 0.7 at baseline to 2.8 ± 0.6 h at 6 months, and was consistently longer compared with baseline throughout the follow-up period (Fig. 3).
Global clinical assessment indicated that, compared with baseline, 77.1% of patients on levodopa/DDCI and entacapone therapy were either improved or unchanged at 6 months. This proportion remained stable (range 67.8–84.0%) for up to 52 months.
Figure 4 shows the mean daily levodopa dose in patients during the study period. Following initiation of levodopa/DDCI and entacapone, the mean daily levodopa dose could be reduced by 68 (±169) mg by 3 months and remained below baseline levels for up to 56 months.
Figure 5 shows the mean number of daily levodopa doses in patients during the study period. The mean number of levodopa doses remained stable throughout the study and did not change by more than 0.6 of a dose at any time point during the study, compared with baseline (Fig. 5).
Safety and tolerability
A total of 861 patients received at least one dose of levodopa/DDCI and entacapone therapy during the double-blind period or the extension phase, and were included in the safety analysis. Maximum follow-up time for safety was over 5 years.
Adverse events and premature discontinuation
In general, levodopa/DDCI and entacapone therapy was well-tolerated. Table 3 summarises the AEs that occurred in >10% of patients. The most commonly reported AEs were aggravation of parkinsonism (worsening of parkinsonian symptoms), dyskinesia and nausea. The majority of the dopaminergic AEs were encountered within the first 4 weeks of exposure to treatment and were generally mild-to-moderate, transient and often managed by adjustment of the levodopa dose. Patient withdrawal due to dopaminergic AEs was uncommon, with low rates of discontinuations due to dyskinesia (2.7%) and nausea (2.0%). Overall, approximately one third (36%) of dyskinesia events occurred within 1 week and about one fifth (22%) occurred >1–4 weeks after initiation of study medication. Similarly, the figures for nausea were one third (34%) of patients within 1 week and one fifth (19%) >1–4 weeks. The most commonly reported non-dopaminergic AE was diarrhoea (14%). This was never explosive and only 1.5% of patients on levodopa/DDCI and entacapone discontinued treatment due to diarrhoea over the 5-year period.
During the 5-year study period a total of 478 serious AEs (SAEs), including 31 deaths, were reported in 274 patients (31.8%). The most frequently reported SAEs were falls (24 patients, 2.8%) and hallucinations (18 patients, 2.1%). No cases of hepatotoxicity or other unexpected serious safety concerns were reported as SAEs.
A total of 248 (28.8%) patients discontinued the study treatment during the 5-year study period due to an AE, most of which were dopaminergic and/or PD-related in nature. An additional one fifth (19.5%; n = 168) of patients discontinued for other reasons such as protocol deviation or were lost to follow up. The remaining decline in the number of patients was not due to discontinuations but due to the administrative termination of the open-label study phase on a country-by-country basis, when marketing authorisation for entacapone was granted.
Vital signs and laboratory parameters
During the 5-year study period, most patients (>80%) showed no clinically relevant changes in blood pressure or heart rate. Similarly, few clinically significant changes in liver function tests, haematological parameters or urinalysis occurred during study treatment, with most changes being transient in nature (Table 4).
Discussion
This analysis is the first to present long-term efficacy (n = 649) and safety (n = 861) data for levodopa/DDCI and entacapone for a period of up to 5 years in a large cohort of PD patients. The benefits of levodopa/DDCI and entacapone therapy seen in the present analysis are consistent with the findings reported in previous studies with shorter duration (Brooks et al. 2003; Larsen et al. 2003; Myllyla et al. 2001; Poewe et al. 2002; PSG 1997; Rinne et al. 1998).
Analysis of the UPDRS III motor scores showed an initial significant improvement on initiation of entacapone, despite optimization of patients’ conventional levodopa/DDCI regimen prior to study entry. Thereafter, UPDRS III motor scores remained below baseline values for up to 2 years despite progression of disease. As the UPDRS III scores were determined during the patients’ best ‘ON’-time (a mean of 1.7 h following drug intake), the results indicate that levodopa/DDCI and entacapone improved patients’ quality of ‘ON’-time. After two years of treatment (up to 5 years), UPDRS III scores deteriorated by about 1.0 point/year. This finding is in line with the average annual deterioration rate reported previously with levodopa in a similar patient population in normal clinical practice (Goetz et al. 2000). Indeed, results from a study by Goetz et al. demonstrated that parkinsonian impairment was linear over time, despite significant increases in both levodopa and dopamine agonist doses over 4 years. Moreover, these increases were progressive over each year of the 4-year study (Goetz et al. 2000). However, in contrast to the study by Goetz et al., no increases in levodopa dose were observed in the present analysis, despite the fact that investigators were encouraged to adjust treatment of their patients during the open-label phase according to the patients’ clinical condition. Overall, the mean daily dose of levodopa was maintained below the baseline levels for a period of up to 56 months, extending the 3 year findings from the study reported by Larsen et al. (2003). Thus, in general, levodopa/DDCI and entacapone showed long-term efficacy without a need to increase levodopa doses. In addition, the benefits of levodopa/DDCI and entacapone were well perceived by patients throughout treatment, as reflected by the fact that the number of patients with similar or improved global evaluation scores remained at a high level (70–80%) throughout the follow-up period.
Our present results indicate that levodopa/DDCI and entacapone treatment not only improved patients’ quality of ‘ON’-time, but also increased the duration of ‘ON’-time. This was shown by assessing the response duration of the morning dose of levodopa. Previously published 3-year results demonstrated that the administration of a single dose of entacapone prolonged the levodopa-induced ‘ON’-time by approximately 40 min compared with baseline (Larsen et al. 2003). Our findings further extend these results demonstrating that the prolonged levodopa-induced ‘ON’-time was maintained for up to 56 months. It should be noted that the current analysis only includes ‘ON’-time data after the first morning dose of levodopa. A previous double-blind study found that the proportion of ‘ON’-time increased towards the end of the day (PSG 1997), suggesting that determining daily ‘ON’- and ‘OFF’-time by patient diary during long-term follow-up (which was unfortunately not available in the extension studies analyzed here) would have shown the full benefit of the study treatment in this respect.
The present long-term safety data did not reveal any new, unexpected safety concerns attributable to levodopa/DDCI and entacapone. Levodopa/DDCI and entacapone was generally well tolerated with most AEs being mild or moderate in intensity. Aggravated parkinsonism was often reported, but this is most likely due to disease progression rather than treatment. As expected, the most common AEs reported with levodopa/DDCI and entacapone therapy were those associated with increased dopaminergic stimulation (e.g. dyskinesia and nausea). The majority of these AEs were encountered within the first 4 weeks of exposure and, in general, were transient and managed by levodopa dose reduction, as shown by the low rates of discontinuations due to dyskinesia and nausea of 2.7 and 2.0%, respectively. The most commonly reported non-dopaminergic AE was diarrhoea (14%), comparable to the incidence reported at 6 months (Brooks et al. 2003; Poewe et al. 2002; PSG 1997; Rinne et al. 1998) and 3 years (Larsen et al. 2003). However, only 1.5% of patients discontinued treatment with levodopa/DDCI and entacapone due to diarrhoea over the 5-year assessment period.
Analysis of vital signs and laboratory parameters rarely revealed any clinically significant changes over the 5-year period. In particular, long-term use with levodopa/DDCI and entacapone was only rarely associated with significant changes in liver function tests; all of these were transient in nature.
There are limitations associated with the retrospective analysis of this pooled data. Although the designs of the individual studies were comparable, they were not identical and there were variations such as in the use of CR levodopa. In addition, the open-label extensions were primarily designed for the collection of long-term safety data, and the lack of a control group precludes comparative statistical analysis. However, both the current efficacy and safety findings provide valuable long-term experience with levodopa/DDCI and entacapone therapy that is relevant to clinical practice.
In conclusion, the present analysis demonstrates that levodopa/DDCI and entacapone therapy offers long-term efficacy for up to 5 years in a safe and well-tolerated manner without the need to increase levodopa doses.
References
Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP (2005) Progression of motor impairment and disability in Parkinson disease: a population-based study. Neurology 65(9):1436–1441
Bracco F, Battaglia A, Chouza C, Dupont E, Gershanik O, Marti Masso JF et al (2004) The long-acting dopamine receptor agonist cabergoline in early Parkinson’s disease: final results of a 5-year, double-blind, levodopa-controlled study. CNS Drugs 18(11):733–746
Brooks DJ, Sagar H (2003) Entacapone is beneficial in both fluctuating and non-fluctuating patients with Parkinson’s disease: a randomised, placebo controlled, double blind, six month study. J Neurol Neurosurg Psychiatry 74(8):1071–1079
Goetz CG, Stebbins GT, Blasucci LM (2000) Differential progression of motor impairment in levodopa-treated Parkinson’s disease. Mov Disord 15(3):479–484
Holloway RG, Shoulson I, Fahn S, Kieburtz K, Lang A, Marek K et al (2004) Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 61(7):1044–1053
Larsen JP, Worm-Petersen J, Siden A, Gordin A, Reinikainen K, Leinonen M (2003) The tolerability and efficacy of entacapone over 3 years in patients with Parkinson’s disease. Eur J Neurol 10(2):137–146
Myllyla V, Kultalahti ER, Haapaniemi H, Leinonen M (2001) Twelve-month safety of entacapone in patients with Parkinson’s disease. Eur J Neurol 8(1):53–60
Oertel WH, Wolters E, Sampaio C, Gimenez-Roldan S, Bergamasco B, Dujardin M et al (2006) Pergolide versus levodopa monotherapy in early Parkinson’s disease patients: The PELMOPET study. Mov Disord 21(3):343–353
Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonuccelli U, Damier P et al (2004) Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord 19(9):997–1005
Olanow CW, Obeso JA, Stocchi F (2006) Continuous dopamine-receptor treatment of Parkinson´s disease: scientific rationale and clinical implications. Lancet Neurol 5:677–687
Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M (2002) Efficacy and safety of entacapone in Parkinson’s disease patients with suboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study). Acta Neurol Scand 105(4):245–255
PSG (1997) Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Parkinson Study Group. Ann Neurol 42(5):747–755
Rajput AH (2001) Levodopa prolongs life expectancy and is non-toxic to substantia nigra. Parkinsonism Relat Disord 8(2):95–100
Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 342(20):1484–1491
Rinne UK, Larsen JP, Siden A, Worm-Petersen J (1998) Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Nomecomt Study Group. Neurology 51(5):1309–1314
Stocchi F (2006) The levodopa wearing-off phenomenon in Parkinson’s disease: pharmacokinetic considerations. Expert Opin Pharmacother 7(10):1399–1407
Acknowledgments
Studies included in this paper were sponsored by Orion Corporation, Orion Pharma. The authors would like to thank Diya Lahiri MSc PhD, for her editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brooks, D.J., Leinonen, M., Kuoppamäki, M. et al. Five-year efficacy and safety of levodopa/DDCI and entacapone in patients with Parkinson’s disease. J Neural Transm 115, 843–849 (2008). https://doi.org/10.1007/s00702-008-0025-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-008-0025-8